Abstract
Background: Numerous studies have shown the presence of multiple defence factors in placental tissue, although their role is partially understood; therefore, the aim of this study was to evaluate the expression of nuclear factor-kappa B (NF-κB); human beta-defensin 2, 3, and 4 (HBD-2,3,4); cathelicidine (LL-37); heat shock protein 60 (HSP60); and interleukin 10 (IL-10) in dissimilar gestational week placental tissue and display correlations between immunoreactive cells. Methods: A total of 15 human placental tissue samples were acquired from mothers with different gestational weeks: 28, 31, and 40. Routine staining and immunohistochemistry for the samples were executed. The evaluation of data was performed with semi-quantitative methods, and, for statistical analysis, the Kruskal–Wallis test was used. Spearman’s rank correlation was used for calculating correlations. Results: NF-κB, HBD- 2,3,4, HSP60, and IL-10 expression were discovered in every examined placental tissue cell type. LL-37 expression was found only in Hofbauer cells. A rise in expression with higher gestational weeks was noted in LL-37-positive Hofbauer cells (p = 0.03), HBD-3-positive cytotrophoblasts (p = 0.007), endothelial cells (p = 0.024), extraembryonic mesodermal cells (p = 0.004), and HBD-4-positive endothelial cells (p = 0.001). Numerous statistically significant moderate and strong positive correlations between defence factors were discovered. Conclusions: The persistence of Hofbauer cell accumulations underlines the growing significance of placental macrophages in placental protection. The expression of positive defence factors and a rise in expression in tissue protection factors (HBD-3, LL-37, HBD-4) in higher gestational weeks may indicate these factors as the most significant protectors of the placenta in ontogenetic aspects. The high number of statistically significant positive and negative correlations between positive cells show a strong network to sustain distressed placental growth and therefore pregnancy.
1. Introduction
The placenta is an important transitional fetal organ and plays a significant role in the health of the fetus and its mother. The placenta sustains fetus growth by delivering oxygen, nutrients, and hormones; moreover, it is also responsible for removing waste products. Flawed early placental development is the main cause of frequent disorders during pregnancy, including fetal growth restriction, pre-eclampsia, stillbirth, and recurrent miscarriage. In addition, poor pregnancy conditions affect the life-long health of the fetus [1,2,3].
The placenta acts as a physical, selective, and protective barrier between fetal and maternal circulation, stopping the possible transfer of different pathogens. Significant factors in this barrier are trophoblasts, and strong evidence reveals that trophoblasts function in organizing signals to optimize transport functions, hormone production in the placenta, and immunological defence mechanisms for the developing fetus [3]. Blood vessels, macrophages such as Hofbauer cells, extraembryonic mesodermal cells, and cytotrophoblasts have a role in protective development mechanisms to sustain healthy placenta function and therefore pregnancy [4,5,6,7].
Multiple tissue factors belong to the local protection systems in the placenta, although they have not been researched enough under different placental developmental times. Cathelicidins are a family of host defence peptides, and they are conserved and play an important role in innate immunity [8]. Cathelicidin’s (LL-37) C-terminal of this protein contains a 37-amino-acid-long peptide starting with two Leu residues, which shows broad antibacterial activity [8,9,10]. LL-37 protein is produced in neutrophils, T cells, natural killer (NK) cells, mast cells, and many other types of tissue cells. LL-37 can also be found in amniotic fluid [11]. LL-37 has multiple functions, including antimicrobial activity against multiple types of microorganisms. It plays a role in organizing the immune response towards infection, locally modulates inflammation, and promotes angiogenesis [8,12].
Nuclear factor kappa B (NF-κB) is a protein transcription factor, and it is considered to be a regulator of innate immunity [13,14]. The functions of NF-κB include regulating multiple pathways that can impact cellular function, including proliferation, differentiation, apoptosis, angiogenesis, epithelial-to-mesenchymal transition, and oxidative stress [15,16,17,18].
Human β Defensin 2 (hBD-2) is a cysteine-rich, cationic, low molecular weight antimicrobial peptide, showing its active antimicrobial activity [19]. hBD-2 is induced by inflammation and the infection of various microorganisms [20]. Human β-Defensins 2 are produced by epithelial cells of the chorionic membrane, amniotic membrane, and vaginal wall, and hBD-2 mRNA expression was noticed in placental, chorion, and villus cells [21,22,23]. High levels of hBD-2 in amniotic fluid have been reported in cases of preterm delivery, but a decrease in hBD-2 was noticed in patients with bacterial vaginosis during pregnancy; furthermore, hBD-2 is more active against gram bacteria [21,24], and hBD-2 could be a marker of intra-amniotic infections [22].
Human β defensin 3 (hBD-3) is a highly basic 45-amino-acid protein that acts both as an antimicrobial agent and as a chemoattractant molecule. It has broad-spectrum antibiotic activity against gram-negative and gram-positive bacteria and can show immunosuppressive activity as well [25,26,27,28]. hBD-3 mRNA expression is upregulated by treatment with inflammatory molecules that include IL-1β+TNFα, IFNγ, and phorbol ester [23]. The expression of hBD-3 in chorioamniotic membranes [29] and amnion and chorio-decidua of placental tissue has been found [30,31]. Furthermore, hBD-3 has been immunolocalized to the syncytiotrophoblast layer of term placental villi [30].
Human β defensin 4 (hBD-4) is a small positively charged cysteine-rich cationic polypeptide [32,33] that shows broad spectrum antimicrobial activity but compared with hBD1-3, it has low ionic strength [33]. There is expression of hBD-4 mRNA in the fetal membranes but, unlike hBD-1 and hBD-3, the expression of hBD-4 mRNA may not be totally affected by proinflammatory cytokine stimuli in chorioamniotic membranes [34].
Heat shock protein 60 (HSP60) are proteins that are chaperones and are mostly localized in the mitochondria of eukaryotic cells; they capture denatured substrate proteins in their central cavity [35,36,37]. HSP60 plays an important role in cell development, reproduction, thermoprotection, and immune defence [35,38]. HSP60 and other HSPs such as HSP90 and HSP70 are found to be localized in cytotrophoblasts, syncytiotrophoblasts, intermediate trophoblasts, Hofbauer, and endothelial cells [39]. Based on an immunostaining study, HSP60 was also immunolocalized in the decidual stromal cells during each trimester of pregnancy [39,40].
Interleukin 10 (IL-10) is a potent anti-inflammatory cytokine that plays a crucial and important role in preventing inflammatory and autoimmune pathologies [41]. IL-10 diminishes the production of inflammatory mediators and inhibits antigen presentation, although it enhances their uptake of antigens [41,42]. IL-10 regulates the differentiation and proliferation of several immune cells, including T cells, B cells, natural killer (NK) cells, antigen-presenting cells, mast cells, and granulocytes [43,44]. IL-10 expression has been found in placental villous trophoblasts, uterine NK cells (uNK cells), monocytes, and regulatory T cells in the decidua, and IL-10 receptors are localized to placental trophoblasts, decidual stromal cells, macrophages, and uterine NK cells (uNK cells) [43,45]. Alternatively activated macrophages (M2 macrophages) secrete high levels of the IL-10 cytokine upon polarization, and Hofbauer cells are thought to have an immunoregulatory phenotype consistent with being an alternatively activated macrophage (M2 macrophage); these cells can be stimulated by glucocorticoids and IL-10 while also secreting Il-10 [46,47].
