Fatty Acid Content and Profile in Ulva lactuca in Response to Exposure to Variable Growth Conditions in Indoor Photobioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Identification of Ulva sp.
2.2. Salinity Experiment
2.3. Temperature Experiment
2.4. Irradiation Experiment
2.5. Nutrient Experiment
2.6. Biomass Collection and Saving
2.7. DW, AC, and Elemental Analysis
2.8. Directly Methylation of FA Followed by Investigation Using Gas Chromatography-Mass Spectrometry (GC-MS)
2.9. Statistical Analysis
3. Results
3.1. Identification of the Ulva Species DNA Marker
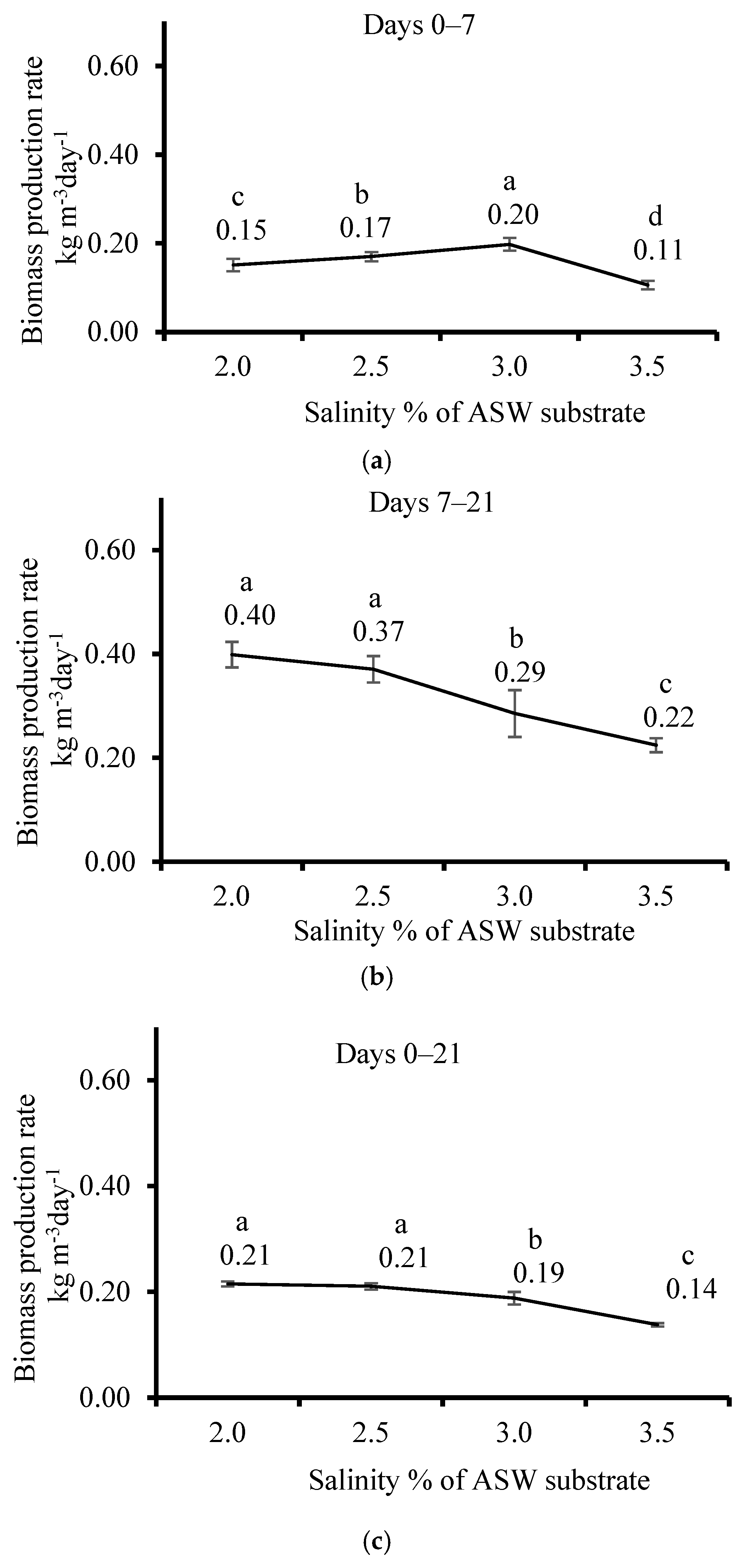
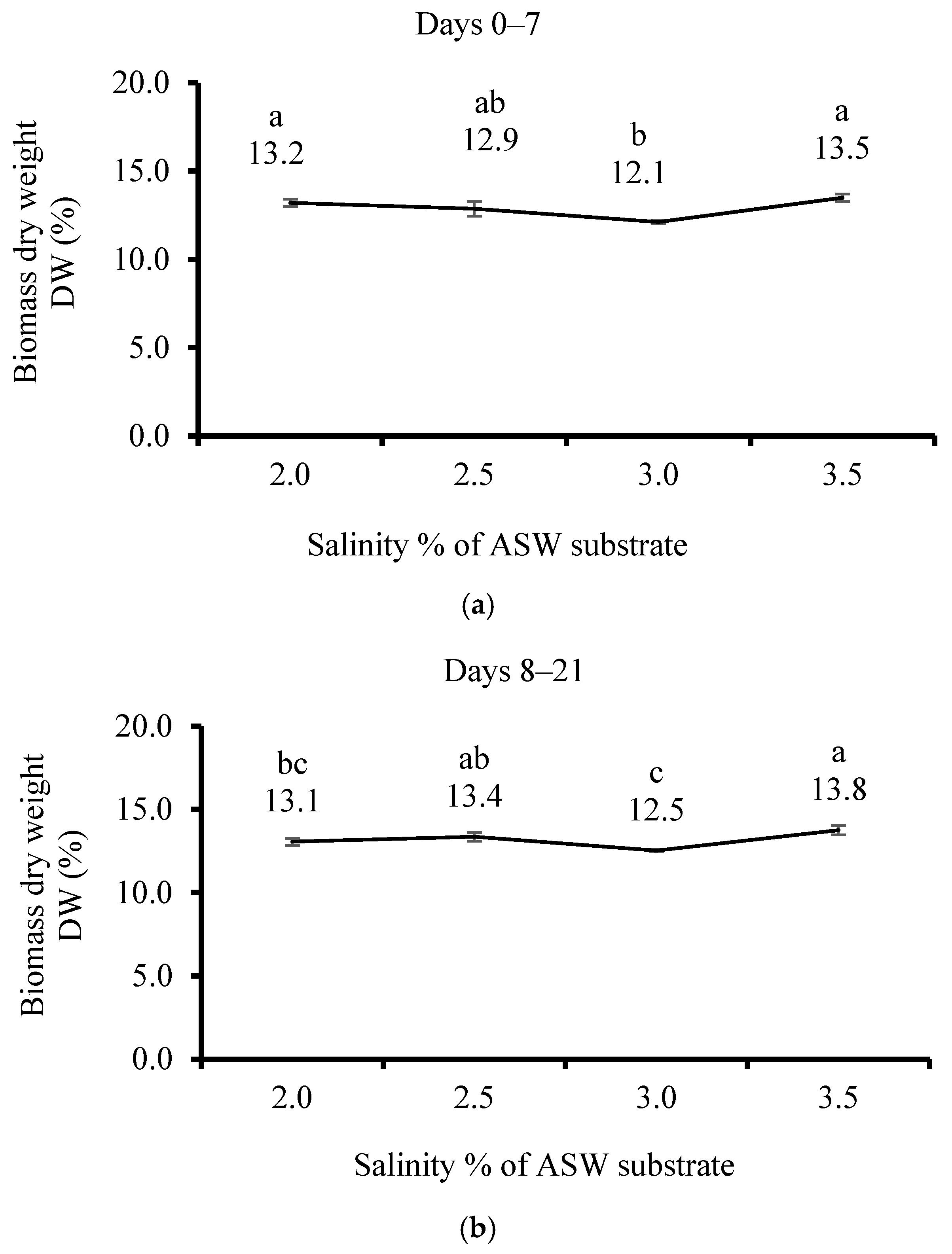
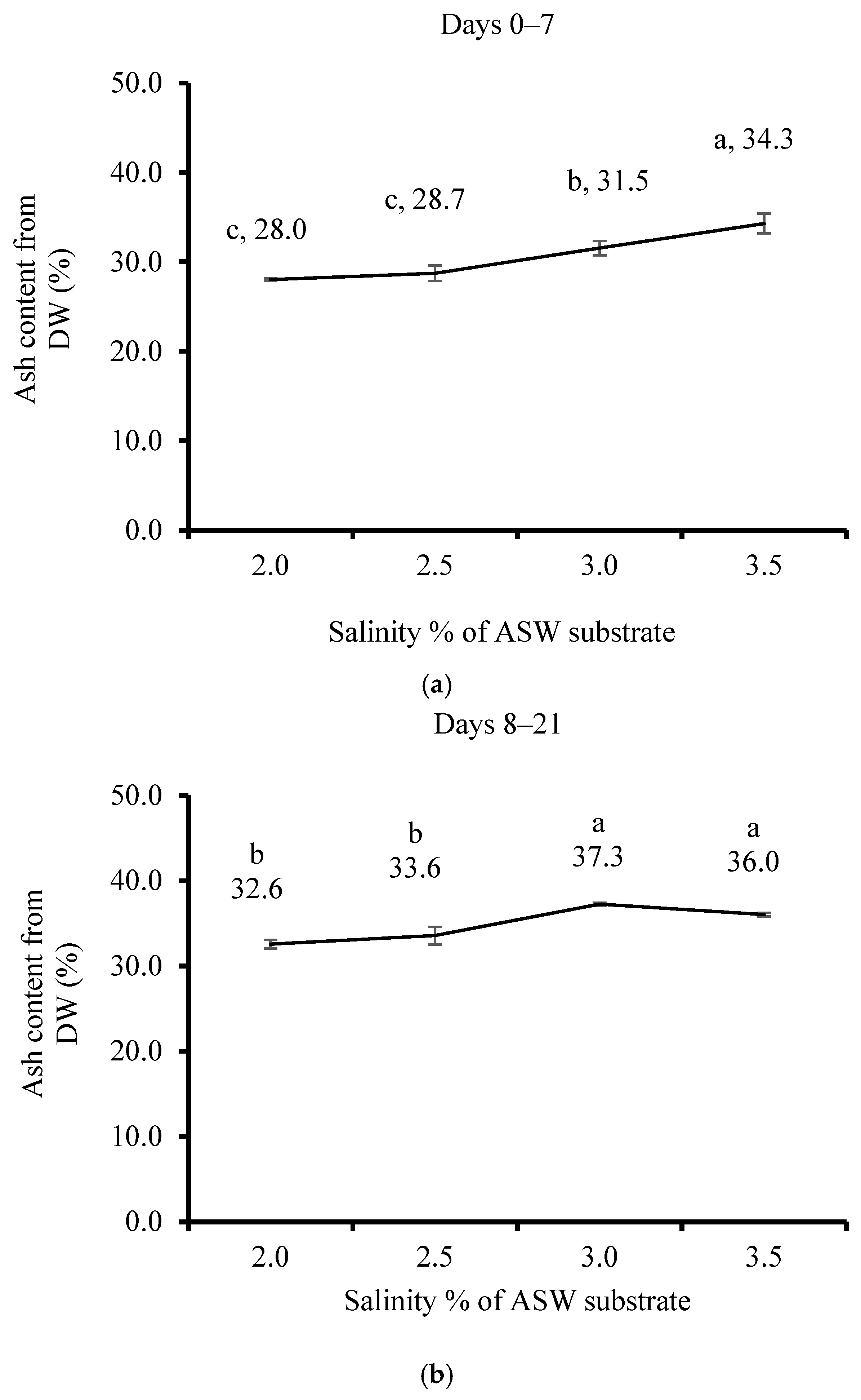
3.2. Effect of Salinity
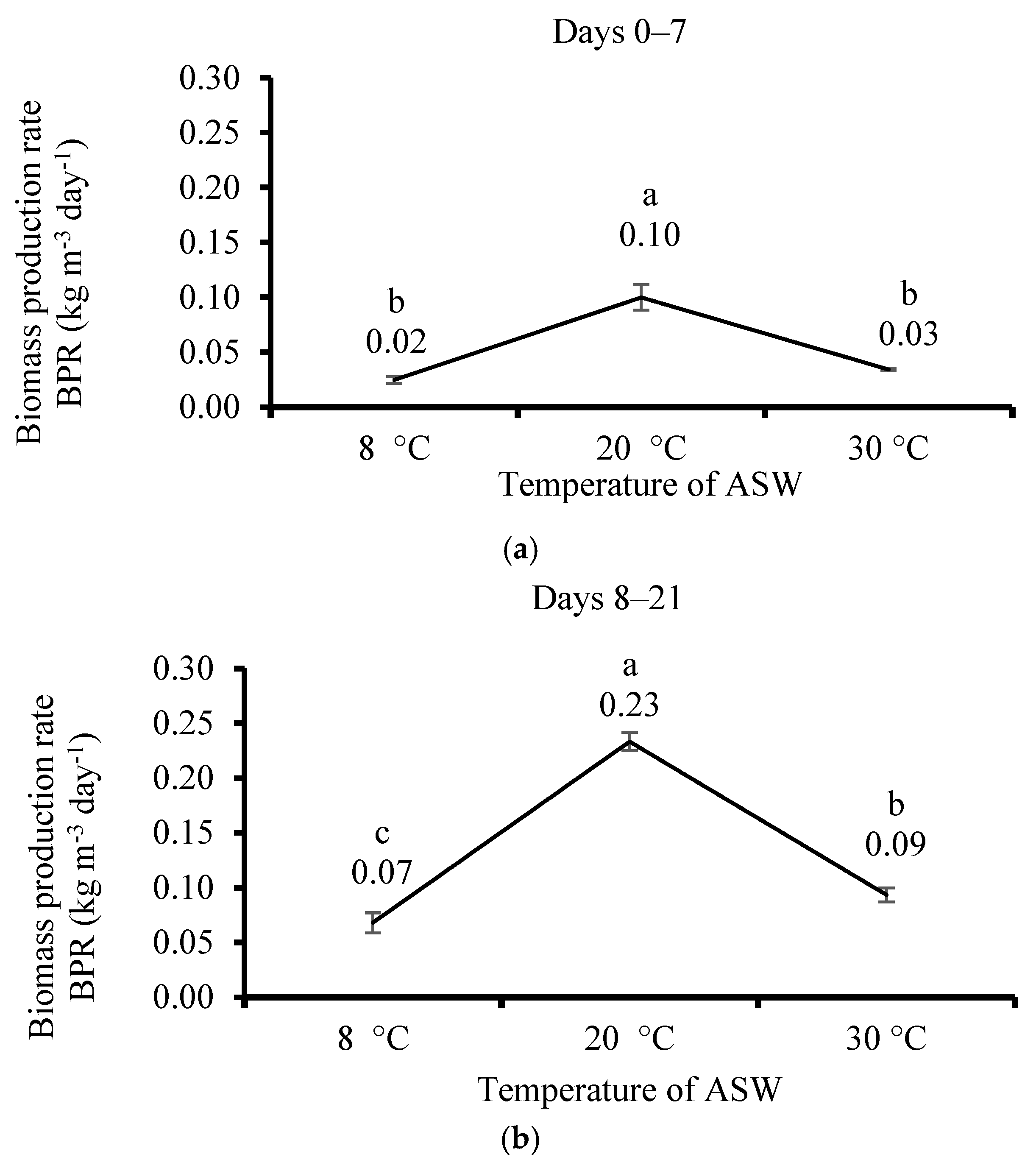
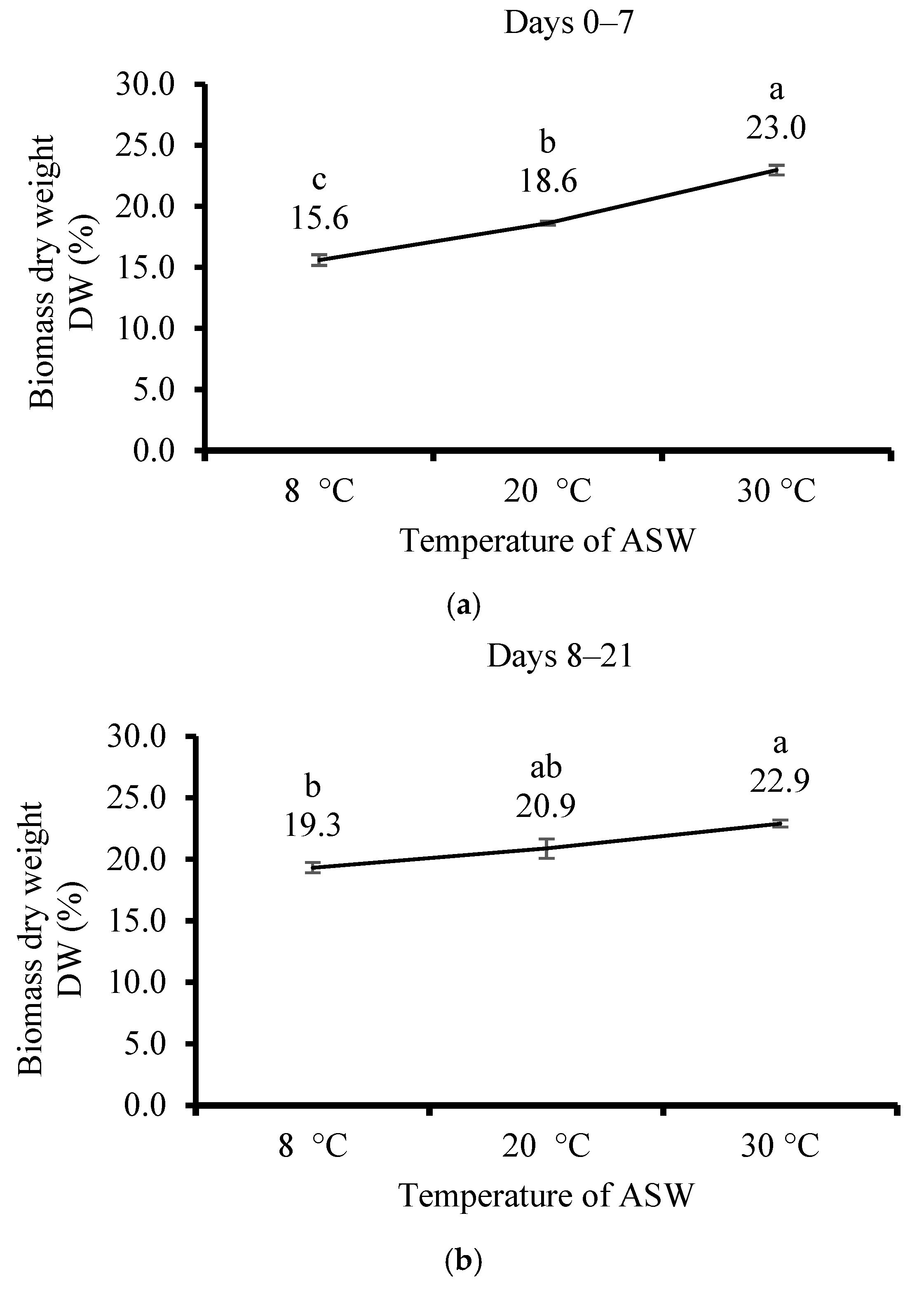
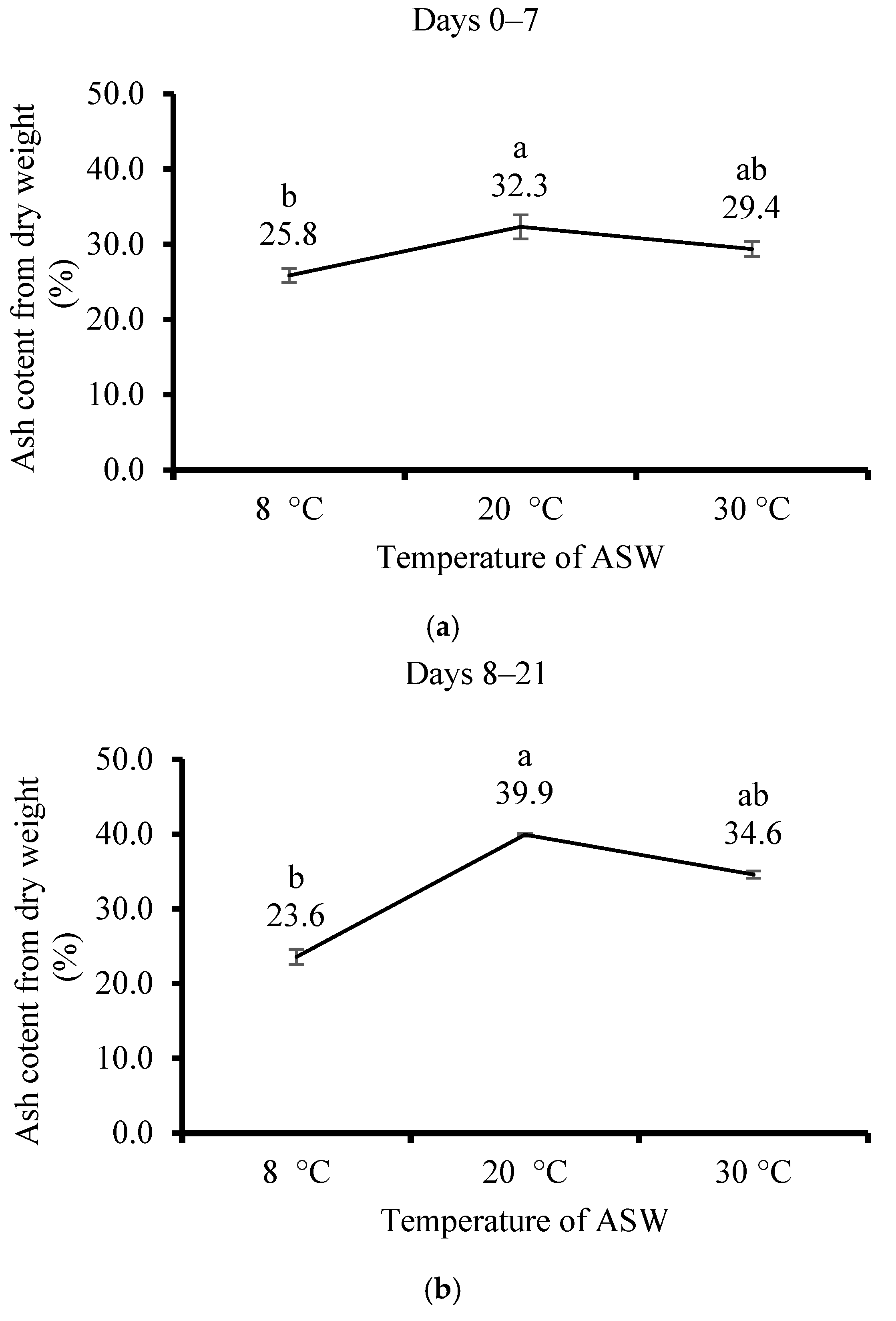
3.3. Effect of Temperature
3.4. Effect of Nutrients
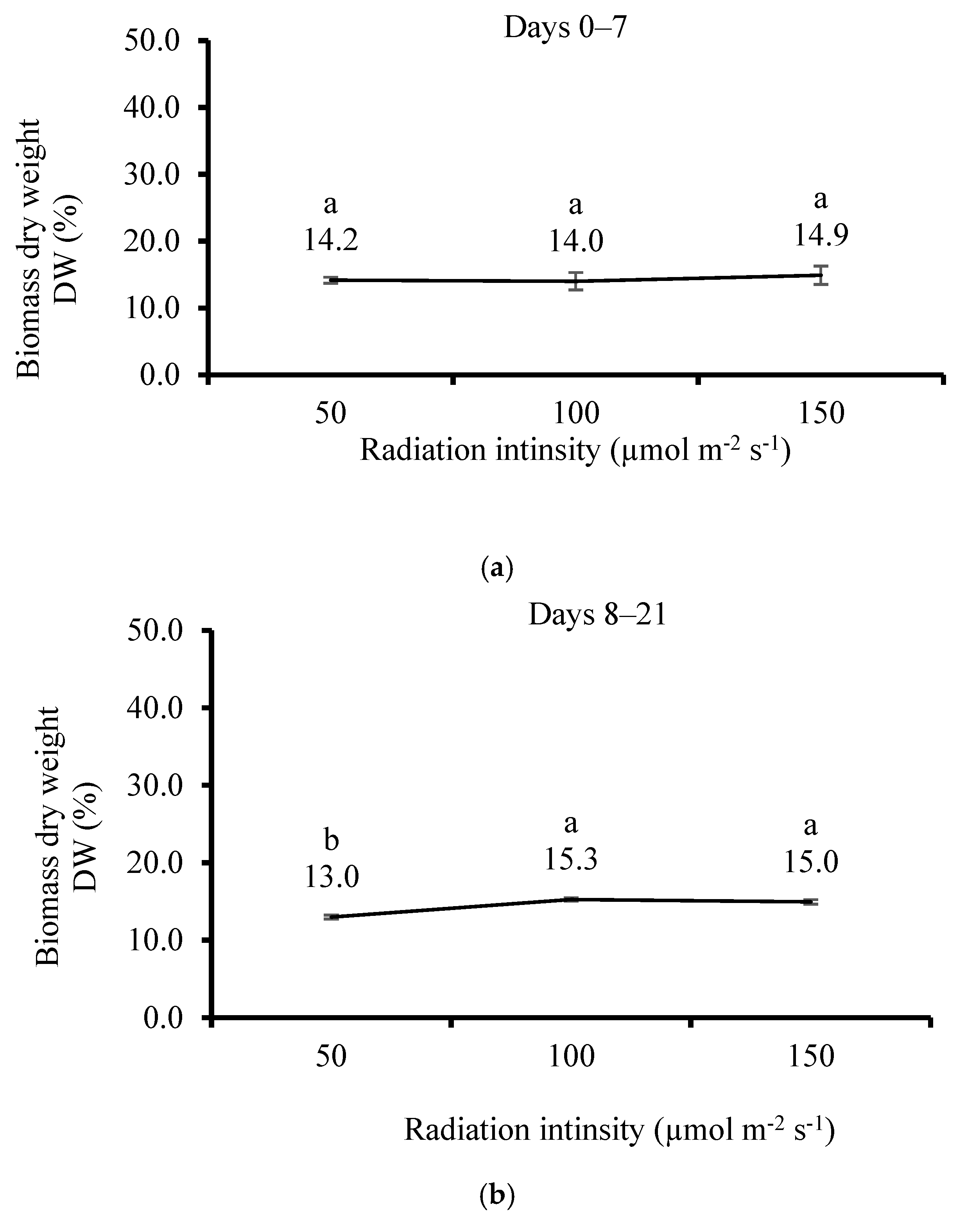
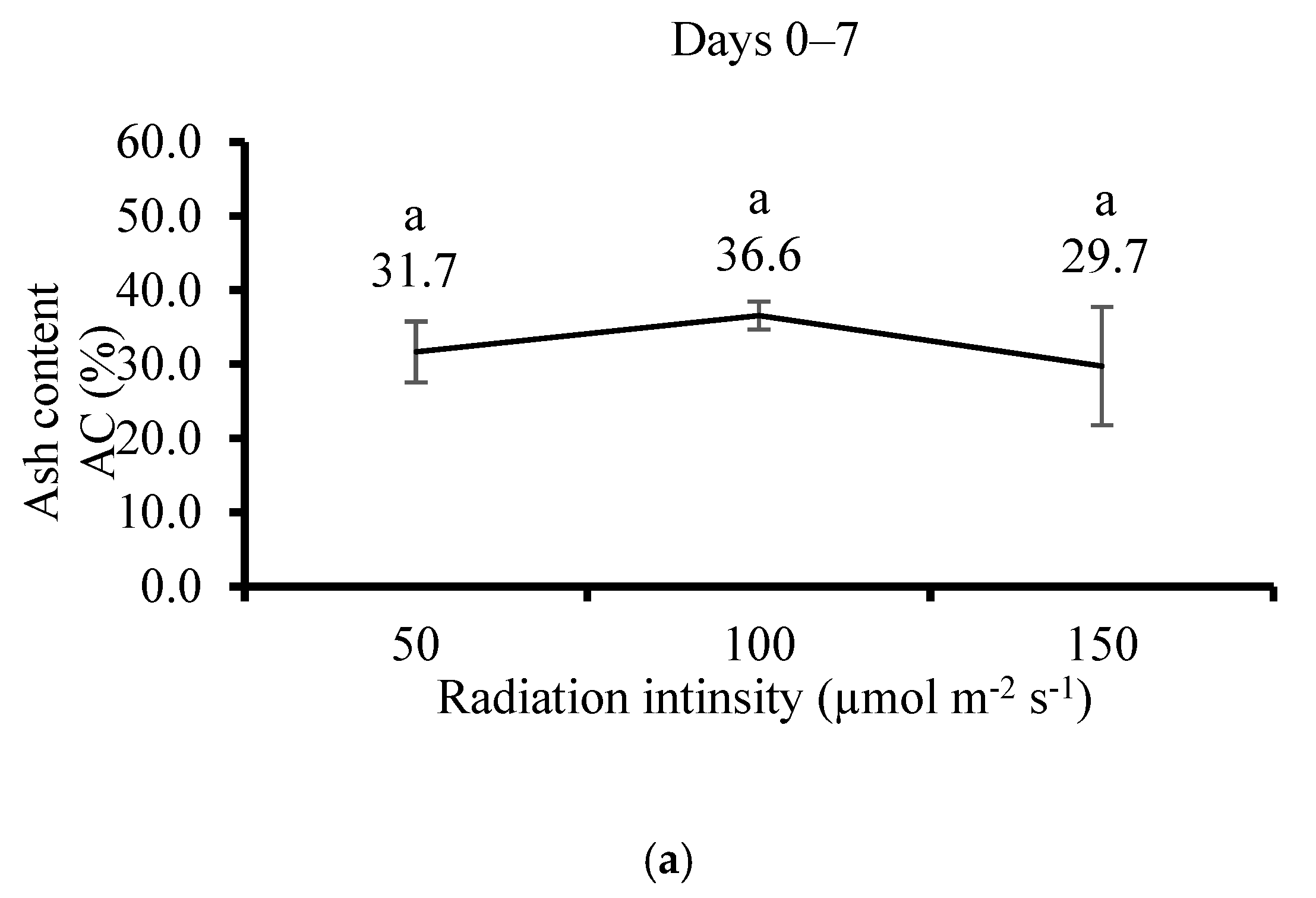
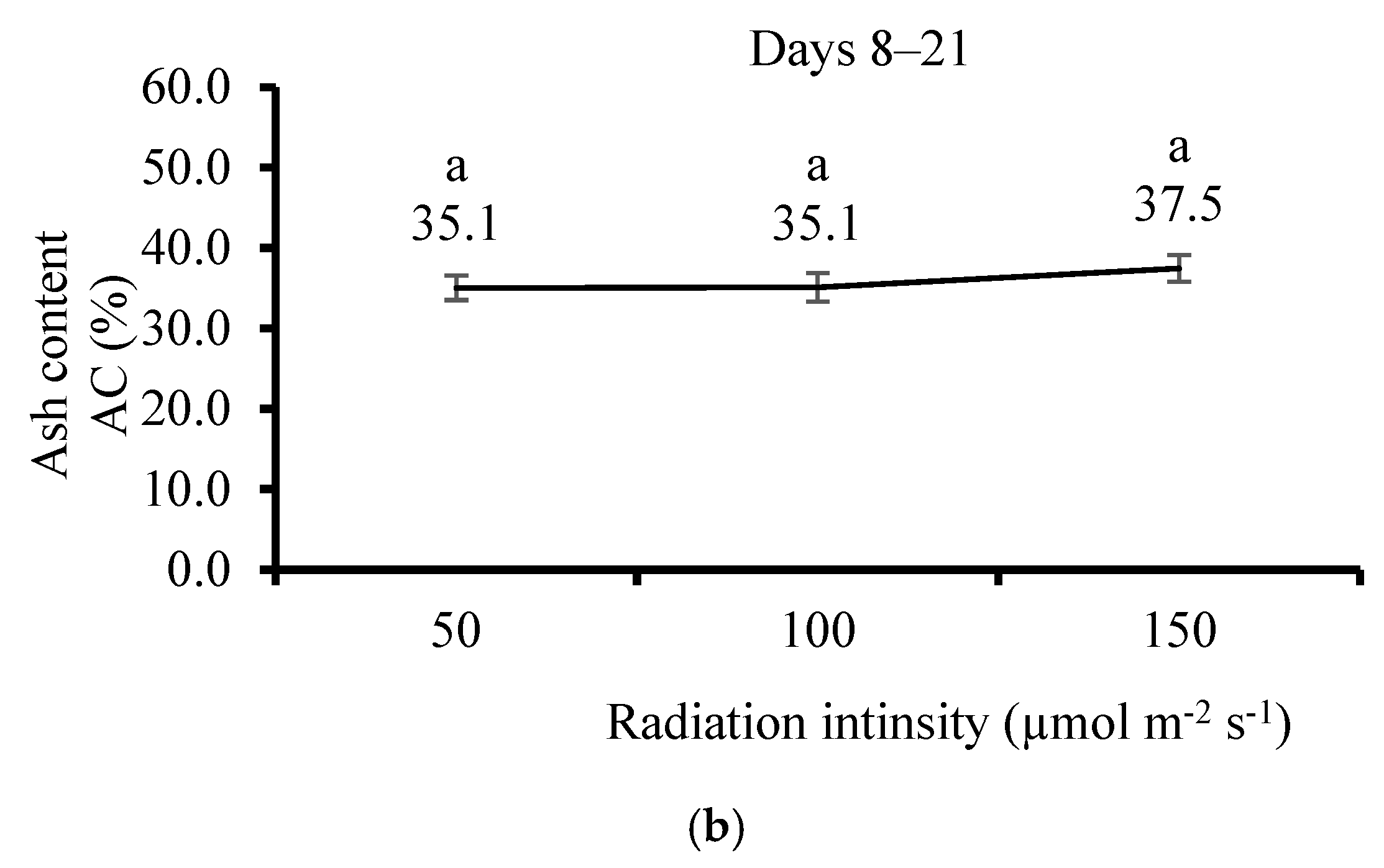
3.5. Effect of Irradiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | ash content |
| ASW | artificial seawater |
| BPR | biomass production rate |
| DHA | docosapentaenoic acid |
| DW | dry weight |
| EPA | eicosapentaenoic acid |
| FA | fatty acids |
| HUFA | highly unsaturated fatty acids |
| MUSFA | monounsaturated fatty acid |
| n-3 | omega-3 fatty acid |
| SFA | saturated fatty acid |
| PAR | photosynthetic active radiation |
| PUFA | polyunsaturated fatty acid |
| TFA | total fatty acid |
| UV | ultra violet radiation |
References
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine macroalgae in a circular economy context: A comprehensive analysis focused on residual biomass. Biotechnol. Adv. 2022, 60, 107987. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An overview on the nutritional and bioactive components of green seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Cotas, J.; Gonçalves, A.M.M. Seaweed proteins: A step towards sustainability? Nutrients 2024, 16, 1123. [Google Scholar] [CrossRef]
- Garcia-Poza, S.; Morais, T.; Leandro, A.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. A comparative study of the fatty acids and monosaccharides of wild and cultivated Ulva sp. J. Mar. Sci. Eng. 2022, 10, 233. [Google Scholar] [CrossRef]
- Sayed, W.; Cabrol, A.; Salma, A.; Amrane, A.; Benoit, M.; Pierre, R.; Djelal, H. Green macroalgae hydrolysate for biofuel production: Potential of Ulva rigida. Appl. Microbiol. 2024, 4, 563–581. [Google Scholar] [CrossRef]
- Rouxel, C.; Bonnabeze, E.; Daniel, A.; Jérôme, M.; Etienne, M.; Fleurence, J. Identification by SDS-PAGE of green seaweeds (Ulva and Enteromorpha) used in the food industry. J. Appl. Phycol. 2001, 13, 215–218. [Google Scholar] [CrossRef]
- Norziah, M.H.; Ching, C.Y. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Einav, R.; Israel, A. Checklist of seaweeds from the Israeli Mediterranean: Taxonomical and ecological approaches. Isr. J. Plant Sci. 2008, 56, 91–127. [Google Scholar] [CrossRef]
- Israel, A.; Golberg, A.; Neori, A. The seaweed resources of Israel in the Eastern Mediterranean Sea. Bot. Mar. 2020, 63, 85–95. [Google Scholar] [CrossRef]
