Associations of Voice Metrics with Postural Function in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Variables
2.3.1. Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III)
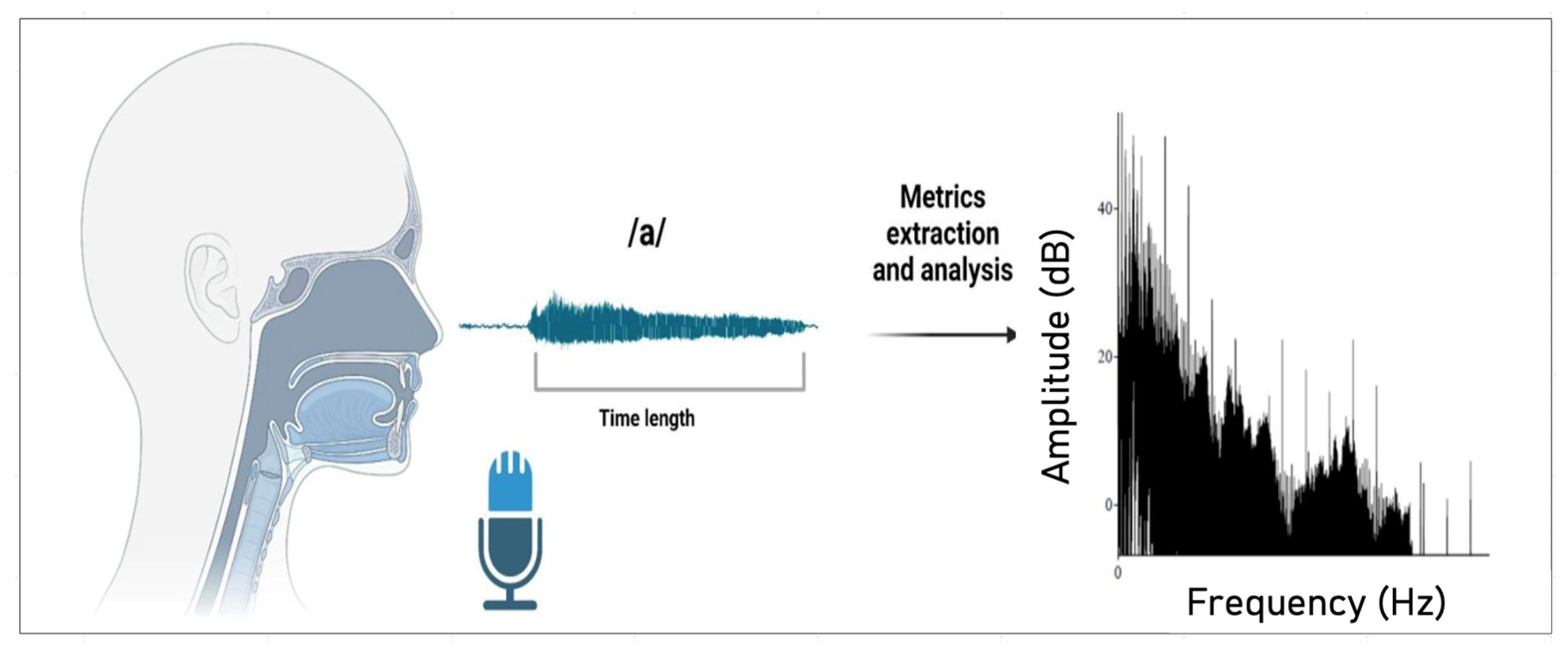
2.3.2. Vocal Recording and Measures
2.3.3. Voice Metrics
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Exploratory Correlations
3.3. Linear Regressions
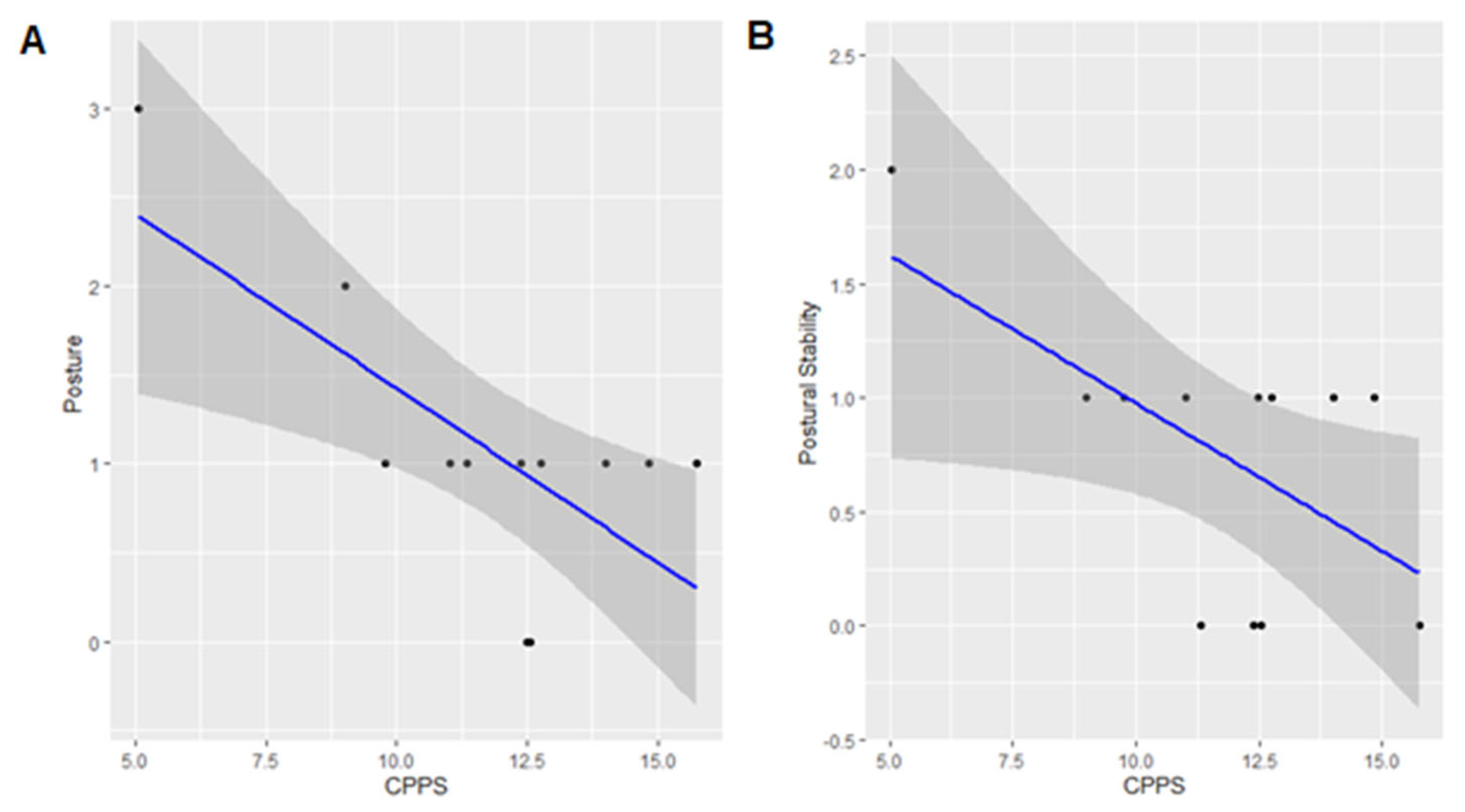
3.4. UPDRS-III Posture and CPPS
3.5. UPDRS-III Postural Stability and CPPS
3.6. Comparison of Voice Measures by Motor Severity Levels
4. Discussion
4.1. Impact of the Study Results on the Field of Otolaryngology
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef]
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Forsaa, E.B.; Larsen, J.P.; Wentzel-Larsen, T.; Herlofson, K.; Alves, G. Predictors and course of health-related quality of life in Parkinson’s disease. Mov. Disord. 2008, 23, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Muslimovic, D.; Post, B.; Speelman, J.D.; Schmand, B.; de Haan, R.J.; CARPA Study Group. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology 2008, 70, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Pickering, R.M.; Grimbergen, Y.A.; Rigney, U.; Ashburn, A.; Mazibrada, G.; Wood, B.; Gray, P.; Kerr, G.; Bloem, B.R. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov. Disord. 2007, 22, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Griffin, H.J.; Quinn, N.P.; Jahanshahi, M. Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov. Disord. 2008, 23, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Hartelius, L.; Svensson, P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: A survey. Folia Phoniatr Logop. 1994, 46, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rusz, J.; Cmejla, R.; Ruzickova, H.; Ruzicka, E. Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson’s disease. J. Acoust. Soc. Am. 2011, 129, 350–367. [Google Scholar] [CrossRef]
- Harel, B.; Cannizzaro, M.; Snyder, P.J. Variability in fundamental frequency during speech in prodromal and incipient Parkinson’s disease: A longitudinal case study. Brain Cogn. 2004, 56, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Sewall, G.K.; Jiang, J.; Ford, C.N. Clinical evaluation of Parkinson’s-related dysphonia. Laryngoscope 2006, 116, 1740–1744. [Google Scholar] [CrossRef]
- Ma, A.; Lau, K.K.; Thyagarajan, D. Voice changes in Parkinson’s disease: What are they telling us? J. Clin. Neurosci. 2020, 72, 1–7. [Google Scholar] [CrossRef]
- Perez, K.S.; Ramig, L.O.; Smith, M.E.; Dromey, C. The Parkinson Larynx: Tremor and Videostroboscopic Findings. J. Voice 1996, 10, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pah, N.D.; Motin, M.A.; Oliveira, G.C.; Kumar, D.K. The Change of Vocal Tract Length in People with Parkinson’s Disease. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2023, 1–4. [Google Scholar] [CrossRef]
