Extra-Anogenital Giant Cutaneous Squamous Cell Carcinomas
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
3.2. Clinical Findings and Disease Stage
3.3. Treatment and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, V.; Trinh, X.B.; An, B.; Julien, L. Extra-anogenital giant cutaneous squamous cell carcinomas require multidisciplinary management. Cancer Treat. Res. Commun. 2021, 28, 100413. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Bayyoud, Y.; Krönert, C.; Nowak, A. Giant epithelial malignancies (Basal cell carcinoma, squamous cell carcinoma): A series of 20 tumors from a single center. J. Cutan. Aesthetic Surg. 2012, 5, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P.; Asgari, M.M.; Green, A.C.; Guhan, S.M.; Arron, S.T.; Proby, C.M.; Rollison, D.E.; Harwood, C.A.; Toland, A.E. Keratinocyte Carcinomas: Current Concepts and Future Research Priorities. Clin. Cancer Res. 2019, 25, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Work Group; Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P.; Alam, M.; Armstrong, A.; Baum, C.; et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef]
- Xiang, F.; Lucas, R.; Hales, S.; Neale, R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: Empirical relationships. JAMA Dermatol. 2014, 150, 1063–1071. [Google Scholar] [CrossRef]
- Christenson, L.J.; Borrowman, T.A.; Vachon, C.M.; Tollefson, M.M.; Otley, C.C.; Weaver, A.L.; Roenigk, R.K. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA 2005, 294, 681–690. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef]
- Higgins, S.; Nazemi, A.; Chow, M.; Wysong, A. Review of Nonmelanoma Skin Cancer in African Americans, Hispanics, and Asians. Dermatol. Surg. 2018, 44, 903–910. [Google Scholar] [CrossRef]
- Ricci, F.; Paradisi, A.; Fossati, B.; Mancini, M.; Curatolo, P.; Guerriero, C.; Capizzi, R. Giant neglected squamous cell carcinoma of the skin. Dermatol. Ther. 2015, 28, 230–234. [Google Scholar] [CrossRef]