- Lundberg, B. Food habits of Siganus rivulatus, a Lessepsian migrant, as adapted to algal resources at the coast of Israel. In Environmental Quality and Ecosystem Stability, Proceedings of the Fourth International Conference of the Israel Society for Ecology & Environmental Quality Sciences, Jerusalem, Israel, 4–8 June 1989; ISEEQS Publication: Jerusalem, Israel, 1989; Volume 5b, pp. 113–124. [Google Scholar]
- Einav, R.; Guiry, M.D.; Israel, Á. A revised list of seaweeds from the Red Sea (1756–2020). Isr. J. Plant Sci. 2021, 68, 175–247. [Google Scholar] [CrossRef]
- Atiba, A.; Abdo, W.; Madkour, F.F. Influence of tropical brown algae (Cystoseira myrica) extract application on wound healing in rats. Kafrelsheikh Vet. Med. J. 2013, 11, 79–90. [Google Scholar] [CrossRef]
- Zollmann, M.; Traugott, H.; Chemodanov, A.; Liberzon, A.; Golberg, A. Deepwater nutrient supply for an offshore Ulva sp. cultivation project in the Eastern Mediterranean Sea: Experimental simulation and modeling. BioEnergy Res. 2019, 12, 1113–1126. [Google Scholar] [CrossRef]
- Carl, C.; de Nys, R.; Paul, N.A. The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS ONE 2014, 9, e98700. [Google Scholar] [CrossRef] [PubMed]
- Carl, C.; Magnusson, M.; Paul, N.A.; de Nys, R. The yield and quality of multiple harvests of filamentous Ulva tepida. J. Appl. Phycol. 2016, 28, 2865–2873. [Google Scholar] [CrossRef]
- Trujillo, E.; Monreal-Escalante, E.; Ramos-Vega, A.; Angulo, C. Macroalgae: Marine players in vaccinology. Algal Res. 2024, 78, 103392. [Google Scholar] [CrossRef]
- Zertuche-González, J.A.; Sandoval-Gil, J.M.; Rangel-Mendoza, L.K.; Gálvez-Palazuelos, A.I.; Guzmán-Calderón, J.M.; Yarish, C. Seasonal and interannual production of sea lettuce (Ulva sp.) in outdoor cultures based on commercial size ponds. J. World Aquac. Soc. 2021, 52, 1047–1058. [Google Scholar] [CrossRef]
- Israel, Á.; Shpigel, M. Photosynthetic CO2 uptake by Ulva (Chlorophyta) as a potential contribution to global warming containment. J. Appl. Phycol. 2023, 35, 1987–1994. [Google Scholar] [CrossRef]
- Fort, A.; Lebrault, M.; Allaire, M.; Esteves-Ferreira, A.A.; McHale, M.; Lopez, F.; Fariñas-Franco, J.M.; Alseekh, S.; Fernie, A.R.; Sulpice, R. Extensive variations in diurnal growth patterns and metabolism amongst Ulva spp. strains. Plant Physiol. 2019, 180, 109–123. [Google Scholar] [CrossRef]
- Unis, R.; Chemodanov, A.; Gnayem, N.; Gnaim, R.; Israel, Á.; Palatnik, R.R.; Golberg, A. Effect of seasonality on the amino acid and monosaccharide profile from the green seaweed Ulva lactuca cultivated in plastic sleeves onshore (Mikhmoret, Israel). J. Appl. Phycol. 2023, 35, 1347–1363. [Google Scholar] [CrossRef]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as valuable sources of essential fatty acids for human nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Proteins involved in fat-soluble vitamin and carotenoid transport across the intestinal cells: New insights from the past decade. Prog. Lipid Res. 2023, 89, 101208. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.A.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. The chemodiversity of polar lipidomes of microalgae from different taxa. Algal Res. 2023, 70, 103006. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, L.M.; Rodríguez-Alcalá, M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition of red, brown, and green macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Moustafa, Y.; Batran, A. Lipid chemistry of green macroalgae Ulva sp.: A potential resource for biotechnological applications in the Southern Mediterranean Sea Coast, Alexandria Shore, Egypt. Egypt. J. Aquat. Biol. Fish. 2014, 18, 9–20. [Google Scholar]