- Lim, W.S.; Chiu, S.I.; Wu, M.C.; Tsai, S.-F.; Wang, P.-H.; Lin, K.-P.; Chen, Y.-M.; Peng, P.-L.; Jang, J.-S.R.; Lin, C.-H. An integrated biometric voice and facial features for early detection of Parkinson’s disease. npj Park. Dis. 2022, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nishio, M.; Niimi, S. Vocal acoustic characteristics of patients with Parkinson’s disease. Folia Phoniatr. Logop. 2011, 63, 223–230. [Google Scholar] [CrossRef]
- Jannetts, S.; Lowit, A. Cepstral analysis of hypokinetic and ataxic voices: Correlations with perceptual and other acoustic measures. J. Voice 2014, 28, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.C.; Motin, M.A.; Pah, N.D.; Drotár, P.; Kempster, P.; Kumar, D. Computerized analysis of speech and voice for Parkinson’s disease: A systematic review. Comput. Methods Programs Biomed. 2022, 226, 107133. [Google Scholar] [CrossRef] [PubMed]
- Maryn, Y.; Weenink, D. Objective dysphonia measures in the program Praat: Smoothed cepstral peak prominence and acoustic voice quality index. J. Voice 2015, 29, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, D.A.; Dahl, K.L.; Perkins, Z.; Abur, D.; Stepp, C.E. Effects of a Concurrent Working Memory Task on Speech Acoustics in Parkinson’s Disease. Am. J. Speech-Lang. Pathol. 2024, 33, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Cantor-Cutiva, L.C.; Ramani, S.A.; Walden, P.R.; Hunter, E.G. Evaluating the Relationship between Vocal Effort and Speech in Parkinson’s Disease Using Cepstral Peak Prominence. Am. J. Speech-Lang. Pathol. 2018, 27, 749–758. [Google Scholar]
- Seifpanahi, M.S.; Ghaemi, T.; Ghaleiha, A.; Sobhani-Rad, D.; Zarabian, M.K. The Association between Depression Severity, Prosody, and Voice Acoustic Features in Women with Depression. Sci. World J. 2023, 2023, 9928446. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.J.; Lopes, L.; Galdino, M.K.C.; Almeida, A.A. Voice Acoustic Parameters as Predictors of Depression. J. Voice 2024, 38, 77–85. [Google Scholar] [CrossRef]
- Geng, P.; Fan, N.; Ling, R.; Li, Z.; Guo, H.; Lu, Q.; Chen, X. Acoustic Characteristics of Mandarin Speech in Male Drug Users. J. Voice 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Icht, M.; Wiznitser-Ressis-Tal, H.; Lotan, M. Can the Vocal Expression of Intellectually Disabled Individuals Be Used as a Pain Indicator? Initial Findings Supporting a Possible Novice Assessment Method. Front. Psychol. 2021, 12, 655202. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Vakhnovetsky, J.; Amanabi, M.; Karamifar, K.; Farhadi, M.; Amini, S.B.; Conte, M. Exploring the Impact of Type II Diabetes Mellitus on Voice Quality. Eur. Arch. Otorhinolaryngol. 