Since the role of protective factors and their possible interaction in placental tissue is still not fully understood, the goal of this study was to discover the appearance and possible interaction of different defence factors in different developmental time placental tissues.
2. Materials and Methods
2.1. Subjects
The study was performed in accordance with the Helsinki Declaration in Latvia at Riga Stradiņs University Institute of Anatomy and Anthropology. Ethical committee at Riga Stradins University approved this research and permit was issued in March 2009 (12.03.2009 No. E-9 (2)). A total of 15 human placental tissue samples were obtained from 15 mothers with gestational weeks from 28 weeks to 40 weeks (Table 1). Mothers were aged 20 to 39 years with a number of graviditas ranging from I to VI. However, six females had one or multiple miscarriages earlier in their lives, resulting in the number of partus not matching the number of graviditas. Five placental tissue samples were obtained from mothers with delivery week 28, five placental tissue samples were from delivery week 31, and five placental tissue samples were from delivery week 40. A total of 10 placental tissue samples that were from week 28 and from week 31 can be classified as preterm delivery [48]. Eight of these deliveries were spontaneous preterm deliveries and two were noted as partum premature operationis. Five tissue samples from delivery week 40 were noted as partus mature with distress acuta. Inclusion criteria for tissue samples were delivery week.

Table 1.
Characteristics of placental tissue samples from different mothers with different delivery weeks and possible associated problems.
2.2. Control Samples
A total of 5 human placental tissue control samples were obtained from 5 mothers with gestational age of 40 weeks. Mothers were aged from 27 to 42 years with a number of graviditas ranging from I to IV. The inclusion criteria for tissue samples was delivery week. Exclusion criteria were presence of any associated problem with pregnancy (Table 2).

Table 2.
Characteristics of placental tissue control samples from mothers with delivery week 40.
2.3. Routine Morphological Assessment
Right after delivery, using single-use surgical knife, a cut was made and two 1 cm × 1 cm samples were acquired from symmetrically situated locations of the placentas, including every single layer of placental tissue. Acquired placental tissue samples were fixated for 24 h using 2% formaldehyde, 0.2% picric acid, and 0.1M phosphate buffer with a pH of 7.2. For 12 h, the samples were processed in Tyrode buffer, which contained 10% saccharose. Following this, the tissues were embedded into paraffin and cut into 5 µm sections. In addition to the morphological evaluation of placental tissue samples, hematoxylin and eosin staining was executed.
For immunohistochemical labelling the Biotin–Streptavidin method [49] was used to detect the following: NF-κB (ab7971 working dilution 1:100, Abcam, Cambridge, UK), HBD-2 (ab203077, working dilution 1:200, Abcam, Cambridge, UK), HBD-3 (orb183268, working dilution 1:100, Biorbyt, St Louis, MO, USA), HBD-4 (ab70215, working dilution 1:100, Abcam, Cambridge, UK), HSP-60 (sc-1052, working dilution 1:100, Santa Cruz Biotechnology, Dallas, TX, USA), Il-10 (ab134742, working dilution 1:50, Abcam, Cambridge, UK), LL-37 (orb88370, working dilution 1:100, Biorbyt LLC, St Louis, MO, USA).
2.4. Immunohistochemical (IHC) Analysis
For the evaluation of immunoreactive cells and their distribution, light microscopy and semi-quantitative non-parametric analysis were used in this study. Positively stained cells in placental tissue in the light microscope visual field were rated using a scale, which had the following labels: 0 no positive cells; 0/+ occasional (less than 10) positive cells; + few (10–15) positive cells; +/++ few to moderate (16–20) positive cells; ++ moderate (21–35) positive cells; ++/+++ moderate to numerous (36–50) positive cells; +++ numerous (51–70) positive cells; +++/++++ numerous to abundant (71–90) positive cells; ++++ abundant (more than 90) positive cells [50]. Positive placental tissue structures were observed in 5 visual fields, and rounded average was stated as the result of the specific sample. For all visual fields, magnification of x100 was used.
2.5. Statistical Analysis
To process obtained data from IHC analysis of positive cells in tissue samples, data were transformed into numerical form: 0: equals 0, 0/+: equals 0.5, +: equals 1, +/++: equals 1.5, ++: equals 2, ++/+++: equals 2.5, +++: equals 3, +++/++++: equals 3.5, ++++: equals 4. Kruskal–Wallis test was used to compare differences in factors based on placenta’s tissue age in distressed placental tissue sample group. The Mann–Whitney U test was used to assess if there were statistically significant differences in defence factor expression in placental structures between 40 gestational week control and distressed group. In addition, Spearman’s rank correlation coefficient was found and used to assess correlations between the chosen defence factors in distressed placental tissue sample group. Correlation with a value R < 0.2 counted as a very weak correlation, R = 0.20–0.39 expressed a weak correlation, R = 0.40–0.59 expressed a moderate correlation, R = 0.60–0.79 expressed a strong correlation, and 0.80–1.00 expressed a very strong correlation. Analyzing statistically significant differences in studied factors based on placental tissue age and correlations between the defence factors, all the results that contained p value < 0.05 were regarded as statistically significant. We processed data using IBM SPSS software version 27.0 (IBM company, North Castle, Armonk, NY, USA).
3. Results
3.1. Routine Changes
Every examined placental tissue revealed numerous accumulations of Hofbauer cells (Figure 1a). An abundant number of inflammatory cell infiltration in a few areas was noticed in almost all examined placental tissue samples except in control samples, where there was no significant inflammatory cell infiltration observed (Figure 1b). Edema was observed in two samples (Figure 1c), and a plethora in five samples (Figure 1d), although all these changes did not differ between the gestational weeks.
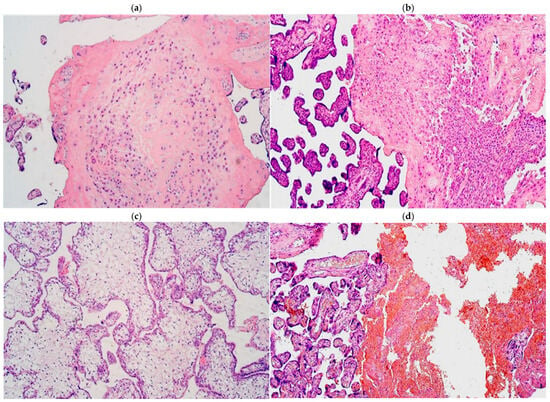
Figure 1.