- Ramírez-Partida, R.A.; García-Cayuela, T.; Amador-Castro, L.F.; Alper, H.S.; Carrillo-Nieves, D. Towards a biorefinery processing Sargassum seaweed: Techno-economic assessment of alginate and fucoidan production through SuperPro Designer® process simulation. Environ. Technol. Innov. 2024, 34, 103587. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, I.; Meiβner, A.; Sawicki, A.; Bartsch, I.; Valentin, K.U.; Steinhagen, S.; Buck, B.H.; Hofmann, L.C. Salinity as a tool for strain selection in recirculating land-based production of Ulva spp. from germlings to adults. J. Appl. Phycol. 2023, 35, 1971–1986. [Google Scholar] [CrossRef]
- Gnayem, N.; Unis, R.; Gnaim, R.; Chemodanov, A.; Israel, Á.; Gnaim, J.; Golberg, A. Seasonal and culture period variations in the lipid and fatty acid content of Ulva lactuca cultivated in Mikhmoret onshore (Israel). Bot. Mar. 2024, 67, 101–114. [Google Scholar] [CrossRef]
- Samarasinghe, M.B.; van der Heide, M.E.; Weisbjerg, M.R.; Sehested, J.; Sloth, J.J.; Bruhn, A.; Vestergaard, M.; Nørgaard, J.V.; Hernández-Castellano, L.E. A descriptive chemical analysis of seaweeds, Ulva sp., Saccharina latissima and Ascophyllum nodosum harvested from Danish and Icelandic waters. Anim. Feed Sci. Technol. 2021, 278, 115005. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Lage, S.; Aluwini, D.F.; Rebours, C.; Brurberg, M.B.; Nitschke, U.; Gentili, F.G. Chemical profiling of the Arctic Sea lettuce Ulva lactuca (Chlorophyta) mass-cultivated on land under controlled conditions for food applications. Food Chem. 2021, 341, 127999. [Google Scholar] [CrossRef]
- Lawton, R.J.; Sutherland, J.E.; Glasson, C.R.; Magnusson, M.E. Selection of temperate Ulva species and cultivars for land-based cultivation and biomass applications. Algal Res. 2021, 56, 102320. [Google Scholar] [CrossRef]
- Robin, A.; Chavel, P.; Chemodanov, A.; Israel, A.; Golberg, A. Diversity of monosaccharides in marine macroalgae from the Eastern Mediterranean Sea. Algal Res. 2017, 28, 118–127. [Google Scholar] [CrossRef]
- De Melo, N.S.M.; Cardoso, L.G.; de Castro Nunes, J.M.; Brito, G.B.; Caires, T.A.; de Souza, C.O.; Druzia, J.I. Effects of dry and rainy seasons on the chemical composition of Ulva fasciata, Crassiphycus corneus, and Sargassum vulgare seaweeds in a tropical environment. Rev. Bras. Bot. 2021, 44, 331–344. [Google Scholar] [CrossRef]
- Tian, S.; Zheng, T.; Wu, M.; Cao, C.; Xu, L.; Gu, Z.; Chen, B.; Ma, Z. Differences of photosynthesis and nutrient utilization in Sargassum fusiforme and its main epiphyte, Ulva lactuca. Aquac. Res. 2022, 53, 3176–3187. [Google Scholar] [CrossRef]
- Jansen, H.M.; Bernard, M.S.; Nederlof, M.A.J.; van der Meer, I.M.; van der Werf, A. Seasonal variation in productivity, chemical composition and nutrient uptake of Ulva spp. (Chlorophyta) strains. J. Appl. Phycol. 2022, 34, 1649–1660. [Google Scholar] [CrossRef]
- Rybak, A.S. Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol. Indic. 2018, 85, 253–261. [Google Scholar] [CrossRef]