2024, 281, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.; Franklin, C.; Peterson, E.; Hunter, E.J.; Robin, D.A.; Halpern, A.; Spielman, J.; Fox, P.T.; Ramig, L.O. Immediate and Long-term Effects of Speech Treatment Targets and Intensive Dosage on Parkinson’s Disease Dysphonia and the Speech Motor Network: Randomized Controlled Trial. Hum. Brain Mapp. 2022, 43, 2328–2347. [Google Scholar] [CrossRef]
- Xiu, N.; Li, W.; Liu, L.; Liu, Z.; Cai, Z.; Li, L.; Vaxelaire, B.; Sock, R.; Ling, Z.; Chen, J.; et al. A Study on Voice Measures in Patients with Parkinson’s Disease. J. Voice 2024, in press. [Google Scholar] [CrossRef]
- Burk, B.R.; Watts, C.R. The effect of Parkinson disease tremor phenotype on cepstral peak prominence and transglottal airflow in vowels and speech. J. Voice 2019, 33, 580.E11–580.E19. [Google Scholar] [CrossRef] [PubMed]
- Goberman, A. Correlation between acoustic speech characteristics and non-speech motor performance in Parkinson Disease. Med. Sci. Monit. 2005, 11, 109–116. [Google Scholar]
- Skodda, S.; Visser, W.; Schlegel, U. Gender-related patterns of dysprosody in Parkinson disease and correlation between speech variables and motor symptoms. J. Voice 2011, 25, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; MacCallum, J.K.; Zhang, Y.; Sprecher, A.; Jiang, J.J. Acoustic analysis of the tremulous voice: Assessing the utility of the correlation dimension and perturbation parameters. J. Commun. Disord. 2010, 43, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.E.; Barbosa, M.T.; Limongi, J.C.P.; Barbosa, E.R. Speech disorders did not correlate with age at onset of Parkinson’s disease. Arq. Neuropsiquiatr. 2016, 74, 117–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillivan-Murphy, P.; Miller, N.; Carding, P. Voice Tremor in Parkinson’s Disease: An Acoustic Study. J. Voice 2019, 33, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Spencer, K.A. The Relationship Between Speech Characteristics and Motor Subtypes of Parkinson’s Disease. Am. J. Speech Lang. Pathol. 2020, 29, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Skodda, S.; Rinsche, H.; Schlegel, U. Progression of dysprosody in Parkinson’s disease over time—A longitudinal study. Mov. Disord. 2009, 24, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.P.; Sundberg, J.; Purdy, S.C.; Rakena, T.O.; Leão, S.H.S. CPPS and Voice-Source Parameters: Objective Analysis of the Singing Voice. J. Voice 2024, 38, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, P.G.; de Jong, F.I.; Oudes, M.J.; Huinck, W.; van Acht, H.; Graamans, K. Muscular Tension and Body Posture in Relation to Voice Handicap and Voice Quality in Teachers with Persistent Voice Complaints. Folia Phoniatr. Logop. 2005, 57, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Bruno, E.; De Padova, A.; Napolitano, B.; Marroni, P.; Batelli, R.; Ottaviani, F.; Alessandrini, M. Voice Disorders and Posturography: Variables to Define the Success of Rehabilitative Treatment. J. Voice 2009, 23, 71–75. [Google Scholar] [CrossRef]