(a–d) Routine staining micrographs of placental tissue samples: (a) numerous Hofbauer cells in 31 gestational week placenta; (b) 28 gestational week placenta containing inflammatory cells; (c) 31 gestational week edematic placenta; (d) 28 gestational week placenta with blood vessel plethora. Hematoxylin and eosin, ×100 for all the slides.
3.2. Immunohistochemistry
3.2.1. Distressed Placental Tissue Samples
The median number of HBD-2-immunopositive cytotrophoblasts ranged from none (0) to few (+) in gestational weeks 28, 31, and 40. Similarly, the median number of HBD-2-immunopositive endothelial cells varied from none (0) to occasional (0/+) in the samples examined across different gestational weeks. However, the median number of HBD-2-immunoreactive extraembryonic mesodermal cells ranged from none (0) to moderate (++), and HBD-2-immunoreactive Hofbauer cells ranged from few (+) to moderate (++) (Table 3, Figure 2a,b).

Table 3.
Median relative number of HBD-2-, HBD-3-, HBD-4-, NF-κB-, HSP60-, and IL-10-positive structures in distressed placental tissue across different gestational weeks.
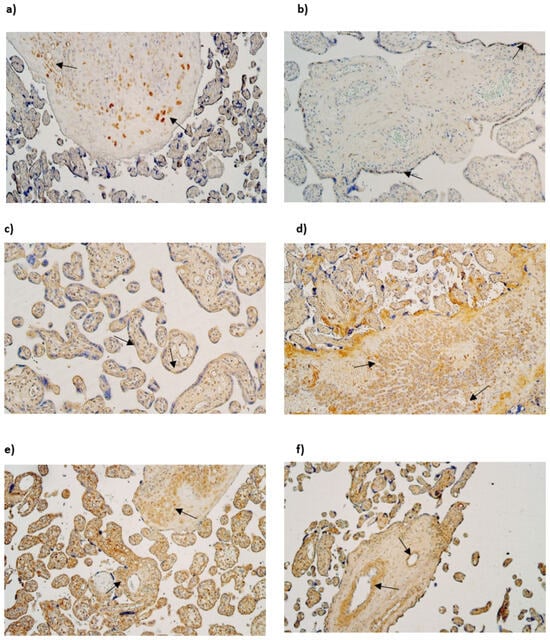
Figure 2.
(a–f) IMH micrographs of placental tissue samples with different gestational weeks: (a) moderate number of HBD-2-positive Hofbauer cells in 28 gestational week placenta (arrows). HBD2 IMH, ×100; (b) few to moderate HBD-2-positive cytotrophoblast cells in tertiary villy in 31 gestational week placenta (arrows). HBD-2 IMH, ×100; (c) moderate number of HBD3-positive cytotrophoblast cells in tertiary villy in 28 gestational week placenta (arrows). HBD-3 IMH, ×100; (d) numerous to abundant HBD-3-positive Hofbauer cells in 40 gestational week placenta (arrows) HBD-3 IMH, ×100; (e) numerous to abundant HBD-4-positive extraembryonic mesodermal and Hofbauer cells in 28 gestational week placenta (arrows). HBD-4 IMH × 100; (f) abundant number of HBD-4-positive endothelial cells of tertiary villy in 40 gestational week placenta (arrows). HBD-4 IMH, ×100.
The median number of HBD-3-immunopositive cytotrophoblasts had a variation from moderate (++) to numerous (+++) in examined different gestational week samples. The median number of HBD-3-immunoreactive endothelial cells in gestational weeks 28 and 31 was few (+), although in week 40 it was moderate (++). The median number of HBD-3-immunopositive extraembryonic mesodermal cells varied from moderate (++) to abundant (++++) in different gestational week samples, although the median number of HBD-3-immunoreactive Hofbauer cells in week 28 was few to moderate (+/++) and in week 31 and 40—moderate (++) (Table 3, Figure 2c,d).
The median number of HBD-4-immunoreactive cytotrophoblasts varied from the lack (0) to moderate (++) in gestational weeks 28, 31 and 40. The median number of HBD-4-immunopositive endothelial cells in weeks 28 and 31 was few (+), although in week 40 it was moderate to numerous (++/+++). In contrast the median number of HBD4-immunoreactive extraembryonic mesodermal cells was moderate to numerous (++/+++) in weeks 28, 31 and 40. The median number of HBD-4-immunoreactive Hofbauer cells in 28 and 31 gestational week samples was numerous to abundant (+++/++++), although in gestational week 40 median number was numerous (+++) (Table 3, Figure 2e,f).
NF-κB was not discovered in cytotrophoblasts. The median number of NF-κB-immunoreactive endothelial cells varied from moderate to numerous (++/+++) in gestational weeks 28, 31 and 40. The median number of NF-κB-immunoreactive extraembryonic mesodermal cells varied from few to moderate (+/++) in examined samples and the median number of immunoreactive Hofbauer cells was occasional (0/+) in gestational week 28, although in gestational week 31 and 40, it was few (+) (Table 3, Figure 3a,b).
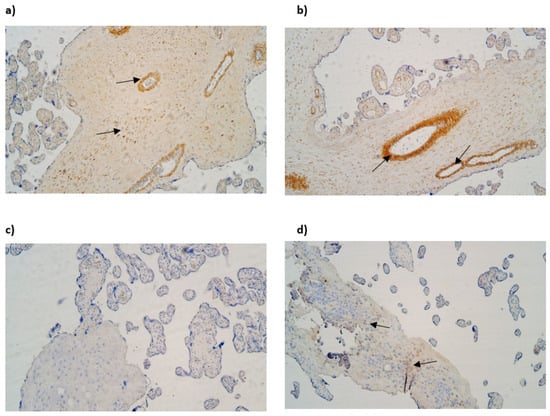
Figure 3.
(a–d) IMH micrographs of placental tissue of different gestational weeks: (a) Numerous to abundant positive NF-κB cells in endothelium and extraembryonic mesoderm in 31 gestational week placenta (arrows). NF-kB IMH, ×100; (b) abundance of NF-kB-positive endothelial cells in 40 gestational week placenta (arrows). NF-κB IMH, ×100; (c) absence of LL-37-positive cells in placental tissue in 28 gestational week placenta. LL-37 IMH, ×100; (d) few to moderate LL-37-positive Hofbauer cells in 40 gestational week placenta (arrows). LL-37 IMH, ×100.
Cathelicidin (LL-37) immunopositivity was observed in Hofbauer cells only, although only in samples of gestation week 40 and it had a variety of the lack (0) to few (+) positive cells (Figure 3c,d).
The median number of HSP60-immunopositive cytotrophoblasts and Hofbauer cells in all examined samples was moderate (++). In contrast, the median number of HSP60-immunoreactive endothelial cells was occasional (0/+) in gestational weeks 28 and 31, while in week 40 it was few to moderate (+/++). The median number of HSP60-immunopositive extraembryonic cells in week 28 was occasional (0/+), while in weeks 31 and 40, it was few (+) (Table 3, Figure 4a,b).