- Traugott, H.; Zollmann, M.; Cohen, H.; Chemodanov, A.; Liberzon, A.; Golberg, A. Aeration and nitrogen modulated growth rate and chemical composition of green macroalgae Ulva sp. cultured in a photobioreactor. Algal Res. 2020, 47, 101808. [Google Scholar] [CrossRef]
- Toth, G.B.; Harrysson, H.; Wahlström, N.; Olsson, J.; Oerbekke, A.; Steinhagen, S.; Kinnby, A.; White, J.; Albers, E.; Edlund, U.; et al. Effects of irradiance, temperature, nutrients, and pCO2 on the growth and biochemical composition of cultivated Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3243–3254. [Google Scholar] [CrossRef]
- Suthar, P.; Gajaria, T.K.; Reddy, C.R.K. Production of quality seaweed biomass through nutrient optimization for the sustainable land-based cultivation. Algal Res. 2019, 42, 101583. [Google Scholar] [CrossRef]
- Bastos, E.; Gouvêa, L.P.; Horta, P.A.; Rörig, L.R. Interaction between salinity and phosphorus availability can influence seed production of Ulva ohnoi (Chlorophyta, Ulvales). Ecol. Evol. Biol. 2019, 167, 103860. [Google Scholar] [CrossRef]
- Chemodanov, A.; Robin, A.; Golberg, A. Design of marine macroalgae photobioreactor integrated into building to support seagriculture for biorefinery and bioeconomy. Bioresour. Technol. 2017, 241, 1084–1093. [Google Scholar] [CrossRef]
- Robin, A.; Sack, M.; Israel, A.; Frey, W.; Müller, G.; Golberg, A. Deashing macroalgae biomass by pulsed electric field treatment. Bioresour. Technol. 2018, 255, 131–139. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Chatzidimitriou, E.; Rose, C.; Caldwell, G.S. Effects of ocean warming and acidification, combined with nutrient enrichment, on chemical composition and functional properties of Ulva rigida. Food Chem. 2018, 258, 71–78. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef]
- Hughey, J.R.; Miller, K.A.; Gabrielson, P.W. Genetic analysis of Ulva (Ulvaceae, Chlorophyta) type specimens resolves northeast Pacific blade-forming species. Bot. Mar. 2023, 67, 165–179. [Google Scholar] [CrossRef]
- Marques, F.; Lopes, D.; Conde, T.; Rego, A.; Ribeiro, A.I.; Abreu, M.H.; Domingues, M.R. Seaweed blends as a valuable source of polyunsaturated and healthy fats for nutritional and food applications. Mar. Drugs 2021, 19, 701. [Google Scholar] [CrossRef] [PubMed]
- Bews, E.; Booher, L.; Polizzi, T.; Long, C.; Kim, J.H.; Edwards, M.S. Effects of salinity and nutrients on metabolism and growth of Ulva lactuca: Implications for bioremediation of coastal watersheds. Mar. Pollut. Bull. 2021, 166, 112199. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.S.; Kang, E.J.; Kim, J.; Kim, K.W. Effect of salinity on growth and nutrient uptake of Ulva pertusa (Chlorophyta) from an eelgrass bed. Algae 2010, 25, 17–26. [Google Scholar] [CrossRef]
- Xia, J.; Li, Y.; Zou, D. Effects of salinity stress on PSII in Ulva lactuca as probed by chlorophyll fluorescence measurements. Aquat. Bot. 2004, 80, 129–137. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Duke, C.S. Biochemical strategies for growth of Gracilaria tikvahiae (Rhodophyta) in relation to light intensity and nitrogen availability. J. Phycol. 1984, 20, 488–495. [Google Scholar] [CrossRef]
