Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential
Abstract
1. Introduction
- Hormone Receptor Status: Breast cancers can be categorized based on the presence or absence of hormone receptors: estrogen receptor (ER) and progesterone receptor (PR). Luminal A represents some subtypes of hormone receptor-positive (ER+ and/or PR+), HER2-negative (HER2-) breast cancer, and typically has low levels of the protein Ki-67, indicating slower cell proliferation. It often has a better prognosis and tends to respond well to hormone-based therapies. ZR-75-1, T-47D, MCF-7, and MDA-MB-415 all represent this subtype [39].
- Luminal B subtypes are also hormone receptor-positive (ER+ and/or PR+), but they might have higher levels of Ki-67, indicating faster cell growth. Some Luminal B tumors may also express HER2 (Luminal B/HER2+), influencing treatment decisions. MDA-MB-330 and ZR-75-30 are often the breast cancer cell lines of choice to represent the Luminal B subtype [39].
- HER2 Status: Human Epidermal Growth Factor Receptor 2 (HER2) is a protein that can promote the growth and division of cancer cells. Breast cancers can be classified as HER2-positive (HER2+) if they overexpress this protein, which can influence treatment decisions. MDA-MB-453, HCC1569, SUM190PT, AU565, and SK-BR-3 represent HER2-enriched breast cancer cell lines that do not have hormonal receptors for ER or PR [39].
- Basal-like/Triple-Negative Breast Cancer (TNBC): This subtype lacks the expression of ER, PR, and HER2 receptors characterized by its gene expression profile, resembling that of basal cells in the breast. A significant portion of TNBC tumors can be classified as basal-like and tend to be the most aggressive presenting fewer targeted treatment options. BT-549, BT-20, CAL148, MDA-MB-157, and MDA-MB-231 are all breast cancer cell lines that have been classified as TNBC [39].
- Claudin-low: These tumors are a subset of the basal-like subtype and are characterized by the low expression of tight junction proteins known as claudins. They have a poorer prognosis since the presence of claudin-low tumors has been associated with higher aggressiveness, exhibiting features associated with epithelial-to-mesenchymal transition (EMT). MDA-MB-157, BT-549, and MDA-MB-231 are used to study claudin-low breast cancer [40].
- Molecular Subtypes (e.g., PAM50), genomic instability and mutations: Molecular profiling using gene expression assays like PAM50 identifies distinct subtypes such as Luminal A, Luminal B, HER2-enriched, and basal-like. These subtypes have different gene expression patterns and respond differently to treatments. Moreover, some classifications consider genomic instability and specific genetic mutations like BRCA1 and BRCA2 mutations, which can predispose individuals to breast cancer since these human genes produce proteins responsible for suppressing tumors and play a crucial role in repairing damaged DNA. Certainly, all these genetic variations are specific to each cell line.
2. Lycium barbarum
3. Bioactive Composition of L. barbarum
3.1. Polyphenolic Compounds
3.2. Carotenoid Compounds
3.3. Polysaccharide Components
3.4. Melatonin
4. Antitumor Activity of Lycium barbarum
4.1. Main Antitumor Activity of Single Components: An Overview
4.1.1. Zeaxanthin
4.1.2. Polyphenol Fraction
4.1.3. Polysaccharide Fraction