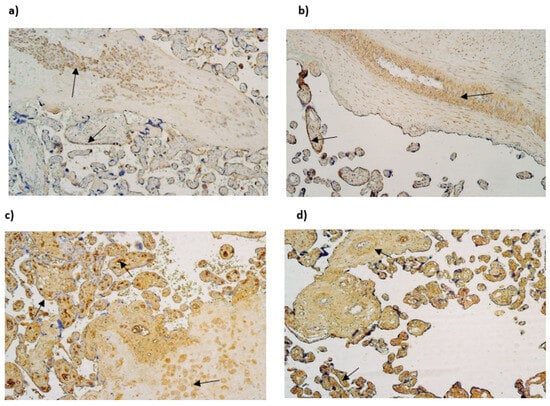
Figure 4.
(a–d) IMH micrographs of placental tissue of different gestational weeks; (a) moderate to numerous number of HSP60-positive Hofbauer and moderate number HSP60-positive cytotrophoblasts in 31 gestational week placenta (arrows). HSP60 IMH × 100; (b) abundant number of HSP60-positive endothelial and cytotrophoblast cells in placental tissue in 40 gestational week placenta (arrows). HSP60 IMH × 100; (c) numerous to abundant number of IL-10 in 28 gestational week placenta (arrows). IL-10 IMH, ×100; (d) moderate to numerous number of IL-10-positive endothelial cells and numerous to abundant number of IL-10-positive extraembryonic mesodermal cells in 40 gestational week placenta (arrows). IL-10 IMH, ×100.
The median number of IL-10-immunopositive cytotrophoblasts was moderate (++) in samples of all gestational weeks. Interestingly, the median number of immunoreactive endothelial and extraembryonic mesodermal cells was moderate to numerous (++/+++) in weeks 28 and 40 and numerous (+++) in weeks 31. The median number of IL-10-immunoreactive Hofbauer cells in weeks 28 was moderate (++), although, in weeks 31 and 40, it was numerous (+++) (Table 3, Figure 4c,d).
3.2.2. Control Placental Tissue Samples
In control samples of gestational week 40, the median number of HBD-2-immunopositive cytotrophoblasts was few (+), the median number of HBD-2-immunopositive endothelial cells was occasional (0/+), the median number of HBD-2-immunoreactive extraembryonic mesodermal was few to moderate (+/++), and the median number of HBD-2-immunoreactive Hofbauer cells was moderate (++) (Table 4, Figure 5a).

Table 4.
Median relative number of HBD-2-, HBD-3-, HBD-4-, NF-κB-, HSP60-, and IL-10-positive structures in control placental tissue.
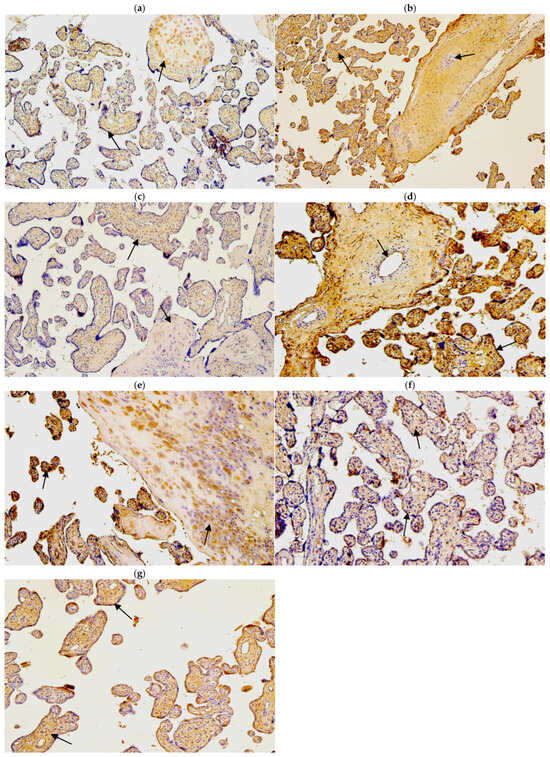
Figure 5.
(a–g). IMH micrographs of control samples of placental tissue; (a) numerous number of HBD-2-positive Hofbauer and few number of HBD-2-positive cytotrophoblasts in 40 gestational week placenta (arrows). HBD-2 IMH, ×100; (b) numerous to abundant number of HBD-3-positive extraembryonic mesodermal cells and few HBD-3-positive endothelial cells in 40 gestational week placenta (arrows). HBD-3 IMH, ×100; (c) moderate number of HBD-4-positive extraembryonic cells and few number of HBD-4-positive Hofbauer cells in 40 gestational week placenta (arrows). HBD-4 IMH, ×100; (d) moderate number of NF-κB-positive endothelial cells and abundant number of NF-κB-positive cytotrophoblasts in 40 gestational week placenta (arrows). NF-κB IMH, ×100; (e) numerous to abundant number of IL-10-positive Hofbauer cells and numerous number of IL-10-positive cytotrophoblasts in 40 gestational week placenta (arrows). IL-10 IMH, ×100; (f) moderate number of HSP60-positive cytotrophoblasts and extraembryonic mesodermal cells in 40 gestational week placenta (arrows). HSP60 IMH, ×100; (g) numerous number of LL-37-positive extraembryonic mesodermal cells and moderate number of LL-37-positive cytotrophoblasts (arrows). LL-37 IMH, ×100.
In control samples of gestational week 40, the median number of HBD-3-immunopositive cytotrophoblasts was moderate to numerous (++/+++). The median number of HBD-3-immunoreactive endothelial cells was few (+). The median number of HBD-3-immunopositive extraembryonic mesodermal cells was numerous to abundant (++/+++), although the median number of HBD-3-immunoreactive Hofbauer cells was few to moderate (+/++) (Table 4, Figure 5b).
In control samples of gestational week 40, the expression of HBD-4 in cytotrophoblasts was occasional (0/+), and the median number of HBD-4-immunopositive endothelial cells and extraembryonic mesodermal cells was few (+). In contrast, the median number of HBD-4-immunoreactive Hofbauer cells was moderate (++) (Table 4, Figure 5c).
The median number of NF-κB-positive cytotrophoblasts was numerous (+++) The median number of NF-κB-immunoreactive extraembryonic mesodermal cells was moderate (++), although the median number of NF-κB-immunoreactive endothelial cells and Hofbauer cells was few to moderate (+/++) (Table 4, Figure 5d).
The median number of IL-10-immunopositive cytotrophoblasts was moderate (++). Interestingly, the median number of immunoreactive extraembryonic mesodermal and Hofbauer cells was moderate to numerous (++/+++), although the median number of IL-10-immunopositive endothelial cells was few (+) (Table 4, Figure 5e).
The median number of HSP60-immunopositive cytotrophoblasts, extraembryonic mesodermal cells, and Hofbauer cells was moderate (++). The median number of HSP60-immunoreactive endothelial cells was few (+) (Table 4, Figure 5f).