- Gelb, D.; Oliver, E.; Gilman, S. Diagnostic Criteria for Parkinson Disease. Arch Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and Recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Marsden, C.; Goldstein, M.; Calne, D. Recent Developments in Parkinson’s Disease; Macmillan Health Care Information: Florham Park, NJ, USA, 1987. [Google Scholar]
- Sauder, C.; Bretl, M.; Eadie, T. Predicting Voice Disorder Status from Smoothed Measures of Cepstral Peak Prominence Using Praat and Analysis of Dysphonia in Speech and Voice (ADSV). J. Voice 2017, 31, 557–566. [Google Scholar] [CrossRef]
- Teixeira, J.P.; Oliveira, C.; Lopes, C. Vocal Acoustic Analysis—Jitter, Shimmer, and HNR Parameters. Procedia Technol. 2013, 9, 1112–1122. [Google Scholar] [CrossRef]
- Eggers, C.; Kahraman, D.; Fink, G.R.; Schmidt, M.; Timmermann, L. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov Disord. 2011, 26, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Murton, O.; Hillman, R.; Mehta, D. Cepstral Peak Prominence Values for Clinical Voice Evaluation. Am. J. Speech-Lang. Pathol. 2020, 29, 1596–1607. [Google Scholar] [CrossRef]
- Chiu, Y.; Neel, A.; Loux, T. Acoustic Characteristics in Relation to Intelligibility Reduction in Noise for Speakers with Parkinson’s Disease. Clin. Linguist. Phon. 2021, 35, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Demers-Peel, M.; Manxhari, C.; Stepp, C.E. Voice Acoustic Instability During Spontaneous Speech in Parkinson’s Disease. J. Voice 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Neuroanatomy and Pathology of Sporadic Parkinson’s Disease. Adv. Neurol. 2009, 118, 1–12. [Google Scholar]
- Estenne, M.; Zocchi, L.; Ward, M.; Macklem, P.T. Chest Wall Motion and Expiratory Muscle Use During Phonation in Normal Humans. J. Appl. Physiol. 1990, 68, 2075–2082. [Google Scholar] [CrossRef]
- Giovanni, A.; Akl, L.; Ouaknine, M. Postural Dynamics and Vocal Effort: Preliminary Experimental Analysis. Folia Phoniatr. Logop. 2008, 60, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Cayreyre, F.; Akl, L.; de la Bretèque, B.A.; Ouaknine, M.; Giovanni, A. Augmentation des Mouvements Respiratoires Abdominaux Lors du Passage Soudain de la Voix Conversationnelle à la Voix Forte [Increase in the Abdominal Respiratory Movements During the Sudden Passage from a Conversational Voice to a Loud Voice]. Rev. Laryngol. Otol. Rhinol. 2005, 126, 347–351. [Google Scholar]