- Carvalho, A.; Braga, Í.; Chaar, F.; Cardozo, A.P.; Monserrat, J.M.; Ramírez, J.R.; Wasielesky, W., Jr.; Poersch, L.H. Production of the macroalga Ulva lactuca integrated with the shrimp Penaeus vannamei in a biofloc system: Effect of total suspended solids and nutrient concentrations. Phycology 2024, 4, 37–52. [Google Scholar] [CrossRef]
- Gadberry, B.; Colt, J.; Maynard, D.; Boratyn, D.; Webb, K.; Johnson, R.; Sounders, G.; Boyer, R. Intensive land-based production of red and green macroalgae for human consumption in the Pacific Northwest: An evaluation of seasonal growth, yield, nutritional composition, and contaminant levels. Algae 2018, 33, 109–125. [Google Scholar] [CrossRef]
- Tan, K.; Ransangan, J.; Tan, K.; Cheong, K.L. The impact of climate change on omega-3 long-chain polyunsaturated fatty acids in bivalves. Crit. Rev. Food Sci. Nutr. 2023, 63, 11661–11671. [Google Scholar] [CrossRef]
- Lubsch, A.; Timmermans, K. Uptake kinetics and storage capacity of dissolved inorganic phosphorus and corresponding N:P dynamics in Ulva lactuca (Chlorophyta). J. Phycol. 2018, 54, 215–223. [Google Scholar] [CrossRef]
- Morgan, K.C.; Simpson, F.J. The cultivation of Palmaria palmata: Effect of light intensity and nitrate supply on growth and chemical composition. Bot. Mar. 1981, 24, 272–277. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Nitrate and phosphate regimes induced lipidomic and biochemical changes in the intertidal macroalga Ulva lactuca (Ulvophyceae, Chlorophyta). Plant Cell Physiol. 2014, 55, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, M.; Shauli, L.; Odintsov, V.; Ben-Ezra, D.; Neori, A.; Guttman, L. The sea urchin, Paracentrotus lividus, in an Integrated Multi-Trophic Aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture 2018, 490, 260–269. [Google Scholar] [CrossRef]
- Monteiro, V.M.M.; Vollet, M.G.; Turgeon, S.L.; Tamigneaux, É.; Beaulieu, L. Physicochemical properties of wild and cultivated Saccharina latissima macroalgae harvested in the Canadian boreal-subarctic transition zone. Heliyon 2024, 10, e29626. [Google Scholar] [CrossRef]
- Krupnik, N.; Paz, G.; Douek, J.; Lewinson, E.; Israel, A.; Carmel, N.; Mineur, F.; Maggs, C.A. Native, invasive, and cryptogenic Ulva species from the Israeli Mediterranean Sea: Risk and potential. Mediterr. Mar. Sci. 2018, 19, 132–146. [Google Scholar] [CrossRef]
- Queirós, A.S.; Circuncisão, A.R.; Pereira, E.; Válega, M.; Abreu, M.H.; Silva, A.M.; Cardoso, S.M. Valuable nutrients from Ulva rigida: Modulation by seasonal and cultivation factors. Appl. Sci. 2021, 11, 6137. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Costa, E.; Melo, T.; Domingues, P.; Fort, A.; Domingues, M.R.; Sulpice, R. Lipidome in-depth characterization highlights the nutritional value and species-specific idiosyncrasies of different Ulva species. Algal Res. 2022, 64, 102694. [Google Scholar] [CrossRef]




| Fatty Acid | Percentage of Fatty Acid (% w/w) of the Total Fatty Acids | |||||||
|---|---|---|---|---|---|---|---|---|
| Salinity | 2.0% 8 | 2.5% | 3.0% | 3.5% | 2.0% | 2.5% | 3.0% | 3.5% |
| Cultivation Period | 0–7 Days 9 | 8–21 Days | ||||||
| C14:0 | 0.8 ± 0.0 a | 0.8 ± 0.0 a | 0.4 ± 0.0 b | 0.1 ± 0.0 c | 0.1 ± 0.0 d | 0.4 ± 0.0 c | 0.8 ± 0.0 b | 1.2 ± 0.0 a |
| C15:0 | 0.9 ± 0.1 a | 0.9 ± 0.1 a | 0.2 ± 0.0 b | 0.1 ± 0.0 c | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.1 ± 0.0 b | 0.3 ± 0.0 a |