- Bajaj, S.; Wolner, Z.J.; Dusza, S.W.; Braun, R.P.; Marghoob, A.A.; DeFazio, J. Total Body Skin Examination Practices: A Survey Study Amongst Dermatologists at High-Risk Skin Cancer Clinics. Dermatol. Pract. Concept. 2019, 9, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Özkur, E.; Altunay, İ.K.; Celayir, M.F.; Çerman, A.A.; Uçak, R. A Giant Squamous Cell Carcinoma Arising in a Patient with Hidradenitis Suppurativa. Adv. Skin Wound Care 2020, 33, 554–556. [Google Scholar] [CrossRef]
- Jourabchi, N.; Fischer, A.H.; Cimino-Mathews, A.; Waters, K.M.; Okoye, G.A. Squamous cell carcinoma complicating a chronic lesion of hidradenitis suppurativa: A case report and review of the literature. Int. Wound J. 2017, 14, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Mufti, A.; Zaaroura, H.; Abduelmula, A.; Lansang, R.P.; Bagit, A.; Alhusayen, R. Squamous cell carcinoma arising within hidradenitis suppurativa: A literature review. Int. J. Dermatol. 2021, 60, e459–e465. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Sparsa, A.; Doffoel-Hantz, V.; Durox, H.; Gaston, J.; Delage-Core, M.; Bédane, C.; Labrousse, F.; Sannajust, J.-P.; Bonnetblanc, J.-M. Tumeur maligne historique: 27 cas [Historic malignant tumour: 27 observations]. Ann. Dermatol. Venereol. 2012, 139, 189–193. [Google Scholar] [CrossRef]
- Reule, R.B.; Golda, N.J.; Wheeland, R.G. Treatment of cutaneous squamous cell carcinoma with perineural invasion using Mohs micrographic surgery: Report of two cases and review of the literature. Dermatol. Surg. 2009, 35, 1559–1566. [Google Scholar] [CrossRef]
- Rosendahl, C.; Cameron, A.; Argenziano, G.; Zalaudek, I.; Tschandl, P.; Kittler, H. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch. Dermatol. 2012, 148, 1386–1392. [Google Scholar] [CrossRef]