- Arora, S.; Venkataraman, V.; Zhan, A.; Donohue, S.; Biglan, K.M.; Dorsey, E.R.; Little, M.A. Detecting and Monitoring the Symptoms of Parkinson’s Disease Using Smartphones: A Pilot Study. Park. Relat. Disord. 2015, 21, 650–653. [Google Scholar] [CrossRef]
- Dipietro, L.; Eden, U.; Elkin-Frankston, S.; El-Hagrassy, M.M.; Camsari, D.D.; Ramos-Estebanez, C.; Fregni, F.; Wagner, T. Integrating Big Data, Artificial Intelligence, and Motion Analysis for Emerging Precision Medicine Applications in Parkinson’s Disease. J. Big Data 2024, 11, 155. [Google Scholar] [CrossRef]
- Marino, J.P.; Johns, M.M., III. The Epidemiology of Dysphonia in the Aging Population. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Cardella, A.; Ottaviani, F.; Luzi, L.; Albera, A.; Schindler, A.; Mozzanica, F. Daily Speaking Time and Voice Intensity Before and After Hearing Aids Rehabilitation in Adult Patients with Hearing Loss. Folia Phoniatr. Logop. 2023, 76, 440–448. [Google Scholar] [CrossRef] [PubMed]
| Authors/ Year | Sample | Goal | Motor Symptoms | Main Voice Metrics | Analyses | Results |
|---|---|---|---|---|---|---|
| Burk & Watts, 2018 [28] | 32 PD (ON period, H&Y: 2.62-2.75), 10 HC | Differentiate tremor and non-tremor phenotypes. | Tremor (UPDRS and self-reporting) | CPP (dB) | Sustained vowel (/a/) and connected speech (Computerized Speech Lab - Pentax Medical, Montvale, NJ) | Tremor dominant exhibited lower CPP than non-tremor subjects and control. |
| Goberman, 2005 [29] | 9 PD (ON period, H&Y) | Examine the associations between voice and motor variables. | Factored UPDRS-III: Axial function/gait, rest tremor, rigidity, bradykinesia, postural tremor | F0 and F0 SD | Sustained vowel (/i/, /u/, /a/, /ae/) and continuous speech (Computerized Speech Lab software - Kay Elemetrics). | F0 SD associated with axial and non-axial motor symptoms. |
| Skodda et al., 2011 [30] | 169 PD (ON period, H&Y: 2.51), 64 HC | Explore correlations of prosodic and motor symptoms. | Total UPDRS-III and sub-scores | F0, SD, and variation range | Continuous speech (Praat, Version 5.1 - Institute of Phonetic Sciences, University of Amsterdam) | Mean F0 associated to axial UPDRS-II sub- scores; F0 variability reduced in PD. |
| Dias et al., 2016 [32] | 50 PD (ON period, H&Y: 2.71-3.18) | Correlate speech impairment and motor symptoms. | UPDRS-III: tremor, rigidity, bradykinesia, axial impairment | Formant frequency values F1 and F2 | Sustained vowel (/a/, /i/, /u/), continuous and spontaneous speech (Praat software v5.3.30 - Phonetic Sciences, University of Amsterdam) | Associations between the metrics and axial, rigidity, and bradykinesia sub- scores. |