| C16:3 n-6 | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.1 ± 0.0 b | 0.0 ± 0.0 b | 0.2 ± 0.0 a | 0.2 ± 0.0 b | 0.1 ± 0.0 c | 0.1 ± 0.0 d |
| C16:4 n-4 | 3.3 ± 0.1 a | 3.3 ± 0.1 a | 2.9 ± 0.0 b | 0.0 ± 0.0 c | 4.6 ± 0.1 b | 3.9 ± 0.1 c | 5.6 ± 0.1 a | 0.2 ± 0.1 d |
| C16:3 n-3 | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.1 ± 0.0 b | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.1 ± 0.0 b | 0.0 ± 0.0 c |
| C16:1 n-7 | 1.5 ± 0.1 b | 1.5 ± 0.1 b | 2.3 ± 0.1 a | 0.3 ± 0.0 c | 1.6 ± 0.0 b | 1.5 ± 0.1 b | 2.0 ± 0.0 a | 1.0 ± 0.1 c |
| C18:2 n-2 | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 b | 0.1 ± 0.0 c | 0.2 ± 0.0 c | 0.3 ± 0.0 b | 0.3 ± 0.0 a | 0.0 ± 0.0 d |
| C16:0 | 68.6 ± 0.4 b | 68.6 ± 0.4 b | 61.3 ± 0.6 c | 77.2 ± 0.3 a | 45.9 ± 0.6 d | 54.2 ± 0.6 c | 61.1 ± 0.6 b | 65.4 ± 0.4 a |
| C17:1 n-7 | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.1 ± 0.0 b | 0.0 ± 0.0 c | 0.5 ± 0.0 a | 0.2 ± 0.0 b | 0.1 ± 0.0 c | 0.5 ± 0.0 a |
| C18:3 n-6 | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.2 ± 0.0 a | 0.1 ± 0.0 c | 0.8 ± 0.0 b | 0.9 ± 0.0 a | 0.4 ± 0.0 c | 0.2 ± 0.0 d |
| C18:4 n-3 | 6.1 ± 0.2 b | 6.1 ± 0.2 b | 7.2 ± 0.4 a | 3.9 ± 0.1 c | 10.1 ± 0.2 a | 9.9 ± 0.0 a | 8.6 ± 0.1 b | 1.3 ± 0.0 c |
| C18:2 n-6 | 2.6 ± 0.0 b | 2.6 ± 0.0 b | 3.3 ± 0.2 a | 2.2 ± 0.1 c | 7.2 ± 0.2 a | 7.2 ± 0.1 a | 4.3 ± 0.2 b | 0.3 ± 0.0 c |
| C18:3 n-3 | 5.0 ± 0.2 b | 5.0 ± 0.2 b | 9.8 ± 0.1 a | 4.8 ± 0.1 b | 14.5 ± 0.2 a | 11.7 ± 0.3 b | 7.8 ± 0.1 c | 4.0 ± 0.0 d |
| C18:1 n-7 | 7.7 ± 0.2 b | 7.7 ± 0.2 b | 9.4 ± 0.2 a | 7.6 ± 0.1 b | 8.5 ± 0.1 a | 6.4 ± 0.1 b | 6.9 ± 0.2 b | 8.7 ± 0.1 a |
| C18:0 | 1.3 ± 0.0 a | 1.3 ± 0.0 a | 0.4 ± 0.0 c | 0.6 ± 0.0 b | 1.4 ± 0.0 a | 0.9 ± 0.0 b | 0.7 ± 0.0 c | 1.4 ± 0.0 a |
| C20:4 n-6 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.8 ± 0.0 a | 0.1 ± 0.0 b | 0.0 ± 0.0 c | 0.0 ± 0.0 c |
| C22:4 n-7 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.6 ± 0.0 a | 0.1 ± 0.0 b | 0.0 ± 0.0 c | 0.1 ± 0.0 b |
| C22:6 n-3 | 0.8 ± 0.0 a | 0.8 ± 0.0 a | 0.1 ± 0.0 b | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 ab |
| C22:0 | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 1.9 ± 0.1 b | 2.7 ± 0.0 a | 2.5 ± 0.0 b | 1.8 ± 0.1 c | 0.9 ± 0.0 d | 15.3 ± 0.4 a |
| TFA 1 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 99.9 ± 0.0 a | 99.8 ± 0.3 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| SFA 2 | 71.6 ± 0.2 b | 71.6 ± 0.2 b | 64.3 ± 0.6 c | 80.6 ± 0.3 a | 49.9 ± 0.6 d | 57.4 ± 0.5 c | 63.7 ± 0.6 b | 83.5 ± 0.1 a |
| MUFA 3 | 9.5 ± 0.2 b | 9.5 ± 0.2 b | 11.8 ± 0.3 a | 8.0 ± 0.1 c | 10.6 ± 0.1 a | 8.1 ± 0.1 c | 9.0 ± 0.2 b | 10.2 ± 0.1 a |
| PUFA 4 | 19.1 ± 0.1 b | 19.1 ± 0.1 b | 23.8 ± 0.6 a | 11.4 ± 0.2 c | 38.9 ± 0.7 a | 33.8 ± 0.4 b | 27.0 ± 0.4 c | 6.6 ± 0.1 d |
| n-3 5 | 12.1 ± 0.1 b | 12.1 ± 0.1 b | 17.2 ± 0.4 a | 8.8 ± 0.1 c | 24.8 ± 0.4 a | 21.9 ± 0.3 b | 16.5 ± 0.2 c | 5.4 ± 0.0 d |
| n-6 6 | 3.2 ± 0.0 b | 3.2 ± 0.0 b | 3.7 ± 0.2 a | 2.4 ± 0.1 c | 9.0 ± 0.2 a | 8.3 ± 0.1 b | 4.9 ± 0.2 c | 0.6 ± 0.0 d |
| n-6/n-3 7 | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.2 ± 0.0 b | 0.3 ± 0.0 a | 0.4 ± 0.0 a | 0.4 ± 0.0 a | 0.3 ± 0.0 b | 0.1 ± 0.0 c |
| Fatty Acid | Percentage of Fatty Acid (%) from the Total Fatty Acids | |||||
|---|---|---|---|---|---|---|
| Temperature | 8 °C 8 | 20 °C | 30 °C | 8 °C | 20 °C | 30 °C |