The median number of Cathelicidin (LL-37)-immunopositive cytotrophoblasts and Hofbauer cells was moderate (++), the median number of Cathelicidin (LL-37)-immunoreactive endothelial cells occasional (0/+), and the median number of immunopositive extraembryonic mesodermal cells was moderate to numerous (++/+++) (Table 4, Figure 5g).
Statistically significant differences between distressed placental tissue samples were evaluated using the Kruskal–Wallis test between different gestational week placental tissue samples in LL-37-positive Hofbauer cells, HBD-3-positive cytotrophoblasts, endothelial cells, extraembryonic mesodermal cells, and HBD-4-containing endothelial cells. The relative median number of LL-37-immunopositive cells in gestational weeks 28 and 31 was 0 (0), although in gestational week 40, it was 1 (+). The relative median number of HBD-3-immunopositive cytotrophoblasts in gestational week 28 was 2 (++), and, in week 31, it was 2.5 (++/+++), although, in week 40, it was 3 (+++). The relative median number of HBD-3-immunoreactive endothelial cells in gestational weeks 28 and 31 was 1 (+), although in week 31, it was 2 (++). The relative median number of HBD-3-immunopositive extraembryonic mesodermal cells in gestational week 28 was 2 (++), 3 in week 31 (+++), and 4 in week 40 (++++). The relative median number of HBD-4-immunoreactive endothelial cells in weeks 28 and 31 was 1 (+), although in week 40, it was 2.5 (++/+++) (Table 5, Figure 6).

Table 5.
Statistically significant differences between relative number of defence factors in placental tissue samples of placentas across different gestational weeks.
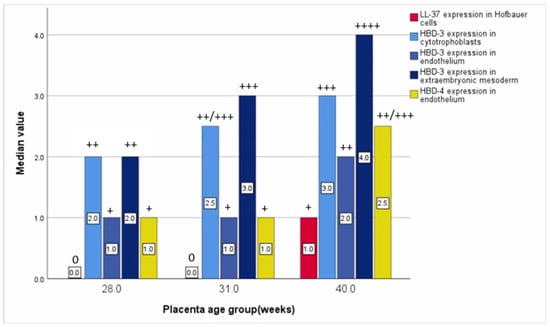
Figure 6.
Relative median number of statistically significant evaluated defence factors based on placenta’s gestational weeks.
Statistically significant differences between control placental tissue samples and distressed placental tissue samples of gestational week 40 were found using the Mann–Whitney U test in NF-κB-positive cytotrophoblasts and endothelial cells, HBD-2-containing extraembryonic mesodermal cells, HBD-4-containing extraembryonic mesodermal and endothelial cells, and LL-37-positive extraembryonic mesodermal cells and cytotrophoblasts. Furthermore, statistically significant differences between IL-10-positive endothelial cells and HSP60-positive extraembryonic mesodermal cells were found. The relative median number of NF-κB-immunopositive cytotrophoblasts in distressed placental tissue of gestational week 40 was 0 (0), although, in control samples of gestational week 40, it was 3 (+++). The relative median number of NF-κB-immunopositive endothelial cells in distressed placental tissue of gestational week 40 was 3 (+++), although in control samples, it was 1.5 (+/++). Furthermore, the relative median number of HBD-2-immunopositive extraembryonic mesodermal cells in distressed placental tissue of gestational week 40 was 0 (0), although, in control samples, it was 1.5 (+/++). The relative median number of HBD-4-immunopositive extraembryonic mesodermal cells and endothelial cells in distressed placental tissue of gestational week 40 was 2.5 (++/+++), which is in contrast to control samples, where both relative median numbers of the expression in these structures were 1 (+). In addition, in distressed placental tissue of gestational week 40, LL-37-positive extraembryonic mesodermal cells and cytotrophoblasts were not found, although the relative median numbers of LL-37-positive extraembryonic mesodermal cells and cytotrophoblasts in control samples were 2 (++) and 1.5 (+/++), respectively. The relative median number of IL-10-positive endothelial cells in distressed placental tissue samples of gestational week 40 was 2.5 (++/+++), although, in control samples, it was 1 (+). The relative median number of HSP60-immunopositive extraembryonic mesodermal cells in distressed placental tissue samples of gestational week 40 was 1 (+), although, in control samples, it was 2 (++) (Table 6).

Table 6.
Statistically significant differences between relative number of defence factors comparing distressed placental tissue samples of gestational week 40 and control placental tissue samples of gestational week 40.
Correlation analysis revealed multiple statistically significant correlations between the factors of immunopositive cells in distressed placental tissue. A statistically notable and very strong positive correlation (R = 0.854; p = <0.001) was calculated between HBD-3-immunoreactive cytotrophoblasts and HBD-3-immunoreactive extraembryonic mesodermal cells. Statistically notable and strong positive correlations (R = 0.6–0.8) were evaluated in multiple immunopositive cells between HBD-2-immunoreactive cytotrophoblasts and HBD-2-immunoreactive extraembryonic mesodermal cells (R = 0.612; p = 0.015); between HBD-2-immunopositive extraembryonic mesodermal cells and HBD-4-immunopositive extraembryonic mesodermal cells (R = 0.616; p = 0.014); between HBD-3-immunoreactive cytotrophoblasts and HBD-4-immunopositive endothelial cells (R = 0.741; p = 0.002); between HBD-3-immunoreactive extraembryonic mesodermal cells and HBD-4-immunoreactive endothelial cells (R = 0.716; p = 0.003); between HBD-4-immunopositive endothelial cells and LL-37-immunopositive Hofbauer cells (R = 0.716; p = 0.003); between NF-κB-immunoreactive endothelial cells and NF-κB-immunoreactive extraembryonic mesodermal cells (R = 0.643; p = 0.010); between NF-κB-immunoreactive Hofbauer cells and IL-10-immunoreactive cytotrophoblasts (R = 0.603; p = 0.017); between NF-κB-immunopositive endothelial cells and HBD-4-immunopositive extraembryonic mesodermal cells (R = 0.755; p = 0.001); between NF-κB-immunoreactive extraembryonic mesodermal cells and IL-10-immunoreactive endothelial cells (R = 0.770; p = <0.001); between NF-κB-immunopositive extraembryonic mesodermal cells and HBD-4-immunopositive extraembryonic mesodermal cells (R = 0.697; p = 0.004); and between IL-10-immunoreactive extraembryonic mesodermal cells and IL-10-immunoreactive cytotrophoblasts (R = 0.771; p = <0.001). Interestingly, a strong negative correlation (R = −0.678; p = 0.005) was calculated between HSP60-positive endothelial cells and HBD-4-positive Hofbauer cells (Table 7, Figure 7).

Table 7.
Statistically notable correlations between factors in placental structures.
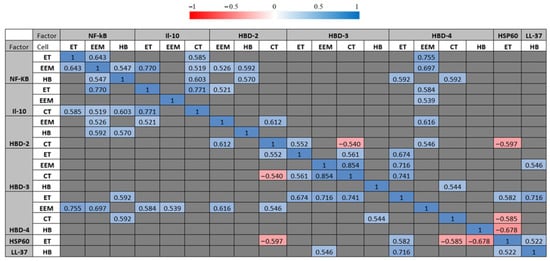
Figure 7.