- Lallas, A.; Pyne, J.; Kyrgidis, A.; Andreani, S.; Argenziano, G.; Cavaller, A.; Giacomel, J.; Longo, C.; Malvestiti, A.; Moscarella, E.; et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br. J. Dermatol. 2015, 172, 1308–1315. [Google Scholar] [CrossRef]
- Karia, P.S.; Morgan, F.C.; Califano, J.A.; Schmults, C.D. Comparison of Tumor Classifications for Cutaneous Squamous Cell Carcinoma of the Head and Neck in the 7th vs 8th Edition of the AJCC Cancer Staging Manual. JAMA Dermatol. 2018, 154, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Dong, Z.M.; Wu, P.C. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma: Clinical experience and review of literature. World J. Surg. Oncol. 2011, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur. J. Cancer 2020, 128, 83–102. [Google Scholar] [CrossRef]
| No. | Age (years) | Sex | Tumor Site | Maximum Clinical Diameter (mm) | Size of the Tumor (cm2) | Risk Factors | Time to Diagnosis (months) | Reason for a Prolonged Time to Diagnosis |
|---|---|---|---|---|---|---|---|---|
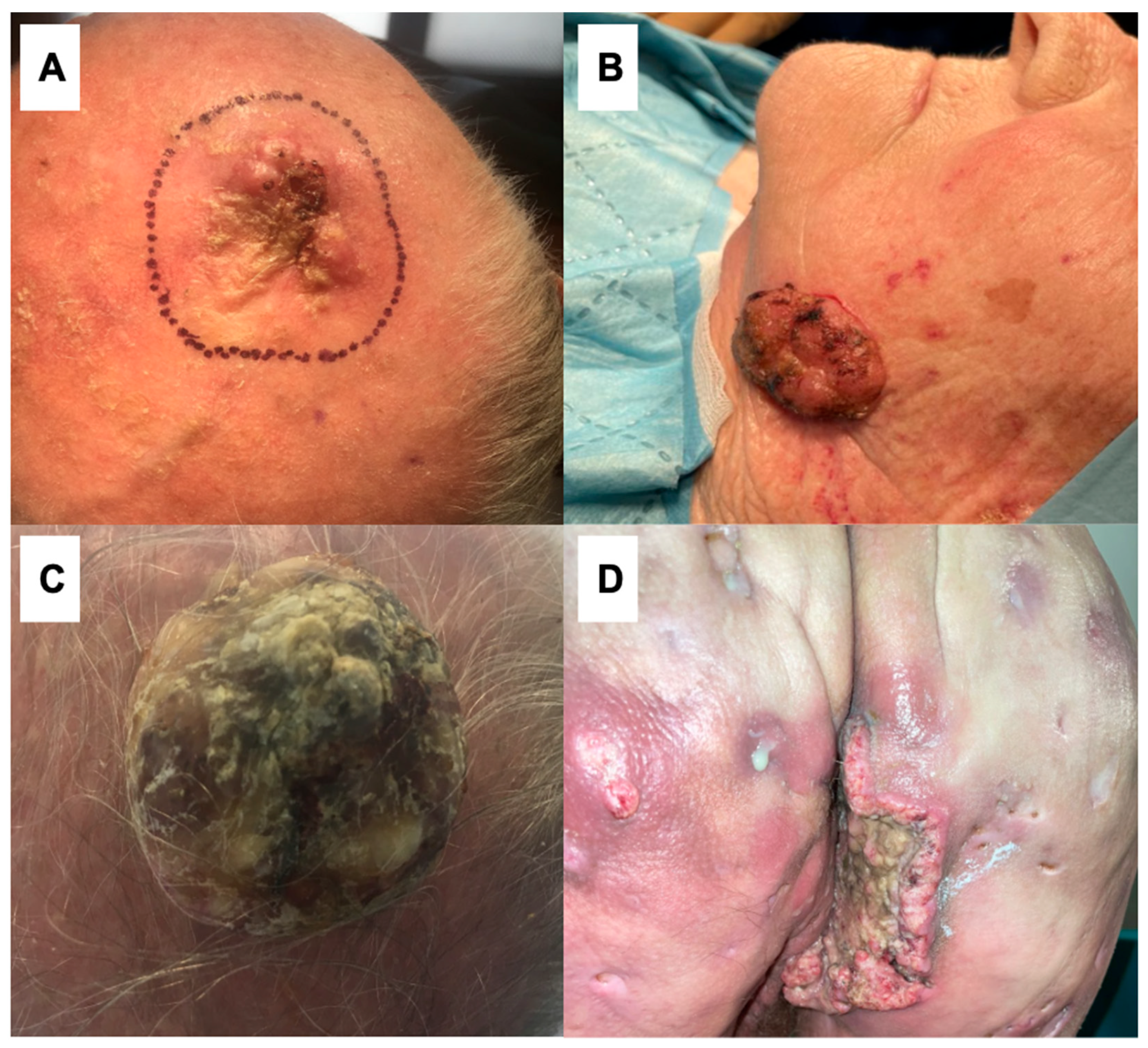
| 1 | 59 | M | Right buttock (Figure 1D) | 103 mm | 271.81 cm2 | Hidradenitis suppurativa, smoking | 18 months | Neglection; the similarity between cSCC and HS |
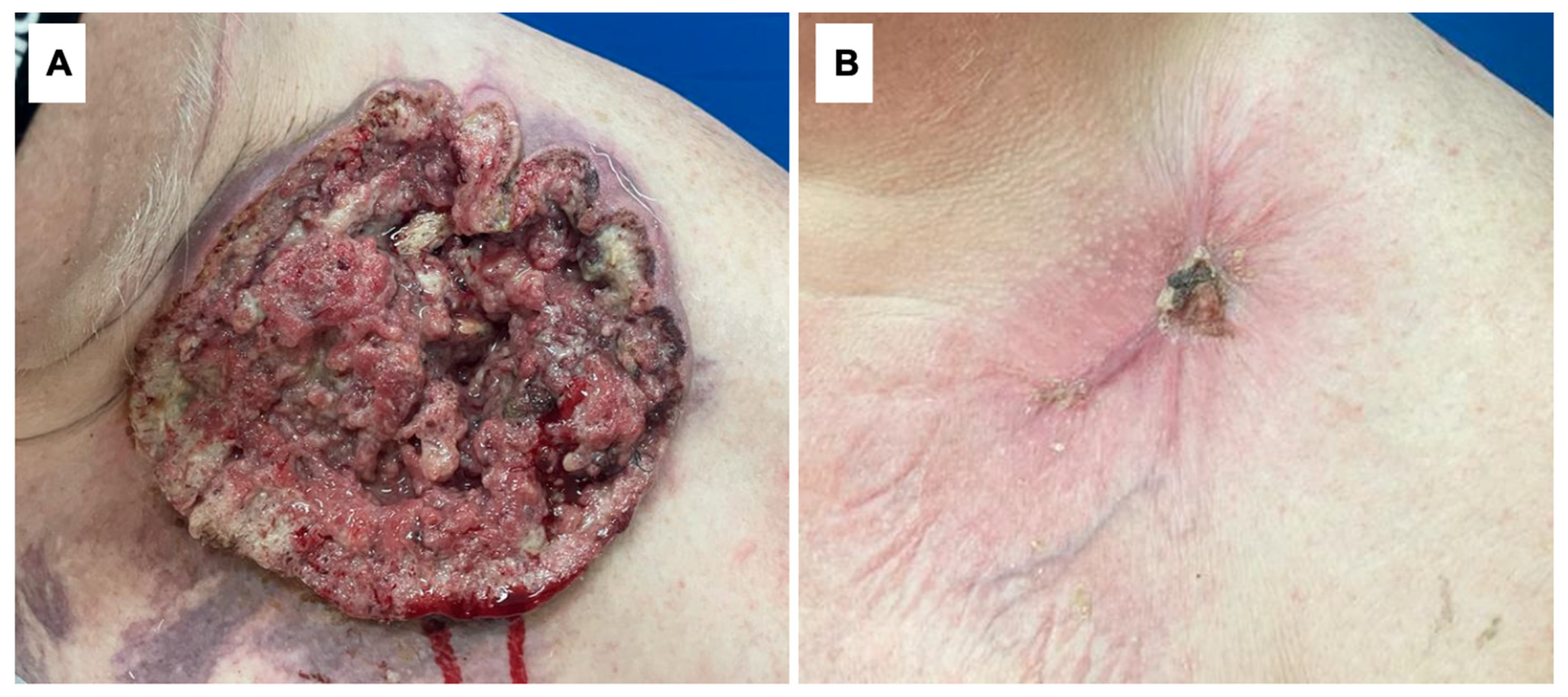
| 2 | 68 | M | Left supraclavicular area (Figure 2) | 181 mm | 562.94 cm2 | Smoking | 26 months | Neglection, fear of being diagnosed |
| 3 | 84 | M | Scalp (Figure 1C) | 115 mm | 390.19 cm2 | UV radiation | 12 months | Neglection |
| 4 | 86 | M | Scalp | 86 mm | 208.04 cm2 | UV radiation, smoking | 36 months | Neglection, fear of being diagnosed |
| 5 | 89 | M | Scalp (Figure 1A) | 81 mm | 160.32 cm2 | UV radiation | 43 months | Neglection, fear of being diagnosed |
| 6 | 83 | F | Left cheek (Figure 1B) | 62 mm | 114.92 cm2 | UV radiation | 59 months | Neglection, fear of being diagnosed |