| Cultivation Period | After 7 Days 9 | After 21 Days | ||||
| C14:0 | 0.8 ± 0.2 a | 0.1 ± 0.2 a | 0.5 ± 0.1 a | 1.2 ± 0.1 a | 1.2 ± 0.1 a | 0.7 ± 0.0 a |
| C15:0 | 1.1 ± 0.2 a | 0.1 ± 1.2 b | 0.7 ± 0.0 ab | 2.3 ± 0.3 a | 0.2 ± 0.3 b | 0.4 ± 0.0 b |
| C16:3 n-6 | 0.3 ± 0.0 a | 0.4 ± 0.0 a | 0.2 ± 0.0 a | 1.1 ± 0.0 a | 0.3 ± 0.0 b | 0.2 ± 0.0 b |
| C16:4 n-4 | 0.9 ± 0.0 a | 0.1 ± 0.0 a | 0.2 ± 0.1 a | 1.9 ± 0.1 a | 1.1 ± 0.3 a | 2.4 ± 0.3 a |
| C16:3 n-3 | 0.2 ± 0.5 b | 2.8 ± 0.0 a | 0.4 ± 1.0 b | 0.4 ± 0.1 a | 0.2 ± 0.0 a | 0.3 ± 0.0 a |
| C16:1 n-7 | 1.9 ± 0.1 a | 2.1 ± 0.3 a | 5.2 ± 0.1 a | 6.3 ± 0.1 a | 3.7 ± 0.4 a | 2.0 ± 0.1 a |
| C18:2 n-2 | 0.0 ± 0.0 b | 3.3 ± 0.0 a | 0.8 ± 0.0 b | 0.7 ± 0.0 a | 0.2 ± 0.0 a | 0.0 ± 0.0 a |
| C16:0 | 62.3 ± 2.5 b | 70.8 ± 0.2 b | 77.4 ± 0.5 a | 57.0 ± 0.5 a | 65.3 ± 2.3 a | 73.8 ± 0.6 a |
| C17:1 n-7 | 0.3 ± 0.0 b | 2.0 ± 0.0 a | 0.4 ± 0.1 b | 1.4 ± 0.0 a | 0.3 ± 0.0 a | 0.5 ± 0.0 a |
| C18:3 n-6 | 0.2 ± 0.0 a | 0.0 ± 0.0 a | 2.2 ± 0.0 a | 0.0 ± 0.0 a | 0.6 ± 0.1 a | 2.0 ± 0.5 a |
| C18:4 n-3 | 8.2 ± 0.3 a | 2.4 ± 0.0 a | 1.6 ± 0.3 a | 14.5 ± 1.0 a | 4.3 ± 0.0 a | 2.5 ± 0.4 a |
| C18:2 n-6 | 3.1 ± 0.4 ab | 4.2 ± 0.2 a | 0.9 ± 0.2 b | 1.3 ± 0.6 b | 2.1 ± 0.2 b | 4.1 ± 0.1 a |
| C18:3 n-3 | 9.5 ± 0.2 a | 3.8 ± 0.4 a | 2.7 ± 0.2 a | 2.1 ± 0.7 a | 8.3 ± 0.9 a | 3.0 ± 0.4 a |
| C18:1 n-7 | 4.8 ± 1.2 a | 3.9 ± 0.2 b | 3.1 ± 0.1 c | 2.5 ± 0.1 b | 6.8 ± 0.2 a | 5.1 ± 0.2 a |
| C18:0 | 1.5 ± 0.4 a | 1.8 ± 0.6 a | 1.7 ± 0.1 a | 1.8 ± 0.1 a | 1.7 ± 0.1 a | 1.1 ± 0.0 a |
| C20:4 n-6 | 2.5 ± 0.0 a | 0.7 ± 0.0 a | 0.1 ± 0.0 a | 0.3 ± 0.3 a | 0.8 ± 0.0 a | 0.2 ± 0.0 a |
| C22:4 n-7 | 0.2 ± 0.0 a | 0.4 ± 0.2 a | 0.4 ± 0.0 a | 0.1 ± 0.2 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| C22:6 n-3 | 0.0 ± 0.0 a | 0.4 ± 1.5 a | 0.8 ± 0.2 a | 0.0 ± 0.2 a | 0.0 ± 0.4 ab | 0.0 ± 0.0 b |
| C22:0 | 2.1 ± 0.7 a | 0.7 ± 0.9 b | 0.6 ± 0.1 b | 5.2 ± 0.3 a | 2.7 ± 0.1 a | 1.9 ± 0.0 a |
| TFA 1 | 100.0 ± 6.7 a | 100.0 ± 5.9 a | 100.0 ± 3.1 a | 100.0 ± 4.8 a | 100.0 ± 5.3 a | 100.0 ± 2.6 a |
| SFA 2 | 67.8 ± 4.0 b | 73.5 ± 3.1 b | 81.0 ± 0.8 a | 67.5 ± 1.4 a | 71.2 ± 2.7 a | 77.9 ± 0.7 a |
| MUFA 3 | 7.0 ± 1.2 a | 8.0 ± 0.5 a | 8.8 ± 0.3 a | 10.2 ± 0.2 a | 10.8 ± 0.6 a | 7.5 ± 0.3 a |
| PUFA 4 | 25.2 ± 1.5 a | 20.5 ± 2.3 a | 8.5 ± 2.1 b | 23.7 ± 3.2 a | 17.7 ± 1.9 a | 13.0 ± 1.2 a |
| n-3 5 | 17.9 ± 1.0 a | 9.4 ± 1.9 a | 5.5 ± 1.7 a | 17.0 ± 2.0 a | 12.8 ± 1.3 a | 5.7 ± 0.8 a |
| n-6 6 | 6.2 ± 0.4 a | 5.3 ± 0.2 a | 3.3 ± 0.2 b | 2.7 ± 0.9 a | 3.7 ± 0.4 a | 6.4 ± 0.6 a |
| n-6/n-3 7 | 0.3 ± 0.0 a | 0.6 ± 0.0 a | 0.6 ± 0.0 a | 0.2 ± 0.0 a | 0.3 ± 0.0 a | 1.1 ± 0.1 a |
| Fatty Acid | Percentage of Fatty Acid (%) of the Total Fatty Acids by Treatments | |||
|---|---|---|---|---|
| Nutrient | N0P0 8 | N6.4P 1 | N0P1 | N6.4P0 |
| Cultivation Period | 0–21 Days 9 | 0–21 Days | 0–21 Days | 0–21 Days |
| C15:0 | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.2 ± 0.0 a | 0.3 ± 0.1 a |
| C16:4 n-4 | 6.5 ± 0.2 b | 6.7 ± 0.4 b | 7.3 ± 0.5 ab | 8.1 ± 0.3 a |
| C16:2 n-6 | 0.6 ± 0.1 a | 0.5 ± 0.2 a | 0.4 ± 0.2 a | 0.4 ± 0.1 a |
| C16:1 n-5 | 0.4 ± 0.0 a | 0.6 ± 0.1 a | 0.8 ± 0.1 a | 0.7 ± 0.3 a |
| C16:1 n-9 | 3.9 ± 0.4 a | 4.5 ± 0.0 a | 4.0 ± 0.1 a | 3.7 ± 0.4 a |
| C16:0 | 58.7 ± 3.0 a | 56.9 ± 2.8 ab | 51.3 ± 0.7 ab | 49.1 ± 2.3 b |