| Gillivan-Murphy, Miller & Carding, 2019 [33] | 30 PD (OFF period), 28 HC | Examine correlations of voice tremor and disease variables. | UPDRS-III total score | Voice tremor rate (rate, periodicity, variation, and amplitude of F0) | Sustained vowel (/a/) (Multi-Dimensional Voice Program, Computerized Speech Laboratory) | Only the rate of amplitude voice tremor correlated negatively with UPDRS-III; voice disability did not correlate with voice tremor; rate of tremor higher in PD than HC. |
| Brown & Spencer, 2020 [34] | 27 PD (ON period) | Investigate whether acoustic dysarthria aligns with non-tremor and tremor-dominant profiles. | MDS-UPDRS-III (classification of tremor profiles) | F0 range (Hz), average pause duration, CPPS (dB) | Continuous speech (Praat - Boersma & Weenink, 2017 and Adobe Audition Version 9.0) | No differences were observed between the motor profiles. |
| Skodda et al., 2009 [35] | 50 PD (ON period), 50 HC | Analyze changes in speech over time (up to 79 months) and correlated with motor impairment. | UPDRS-III total score | F0, SD, and variation range (Hz) | Continuous speech (Praat - Phonetic Sciences, University of Amsterdam) | No association between the changes in the vocal metrics and changes in UPDRS-III. |
| Units | Objective | Clinical Relevance | Interpretation | |
|---|---|---|---|---|
| CPPS | decibels (dB) | To measure the regularity and periodicity of the voice signal, focusing on the fundamental frequency and its prominence in the cepstrum. | Useful for detecting subtle changes in voice periodicity and diagnosing voice disorders affecting vocal fold vibrations. | High CPP suggests a highly regular voice signal and good vocal quality, while low CPP suggests aperiodicity, which may be associated with voice disorders. |
| Harmonic–Noise Ratio (HNR) | decibels (dB) | To measure the relative amount of periodic (harmonic) energy to aperiodic (noise). | Provides a broader measure of overall voice quality and is useful for diagnosing voice disorders that introduce noise. | High HNR indicates a clear and stable voice, while low HNR suggests potential pathologies. |
| Shimmer | milliseconds (ms) or percentages (%) | To measure variations in the amplitude. | Important parameter for assessing vocal quality and health. | Low shimmer translates to loudness stability, while high shimmer suggests an unhealthy voice. |
| Jitter | decibels (dB) or percentages (%) | To measure variations in the fundamental frequency. | Used to diagnose and monitor voice disorders. | Low jitter reflects pitch stability, while high jitter indicates possible disorders. |
| Fundamental Frequency (F0) | hertz (Hz) | To measure the rate at which vocal folds vibrate, representing the pitch of the voice. | Vital for understanding pitch control, voice quality, and diagnosing voice disorders related to pitch regulation. | A low F0 indicates a slower vibration of the vocal folds, producing a lower-pitched voice. |