Statistically significant correlation values between examined factors (p < 0.05). Spearman’s rank correlation test was used for analysis. Defence factors with one or more statistically significant correlations are shown. Correlation values are coloured based on correlation legend. Correlation legend is shown on top. Red colours indicate a negative correlation between defence factors, while blue colours show a positive correlation between defence factors. The saturation of the colour indicates stronger (negative or positive) Spearman’s rank correlation coefficients. Correlation values that were not statistically significant are marked as grey cells.
4. Discussion
In this study, HBD-2, HBD-3 and HBD-4 expression was observed in all distressed placental structures. However, we found that the relative number of HBD-2-positive cytotrophoblasts and extraembryonic mesodermal and endothelial cells had a tendency to decrease with higher gestational weeks. In contrast, there were statistically significant differences in the relative number of HBD-3-positive extraembryonic mesodermal and endothelial cells that increased with higher gestational weeks. According to these findings, we suspect that HBD-2 expression is not essential for placenta maturation, and it is substituted by an increase in selective HBD-3 expression mainly in the endothelium and extraembryonic mesoderm. It is known that in placental tissue, fungi and bacteria act as stimuli for HBD-2 expression [22], and, during early pregnancy, low levels of HBD-2 suggest the presence of a poor vaginal environment, thus increasing the possibility of developing (premature rupture of membranes (PROM)), affecting placental tissue as well [20]. Therefore, in this study, the higher expression of HBD-2 in gestational weeks 28 and 31 could be linked with the absence of a poor vaginal environment and probably the presence of pathogenic microorganisms. Our findings that show HBD-3 expression increases with higher gestational weeks are similar to the findings by Anne E King et al. in 2003 [23], which showed that the amniotic fluid concentrations of HBD-3 were higher in women that have spontaneous labour at term; moreover, HBD-3 expression does not change with gestational age in normal pregnancies [23], which is contradictory to our findings of various expression levels in different gestational weeks groups. The expression of HBD-4 between three gestational weeks groups varied in placental structures, indicating the presence of some individual factor affecting the expression, although no significant differences between these groups were noted. In the extraembryonic mesoderm and Hofbauer cells, it was consistently high throughout all three gestational weeks groups. HBD-4 expression showed a tendency to decrease in cytotrophoblasts in higher gestational weeks, although a statistically significant increase in HBD-4-positive endothelial cells with higher gestational weeks was observed. These are completely new findings and the variation in expression could be linked with circumstances of placenta distress. It is known that compared with other β -defensins, which are associated with strong chemotaxis, HBD-4 does not induce CCR6-mediated chemotaxis [51], although HBD-4 can induce a significant migration of monocytes [52]. The overall expression of HBD (2,3,4) in placental structures was selective. HBD-3 and HBD-4 were mainly produced in extraembryonic mesoderm and Hofbauer cells, although HBD-2 was largely produced only in Hofbauer cells. Interestingly, a very strong correlation was calculated between HBD-3-immunoreactive cytotrophoblasts and HBD-3-immunoreactive extraembryonic mesodermal cells, which reveals that HBD-3 function is strongly linked with various placental structures.
In this study, high IL-10 expression was observed in all distressed placental structures, and no statistically significant differences in IL-10 expression were observed between the groups. It was consistent without great variation throughout all gestational weeks, indicating that IL-10 activity in the placenta is consistent during pregnancy. We suspect that IL-10 is important in sustaining the successful growth of the placenta throughout pregnancy. Different studies suggest that decidual macrophages produce Il-10 and are the major source of IL-10; apart from the location, there are no specific markers to distinguish decididual macrophages from Hofbauer cells [47,53]. There are reports that IL-10 is a critical molecule for successful pregnancy outcomes; in addition, in contrast to this study, where IL-10 expression was consistent between all three gestational week groups, the placental expression of IL-10 was found to be reduced in spontaneous abortions and preterm births [43].
In this study, LL-37 expression in distressed placental structures was detected only in a few Hofbauer cells in gestational week 40 placenta, although it was a statistically significant result compared with earlier gestational weeks. Also, it is known that LL-37 expression is higher in fetal membranes and myometrium after term labour. LL-37 also induces proinflammatory and pro-labour mediators via the MyD88/NF-kB pathway [54]. Higher LL-37 presence in higher gestational weeks placenta and only in Hofbauer cells may indicate that LL-37 is not an important component in protective mechanisms in the placenta; however, a study in 2015 by Ratana Lim et al. [54] showed that in vitro LL-37 boosts the immunosuppressive function of placenta-derived mesenchymal stromal cells and modulates TLR3 expression, promoting higher levels of anti-inflammatory factors. A study in 2016 by Martha Oliveira-Bravo et al. showed that increased levels of LL-37 can lead to an increased expression of IL-10; however, in our study, there was no statistically significant correlation between LL-37 and IL-10 expression [55].
The expression of NF-kB varied between distressed placental structures. A high relative number of NF-kB-positive cells was seen mainly in the endothelium, and it showed a tendency to increase in higher gestational weeks; therefore, we suspect that NF-kB plays an important role in advanced stages of pregnancy, modulating vascular function in the placenta. These findings are supported by a study in 2020 by Armistead et al. [56], which showed that NF-kB is highly expressed in the placentas of women with pre-eclampsia where there is vascular dysfunction. It is also known that there are molecules that can activate NF-kB, such as damage-associated molecular patterns (DAMPs). They are molecules released when there is cellular stress, and they activate NF-KB-1 through TLR receptor pathways, inducing proinflammatory cascades [16,56,57]. NF-kB is associated with preterm birth when there is an interaction with activator protein 1 (AP-1) [56]. Extravillous trophoblast invasion is partially regulated by NF-kB [55]. Epithelial-to-mesenchymal transition is regulated by NF-kB and it plays a significant role in extravillous trophoblasts [58]. Our findings of increased expression in higher gestational week placenta can be supported by a study in 2018 by Sakowicz [38], which showed that higher NF-kB expression is seen in the third trimester of normal pregnancy in the decididua, where this factor induces cervical ripening and the degradation of the extracellular matrix to initiate the rupture of placental membranes. Lower NF-Kb expression was seen in the extraembryonic mesoderm and Hofbauer cells throughout all three gestational weeks groups. These findings indicate NF-kB’s capabilities to support placenta function during pregnancy. A lack of NF-kB expression was observed in cytotrophoblasts across all gestational week placentas.