| C18:4 n-3 | 8.9 ± 1.6 a | 9.1 ± 1.5 a | 11.6 ± 0.6 a | 12.8 ± 0.9 a |
| C18:2 n-6 | 6.2 ± 0.7 a | 4.3 ± 0.9 a | 4.1 ± 1.2 a | 3.9 ± 1.1 a |
| C18:3 n-3 | 7.2 ± 1.3 c | 8.5 ± 0.6 bc | 10.6 ± 0.4 ab | 11.8 ± 0.8 a |
| C18:1 n-7 | 6.8 ± 0.3 a | 7.9 ± 0.9 a | 8.9 ± 1.9 a | 8.5 ± 0.3 a |
| C22:0 | 0.5 ± 0.0 a | 0.8 ± 0.2 a | 0.7 ± 0.1 a | 0.8 ± 0.1 a |
| TFA 1 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| SFA 2 | 59.5 ± 3.0 a | 57.9 ± 2.9 ab | 52.2 ± 0.7 bc | 50.2 ± 2.5 c |
| MUFA 3 | 11.1 ± 0.5 b | 13.0 ± 0.9 ab | 13.7 ± 2.1 a | 12.8 ± 0.3 ab |
| PUFA 4 | 29.4 ± 3.2 ab | 29.1 ± 2.6 b | 34.1 ± 2.5 ab | 37.0 ± 2.8 a |
| n-3 5 | 16.1 ± 2.9 b | 17.6 ± 1.9 ab | 22.2 ± 1.0 ab | 24.6 ± 1.7 a |
| n-6 6 | 6.8 ± 0.8 a | 4.8 ± 1.0 a | 4.6 ± 1.4 a | 4.2 ± 1.2 a |
| n-6/n-3 7 | 0.4 ± 0.1 a | 0.3 ± 0.1 b | 0.2 ± 0.1 b | 0.2 ± 0.0 b |
| Fatty Acid | Percentage of Fatty Acid (%) from the Total Fatty Acids | ||
|---|---|---|---|
| Irradiation Intensity | 50 µmol m2 s−1 8 | 100 µmol m2 s−1 | 150 µmol m2 s−1 |
| Cultivation Period | 0–21 Days 9 | 0–21 Days | 0–21 Days |
| C15:0 | 0.6 ± 0.2 a | 0.7 ± 0.2 a | 0.6 ± 0.0 a |
| C16:4 n-4 | 4.9 ± 0.9 a | 2.9 ± 0.4 b | 5.2 ± 0.3 a |
| C16:2 n-6 | 0.3 ± 0.0 a | 0.2 ± 0.1 a | 0.2 ± 0.0 a |
| C16:1 n-5 | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| C16:1 n-9 | 3.3 ± 0.0 a | 3.0 ± 0.3 a | 3.4 ± 0.2 a |
| C16:0 | 56.8 ± 2.0 b | 63.3 ± 1.4 a | 56.3 ± 1.8 b |
| C18:4 n-3 | 9.1 ± 0.9 a | 6.0 ± 0.3 b | 9.9 ± 0.9 a |
| C18:2 n-6 | 3.5 ± 0.3 a | 3.8 ± 0.2 a | 3.1 ± 0.4 a |
| C18:3 n-3 | 11.6 ± 1.2 a | 8.8 ± 0.8 a | 12.0 ± 1.0 a |
| C18:1 n-7 | 8.0 ± 0.7 a | 9.1 ± 1.5 a | 8.0 ± 0.1 a |
| C22:0 | 1.8 ± 0.4 a | 2.1 ± 0.9 a | 1.1 ± 0.2 a |
| TFA 1 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| SFA 2 | 59.2 ± 2.2 b | 66.1 ± 0.3 a | 57.9 ± 2.0 b |
| MUSFA 3 | 11.4 ± 0.7 a | 12.2 ± 1.7 a | 11.6 ± 0.2 a |
| PUFA 4 | 29.4 ± 2.7 a | 21.7 ± 1.4 b | 30.5 ± 2.1 a |
| n-3 5 | 20.7 ± 2.1 a | 14.8 ± 1.0 b | 21.9 ± 1.9 a |
| n-6 6 | 3.9 ± 0.3 a | 4.0 ± 0.1 a | 3.3 ± 0.4 a |
| n-6/n-3 7 | 0.2 ± 0.0 b | 0.3 ± 0.0 a | 0.2 ± 0.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnayem, N.; Unis, R.; Gnaim, R.; Chemodanov, A.; Israel, Á.; Gnaim, J.; Golberg, A. Fatty Acid Content and Profile in Ulva lactuca in Response to Exposure to Variable Growth Conditions in Indoor Photobioreactors. Life 2025, 15, 57. https://doi.org/10.3390/life15010057
Gnayem N, Unis R, Gnaim R, Chemodanov A, Israel Á, Gnaim J, Golberg A. Fatty Acid Content and Profile in Ulva lactuca in Response to Exposure to Variable Growth Conditions in Indoor Photobioreactors. Life. 2025; 15(1):57. https://doi.org/10.3390/life15010057
Chicago/Turabian StyleGnayem, Nabeel, Razan Unis, Rima Gnaim, Alexander Chemodanov, Álvaro Israel, Jallal Gnaim, and Alexander Golberg. 2025. "Fatty Acid Content and Profile in Ulva lactuca in Response to Exposure to Variable Growth Conditions in Indoor Photobioreactors" Life 15, no. 1: 57. https://doi.org/10.3390/life15010057
APA StyleGnayem, N., Unis, R., Gnaim, R., Chemodanov, A., Israel, Á., Gnaim, J., & Golberg, A. (2025). Fatty Acid Content and Profile in Ulva lactuca in Response to Exposure to Variable Growth Conditions in Indoor Photobioreactors. Life, 15(1), 57. https://doi.org/10.3390/life15010057