| 7 | 93 | M | Left temple | 84 mm | 208.48 cm2 | UV radiation | 51 months | Neglection, fear of being diagnosed |
| No. | Histologic Subtype | Nodal Metastasis and/or in-Transit | Distant Metastasis | Treatment | Stage (TNM UICC/AJCC 8th Edition) | Outcome |
|---|---|---|---|---|---|---|
| 1 | Verrucous | Yes, nodal metastasis | No | Cemiplimab and RP1 injection | T3N1M0 | DOD |
| 2 | Verrucous | Yes, nodal metastasis | No | Surgery + lymph node dissection + cemiplimab and RP1 injection | T3N1M0 | AWD, during therapy |
| 3 | Verrucous | No | No | Surgery + 2xRT + cemiplimab and RP1 injection | T3N0M0 | AWD, during therapy |
| 4 | Verrucous | No | No | Surgery (graft), R0 | T3N0M0 | NED |
| 5 | Verrucous | Yes, nodal metastasis | No | Surgery (graft), R0 + lymph node dissection | T3N1M0 | NED |
| 6 | Verrucous | No | No | Surgery (primary closure), R0 | T3N0M0 | NED |
| 7 | Verrucous | No | No | Surgery (graft), R0 | T3N0M0 | NED |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateuszczyk, M.K.; Chlebicka, I.; Łyko, M.; Maj, J.; Szepietowski, J.C. Extra-Anogenital Giant Cutaneous Squamous Cell Carcinomas. Life 2024, 14, 421. https://doi.org/10.3390/life14030421
Mateuszczyk MK, Chlebicka I, Łyko M, Maj J, Szepietowski JC. Extra-Anogenital Giant Cutaneous Squamous Cell Carcinomas. Life. 2024; 14(3):421. https://doi.org/10.3390/life14030421
Chicago/Turabian StyleMateuszczyk, Mateusz K., Iwona Chlebicka, Magdalena Łyko, Joanna Maj, and Jacek C. Szepietowski. 2024. "Extra-Anogenital Giant Cutaneous Squamous Cell Carcinomas" Life 14, no. 3: 421. https://doi.org/10.3390/life14030421
APA StyleMateuszczyk, M. K., Chlebicka, I., Łyko, M., Maj, J., & Szepietowski, J. C. (2024). Extra-Anogenital Giant Cutaneous Squamous Cell Carcinomas. Life, 14(3), 421. https://doi.org/10.3390/life14030421