A higher expression of HSP60 was steadily seen in the cytotrophoblasts and Hofbauer cells, although expression levels did not change between the three gestational week groups. A lower expression of HSP60 was seen in the endothelium and extraembryonic mesoderm and the expression levels were unvarying in the three gestational weeks groups as well. Interestingly, a strong negative correlation was calculated between HSP60-positive endothelial cells and HBD-4-positive Hofbauer cells, which shows possible negative feedback loop actions between these factors. The findings in this study are newly discovered, and we suspect that HSP60 plays an important role in supporting protective mechanisms in the placenta during pregnancy. It is also acknowledged that in pregnancy, HSP60 plays a role in inducing the synthesis of steroid hormones, particularly progesterone synthesis. Preterm premature rupture of the membranes and spontaneous preterm labour are associated with the dysregulated expression of HSP60 and other HSPs, and expression is found to be severely altered [40]. One of the mechanisms of action for HSP60 includes the ability to interact with HSP70 to form an HSP60-HSP70 complex, and this complex allows the transport of proteins [59].
There were multiple statistically significant differences in factor expression in various placental structures between distressed placental tissue samples of 40 gestational weeks and control samples at the same gestational week. All factors except HBD-3 had a significant change in expression in at least one of the placental structures. Control samples showed a statistically significantly higher expression of NF-kB and LL-37 in cytotrophoblasts and HBD-2, HSP60, and LL-37 in the extraembryonic mesoderm. In contrast, control samples showed a statistically significant lower expression of NF-kB, IL-10, and HBD-4 in the endothelium and HBD-4 in the extraembryonic mesoderm.
With everything taken into account, the expression of HBD-3 and NF-kB was observed to a greater extent in higher gestational weeks with distressed placental structures, which could indicate the role of these factors in later periods of pregnancy. However, the expression of IL-10 and HSP60 was more constant throughout all three gestational weeks and shows the role of these factors throughout the entire period of pregnancy. In addition, the expression of HBD-2 and HBD-4 had great variety between different gestational weeks, which may indicate that there is another factor influencing the expression of these defence factors.
Limitations and Future Perspectives
This study has limitations. There was a small number of placental tissue samples, and a higher number of samples could provide more illustrative results. Additionally, there was limited demographic and clinical data about participants, and more in-depth information could be helpful in determining possible additional reasons for the different results regarding defence factor expression levels in placental tissue. Furthermore, it is difficult to gather placental tissues and obtain control samples due to ethical reasons. The next issue is the evaluation of the concentration of the abovementioned factors by ELISA, which could give additional information about the common levels of them in the placenta at different gestational times. Finally, the combination of all the abovementioned factors with other cytokines and remodelling factor expression might give a more complete picture of specific molecular events in the placenta at different developmental times.
As for future perspectives, there is still the possibility of discovering defence factors and their role and functional significance in placental tissue; understanding their role could have clinical implications for immune response processes and may possibly affect pregnancy outcomes. Our study could be expanded in the future by observing levels of defence factors during different gestational week placental tissue and how they interact with other processes occurring in the protection mechanisms of the placenta, which could help understand the functional significance of these defence factors.
5. Conclusions
The persistence of Hofbauer cell accumulations underlines the growing significance of placental macrophages in placental protection.
The expression of positive defence factors and an increase in the expression of tissue protection factors (HBD-3, LL-37, HBD-4) in later gestational weeks may indicate these factors as the most significant protectors of the placenta in the ontogenetic aspect.
The high number of statistically significant positive and negative correlations between factor-positive cells show a strong network to sustain distressed placental growth and therefore pregnancy.
Author Contributions
Conceptualization, M.P.; methodology, M.P.; software, A.K.; validation, A.J. and M.P.; formal analysis, A.K.; investigation, A.K.; resources, M.P.; data curation, A.K.; writing—original draft preparation, A.K.; writing—review and editing, M.P. and A.J.; visualization, A.K.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Riga Stradins University; the permit was issued in March 2009 (12 March 2009 No. E-9 (2)).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
All the data presented in this study are available upon request from the corresponding author.
Acknowledgments
Ilze Kreicberga is kindly acknowledged for providing the material and Natalia Moroza is acknowledged for providing technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cindrova-Davies, T.; Sferruzzi-Perri, A.M. Human placental development and function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef]
- Hoo, R.; Nakimuli, A.; Vento-Tormo, R. Innate Immune Mechanisms to Protect Against Infection at the Human Decidual-Placental Interface. Front. Immunol. 2020, 11, 2070. [Google Scholar] [CrossRef] [PubMed]
- Zaga-Clavellina, V.; Diaz, L.; Olmos-Ortiz, A.; Godínez-Rubí, M.; Rojas-Mayorquín, A.E.; Ortuño-Sahagún, D. Central role of the placenta during viral infection: Immuno-competences and miRNA defensive responses. Biochim. Biophys. Acta. Mol. Basis. Dis. 2021, 1867, 166182. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Wolfe, B.; Golos, T. Hofbauer Cells: Placental Macrophages of Fetal Origin. Results Probl. Cell Differ. 2017, 62, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.J. The placental problem: Linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod. Biol. Endocrinol. 2004, 2, 53. [Google Scholar] [CrossRef]
- Su, E.J. Role of the fetoplacental endothelium in fetal growth restriction with abnormal umbilical artery Doppler velocimetry. Am. J. Obstet. Gynecol. 2015, 213, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.D.; Cross, J.C. Development of structures and transport functions in the mouse placenta. Physiology 2005, 20, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef]
- Bandurska, K.; Berdowska, A.; Barczyńska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- Yoshio, H.; Tollin, M.; Gudmundsson, G.H.; Lagercrantz, H.; Jornvall, H.; Marchini, G.; Agerberth, B. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: Implications for newborn innate defense. Pediatr. Res. 2003, 53, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Büchau, A.S.; Morizane, S.; Trowbridge, J.; Schauber, J.; Kotol, P.; Bui, J.D.; Gallo, R.L. The host defense peptide cathelicidin is required for NK cell-mediated suppression of tumor growth. J. Immunol. 2010, 184, 369–378. [Google Scholar] [CrossRef]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Baltimore, D. Discovering NF-kappaB. Cold Spring Harb. Perspect. Biol. 2009, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Torchinsky, A.; Toder, V. To die or not to die: The function of the transcription factor NF-kappaB in embryos exposed to stress. Am. J. Reprod. Immunol. 2004, 51, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Cummins, E.P.; Comerford, K.M.; Scholz, C.; Bruning, U.; Taylor, C.T. Hypoxic regulation of NF-kappaB signaling. Methods Enzymol. 2007, 435, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.M.; Harder, J. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 1999, 31, 645–651. [Google Scholar] [CrossRef]