| UPDRS-III variables | Mean ±SD |
|---|---|
| Speech | 1.6 ± 0.9 |
| Facial expression | 1.2 ± 1.0 |
| Rigidity | 3.2 ± 2.0 |
| Finger tapping | 1.8 ± 1.2 |
| Hand movements | 1.9 ± 1.5 |
| Alternating movements | 1.8 ± 1.5 |
| Leg agility | 1.8 ± 1.7 |
| Posture | 1.1 ± 0.8 |
| Gait | 0.8 ± 0.7 |
| Postural stability | 0.7 ± 0.6 |
| Bradykinesia | 1.5 ± 1.0 |
| UPDRS-III | 18.0 (13.0) |
| Arising from chair | 0 (0) |
| Kinetic Tremor | 2.0 (1.0) |
| Tremor | 1.0 (3.0) |
| Voice metrics | Mean (±SD) |
| Jitter | 0.67 ± 0.37 |
| Shimmer | 6.56 ± 2.12 |
| CPPS | 11.75 ± 2.86 |
| HNR | 21.11 ± 5.94 |
| UPDRS-III | Voice | β-Coefficient | 95% CI | SE | p Value | |
|---|---|---|---|---|---|---|
| Postural stability | CPPS | −0.130 | −0.252 | −0.007 | 0.055 | 0.040 * |
| Jitter | 0.326 | −0.719 | 1.371 | 0.453 | 0.493 | |
| Shimmer | −0.041 | −0.223 | 0.141 | 0.079 | 0.618 | |
| HNR | 0.015 | −0.050 | 0.080 | 0.028 | 0.612 | |
| Posture | CPPS | −0.196 | −0.334 | −0.058 | 0.061 | 0.010 * |
| Jitter | 0.293 | −0.952 | 1.538 | 0.539 | 0.602 | |
| Shimmer | −0.013 | −0.230 | 0.205 | 0.094 | 0.896 | |
| HNR | 0.015 | −0.063 | 0.092 | 0.033 | 0.675 | |
| Speech | CPPS | −0.108 | −0.307 | 0.091 | 0.089 | 0.255 |
| Jitter | 0.247 | −1.795 | 2.289 | 0.885 | 0.787 | |
| Shimmer | −0.001 | −0.354 | 0.352 | 0.153 | 0.995 | |
| HNR | −0.007 | −0.134 | 0.119 | 0.054 | 0.894 | |
| Gait | CPPS | −0.088 | −0.240 | 0.065 | 0.068 | 0.228 |
| Jitter | −0.088 | −1.353 | 1.178 | 0.548 | 0.877 | |
| Shimmer | 0.066 | −0.146 | 0.277 | 0.091 | 0.494 | |
| HNR | −0.054 | −0.118 | 0.010 | 0.027 | 0.088 | |
| Tremor | CPPS | 0.274 | −0.431 | 0.978 | 0.316 | 0.407 |
| Jitter | −1.131 | −8.303 | 6.041 | 3.110 | 0.726 | |
| Shimmer | 0.378 | −0.826 | 1.583 | 0.522 | 0.489 | |
| HNR | −0.265 | −0.654 | 0.123 | 0.168 | 0.154 | |
| Body bradykinesia | CPPS | −0.161 | −0.379 | 0.056 | 0.097 | 0.129 |
| Jitter | 0.858 | −1.195 | 2.912 | 0.890 | 0.363 | |
| Shimmer | 0.046 | −0.325 | 0.417 | 0.160 | 0.783 | |
| HNR | −0.034 | −0.165 | 0.096 | 0.056 | 0.560 | |
| Facial expression | CPPS | −0.086 | −0.321 | 0.149 | 0.105 | 0.433 |
| Jitter | 0.190 | −2.170 | 2.550 | 1.023 | 0.857 | |
| Shimmer | −0.160 | −0.545 | 0.225 | 0.167 | 0.367 | |
| HNR | 0.041 | −0.101 | 0.182 | 0.061 | 0.525 | |
| Rapid alternating | CPPS | −0.297 | −0.631 | 0.038 | 0.150 | 0.076 |
| Jitter | −0.860 | −4.311 | 2.592 | 1.496 | 0.582 | |
| Shimmer | −0.105 | −0.705 | 0.495 | 0.260 | 0.697 | |
| HNR | 0.016 | −0.200 | 0.232 | 0.093 | 0.866 | |
| Kinetic tremor | CPPS | 0.076 | −0.242 | 0.395 | 0.143 | 0.604 |