- Kotani, H.; Koshizuka, T.; Matsubara, K.; Nishiyama, K.; Sugiyama, T.; Suzutani, T. Relationship Between Human β-Defensin 2 and the Vaginal Environment. Jpn. J. Infect. Dis. 2020, 73, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Matsubara, K.; Koshizuka, T.; Nishiyama, K.; Kaneko, H.; Tasaka, M.; Sugiyama, T.; Suzutani, T. Human β-defensin-2 as a biochemical indicator of vaginal environment in pregnant women. Hypertens. Res. Pregnancy 2018, 6, 68–72. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Human β-Defensin 2 and Its Postulated Role in Modulation of the Immune Response. Cells 2021, 10, 2991. [Google Scholar] [CrossRef]
- King, A.E.; Fleming, D.C.C.; Hilary, O.D.; Critchley, H.; Kelly, R.W. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J. Reprod. Immunol. 2003, 59, 1–16. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.M.; Wu, Z.; Tucker, K.; Lu, W.; Lubkowski, J. Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob. Agents Chemother. 2003, 47, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, H.; Chen, R.; Zhou, J.; Zhang, Y.; Yan, F. Human β-defensin 3 gene modification promotes the osteogenic differentiation of human periodontal ligament cells and bone repair in periodontitis. Int. J. Oral. Sci. 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Semple, F.; Webb, S.; Li, H.N.; Patel, H.; Perretti, M.; Jackson, I.J.; Gray, M.; Davidson, D.J.; Dorin, J.R. Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 2010, 40, 1073–1078. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, G.; Flores-Espinosa, P.; Zaga-Clavellina, V. Tissue-specific human beta-defensins (HBD)1, HBD2, and HBD3 secretion from human extra-placental membranes stimulated with Escherichia coli. Reprod. Biol. Endocrinol. 2010, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef]
- Bai, X.; Tian, T.; Wang, P.; Yang, X.; Wang, Z.; Dong, M. Potential roles of placental human beta-defensin-3 and apolipoprotein B mRNA-editing enzyme catalytic polypeptide 3G in prevention of intrauterine transmission of hepatitis B virus. J. Med. Virol. 2015, 87, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Yuan, X.; Zhao, Y.; Ge, L.; Wang, Y. Potential Application of Human β-Defensin 4 in Dental Pulp Repair. Front. Physiol. 2020, 11, 1077. [Google Scholar] [CrossRef]
- Sharma, H.; Nagaraj, R. Antimicrobial activity of human β-defensin 4 analogs: Insights into the role of disulfide linkages in modulating activity. Peptides 2012, 38, 255–265. [Google Scholar] [CrossRef]
- Polettini, J.; Takitane, J.; Peraçoli, J.C.; Silva, M.G. Expression of β defensins 1, 3 and 4 in chorioamniotic membranes of preterm pregnancies complicated by chorioamnionitis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 150–155. [Google Scholar] [CrossRef]
- Ding, J.; Li, J.; Yang, D.; Yang, F.; Nie, H.; Huo, Z.; Yan, X. Molecular characteristics of a novel HSP60 gene and its differential expression in Manila clams (Ruditapes philippinarum) under thermal and hypotonic stress. Cell Stress Chaperones 2018, 23, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Baker, M.L.; Schröder, G.F.; Douglas, N.R.; Reissmann, S.; Jakana, J.; Dougherty, M.; Fu, C.J.; Levitt, M.; Ludtke, S.J.; et al. Mechanism of folding chamber closure in a group II chaperonin. Nature 2010, 463, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Neuer, A.; Spandorfer, S.D.; Giraldo, P.; Dieterle, S.; Rosenwaks, Z.; Witkin, S.S. The role of heat shock proteins in reproduction. Hum. Reprod. Update 2000, 6, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Krishnan-Sivadoss, I.; Mijares-Rojas, I.A.; Villarreal-Leal, R.A.; Torre-Amione, G.; Knowlton, A.A.; Guerrero-Beltrán, C.E. Heat shock protein 60 and cardiovascular diseases: An intricate love-hate story. Med. Res. Rev. 2021, 41, 29–71. [Google Scholar] [CrossRef]
- Shah, M.; Stanek, J.; Handwerger, S. Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem. J. 1998, 30, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.; Dhar, R.; Singh, S.; Karmakar, S. Heat Shock Proteins and Their Role in Pregnancy: Redefining the Function of “Old Rum in a New Bottle”. Front. Cell. Dev. Biol. 2021, 9, 648463. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Cheng, S.B.; Sharma, S. Interleukin-10: A pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 2015, 73, 487–500. [Google Scholar] [CrossRef]
- Asadullah, K.; Sterry, W.; Volk, H.D. Interleukin-10 therapy—Review of a new approach. Pharmacol. Rev. 2003, 55, 241–269. [Google Scholar] [CrossRef]
- Ozörnek, M.H.; Bielfeld, P.; Krüssel, J.S.; Moustafa, M.; Mikat-Drozdzynski, B.; Koldovsky, U.; Kuhn, U. Interferon gamma and interleukin 10 levels in preimplantation embryo culture media. J. Assist. Reprod. Genet. 1995, 12, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Zulu, M.Z.; Martinez, F.O.; Gordon, S.; Gray, C.M. The Elusive Role of Placental Macrophages: The Hofbauer Cell. J. Innate. Immun. 2019, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Collaboration Preterm Birth Working Group. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047–6056. [Google Scholar] [CrossRef]
- Hsu, S.M.; Raine, L.; Fanger, H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Pilmane, M.; Rumba, I.; Sundler, F.; Luts, A. Patterns of distribution and occurrence of neuroendocrine elements in lungs of humans with chronic lung disease. Proc. Latv. Acad. Sci. 1998, 52, 144–152. [Google Scholar]
- Prahl, A.; Pazgier, M.; Alexandratos, J.; Lubkowski, J. Human β-defensin 4—Defensin without the “twist”. Postepy Biochem. 2016, 62, 349–361. [Google Scholar] [CrossRef] [PubMed]
- García, J.R.; Krause, A.; Schulz, S.; Rodríguez-Jiménez, F.J.; Klüver, E.; Adermann, K.; Forssmann, U.; Frimpong-Boateng, A.; Bals, R.; Forssmann, W.G. Human beta-defensin 4: A novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001, 15, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Liu, H.; Lash, G.E. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Lappas, M. Human cathelicidin antimicrobial protein 18 (hCAP18/LL-37) is increased in foetal membranes and myometrium after spontaneous labour and delivery. J. Reprod. Immunol. 2015, 107, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Bravo, M.; Sangiorgi, B.B.; Schiavinato, J.L.; Carvalho, J.L.; Covas, D.T.; Panepucci, R.A.; Neves, F.A.; Franco, O.L.; Pereira, R.W.; Saldanha-Araujo, F. LL-37 boosts immunosuppressive function of placenta-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.R. The Role of NFκB in Healthy and Preeclamptic Placenta: Trophoblasts in the Spotlight. Int. J. Mol. Sci. 2020, 21, 1775. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Liu, J.; Lv, S.S.; Fu, Z.Y.; Hou, L.L. Baicalein Enhances Migration and Invasion of Extravillous Trophoblasts via Activation of the NF-κB Pathway. Med. Sci. Monit. 2018, 24, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, L.; Ivankova, K.; Krofta, L.; Hromadnikova, I. Expression profile of heat shock proteins in placental tissues of patients with preterm prelabor rupture of membranes and spontaneous preterm labor with intact membranes. Am. J. Reprod. Immunol. 2017, 78, 10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).