| Jitter | −0.037 | −3.273 | 3.198 | 1.403 | 0.980 | |
| Shimmer | −0.042 | −0.598 | 0.513 | 0.240 | 0.864 | |
| HNR | 0.048 | −0.147 | 0.243 | 0.084 | 0.586 | |
| Rigidity | CPPS | −0.356 | −0.815 | 0.102 | 0.205 | 0.114 |
| Jitter | −1.573 | −5.857 | 2.711 | 1.858 | 0.422 | |
| Shimmer | 0.259 | −0.480 | 0.998 | 0.320 | 0.443 | |
| HNR | −0.177 | −0.411 | 0.057 | 0.101 | 0.119 | |
| Finger tapping | CPPS | −0.085 | −0.391 | 0.221 | 0.137 | 0.548 |
| Jitter | −1.337 | −4.067 | 1.393 | 1.183 | 0.292 | |
| Shimmer | −0.250 | −0.713 | 0.212 | 0.200 | 0.247 | |
| HNR | 0.013 | −0.167 | 0.194 | 0.078 | 0.871 | |
| Hand movements | CPPS | −0.184 | −0.547 | 0.178 | 0.162 | 0.283 |
| Jitter | −1.899 | −5.036 | 1.239 | 1.361 | 0.200 | |
| Shimmer | −0.310 | −0.856 | 0.236 | 0.236 | 0.227 | |
| HNR | 0.046 | −0.165 | 0.258 | 0.091 | 0.627 | |
| Leg agility | CPPS | −0.144 | −0.558 | 0.269 | 0.185 | 0.455 |
| Jitter | −1.200 | −4.986 | 2.585 | 1.642 | 0.486 | |
| Shimmer | −0.232 | −0.877 | 0.413 | 0.279 | 0.431 | |
| HNR | −0.051 | −0.287 | 0.186 | 0.102 | 0.635 | |
| Arising from chair | CPPS | - | - | - | - | |
| UPDRS-III total | CPPS | −1.560 | −4.113 | 0.993 | 1.146 | 0.203 |
| Jitter | −6.210 | −30.212 | 17.792 | 10.409 | 0.567 | |
| Shimmer | −0.405 | −4.611 | 3.800 | 1.823 | 0.830 | |
| HNR | −0.395 | −1.869 | 1.079 | 0.639 | 0.554 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianlorenço, A.C.; Costa, V.; Fabris-Moraes, W.; Teixeira, P.E.P.; Gonzalez, P.; Pacheco-Barrios, K.; Ramos-Estebanez, C.; Di Stadio, A.; El-Hagrassy, M.M.; Camsari, D.D.; et al. Associations of Voice Metrics with Postural Function in Parkinson’s Disease. Life 2025, 15, 27. https://doi.org/10.3390/life15010027
Gianlorenço AC, Costa V, Fabris-Moraes W, Teixeira PEP, Gonzalez P, Pacheco-Barrios K, Ramos-Estebanez C, Di Stadio A, El-Hagrassy MM, Camsari DD, et al. Associations of Voice Metrics with Postural Function in Parkinson’s Disease. Life. 2025; 15(1):27. https://doi.org/10.3390/life15010027
Chicago/Turabian StyleGianlorenço, Anna Carolyna, Valton Costa, Walter Fabris-Moraes, Paulo Eduardo Portes Teixeira, Paola Gonzalez, Kevin Pacheco-Barrios, Ciro Ramos-Estebanez, Arianna Di Stadio, Mirret M. El-Hagrassy, Deniz Durok Camsari, and et al. 2025. "Associations of Voice Metrics with Postural Function in Parkinson’s Disease" Life 15, no. 1: 27. https://doi.org/10.3390/life15010027
APA StyleGianlorenço, A. C., Costa, V., Fabris-Moraes, W., Teixeira, P. E. P., Gonzalez, P., Pacheco-Barrios, K., Ramos-Estebanez, C., Di Stadio, A., El-Hagrassy, M. M., Camsari, D. D., Wagner, T., Dipietro, L., & Fregni, F. (2025). Associations of Voice Metrics with Postural Function in Parkinson’s Disease. Life, 15(1), 27. https://doi.org/10.3390/life15010027