4.2. Lycium barbarum and Breast Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| N. | Phenolic Compounds | Classification | Refs. |
|---|---|---|---|
| 1 | Catechin | Flavan-3-ols | [63,64] |
| 2 | Epicatechin | Flavan-3-ols | [63,64] |
| 3 | Naringenin | Flavonones | [63,65] |
| 4 | Hesperidin | Flavonones | [63,65] |
| 5 | Apigenin | Flavones | [63,66] |
| 6 | Luteolin | Flavones | [63,66] |
| 7 | Derrone | Isoflavones | [63,67] |
| 8 | Alpinumisoflavone | Isoflavones | [63,67] |
| 9 | Auriculasin | Isoflavones | [63,67] |
| 10 | Kaempferol | Flavonols | [63,68] |
| 11 | Quercitin | Flavonols | [63,68] |
| 12 | Myricetin | Flavonols | [63,68] |
| 13 | Rutin | Flavonols | [63,68] |
| 14 | Quercetin-rhamno-di-hexoside | Flavonols | [63,68] |
| 15 | 7-O-β-D-Glucopyranosyl-rutin | Flavonols | [63,67] |
| 16 | Hyperoside | Flavonols | [63,66] |
| 17 | Morin | Flavonols | [63,66,69,70] |
| 18 | Nicotiflorin | Flavonols | [63,66,69,70] |
| 19 | Narcissin | Flavonols | [63,66,69,70] |
| 20 | Isoquercitrina | Flavonols | [63,66] |
| 21 | Proanthocyanidin-A2 | Tannins | [63,65] |
| 22 | Proanthocyanidin-B2 | Tannins | [63,65] |
| 23 | Ellagic acid | Tannins | [63,65] |
| 24 | Gallic acid | Tannins | [63,65] |
| 25 | Scopoletin | Coumarins | [63,67,69] |
| 26 | Fabiatrin | Coumarins | [63,67,69] |
| 27 | Scopolin | Coumarins | [63,67,69] |
| 28 | Esculetin | Coumarins | [62,67] |
| 29 | Lycibarbarcoumarin A | Coumarins | [63,67,69] |
| 30 | Pinoresinol | Lignans | [63,67] |
| 31 | Medioresinol | Lignans | [63,67] |
| 32 | Syringaresinol | Lignans | [63,67] |
| 33 | 4-O-(β-d-glucopyranosyl)syringaresinol | Lignans | [62,67] |
| 34 | Acanthoside B | Lignans | [63,67] |
| 35 | Arctigenin | Lignans | [63,67] |
| 36 | Arctiin | Lignans | [63,67] |
| 37 | Neolignan threo-1,2-bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | Lignans | [63,67] |
| 38 | Neolignan erythro-1,2-bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | Lignans | [63,67] |
| 39 | (β)-Lyoniresinol 3-O-β-d-glucopyranoside | Lignans | [62,67] |
| 40 | Medioresinol | Lignans | [62,67] |
| 41 | Cannabisin D | Lignanamides | [63] |
| 42 | Cannabisin E | Lignanamides | [63] |
| 43 | Cannabisin F | Lignanamides | [63] |
| 44 | Threo-Canabisine H | Lignanamides | [63] |
| 45 | Erythro-Canabisine H | Lignanamides | [63] |
| 46 | Melongenamide D | Lignanamides | [63] |
| 47 | Grossamide | Lignanamides | [63] |
| 48 | Lyciumamide A | Lignanamides | [63,71] |
| 49 | Lyciumamide B | Lignanamides | [63,71] |
| 50 | Lyciumamide C | Lignanamides | [63,71] |
| 51 | p-Hydroxybenzoic acid | Hydroxybenzoic acids | [63,65,68,71,72] |
| 52 | Vanillic acid | Hydroxybenzoic acids | [63,65,68] |
| 53 | 2,4-Dihydroxybenzoic | Hydroxybenzoic acids | [63,65,68] |
| 54 | Veratronic acid | Hydroxybenzoic acids | [63,65,68] |
| 55 | Benzoic acid | Hydroxybenzoic acids | [63,65,68] |
| 56 | Salicylic acid | Hydroxybenzoic acids | [63,65,68] |
| 57 | Syringic acid | Hydroxybenzoic acids | [63,65,68] |
| 58 | Chlorogenic acid | Hydroxycinnamic acids | [63,68,69,73,74] |
| 59 | Caffeic acid | Hydroxycinnamic acids | [63,68,69,73,74] |
| 60 | Coumaric acid | Hydroxycinnamic acids | [63,68,69,73,74] |
| 61 | Ferulic Acid | Hydroxycinnamic acids | [63,68,69,73,74] |
| 62 | Dicaffeoylquinic acid | Hydroxycinnamic acids | [63,74] |
| 63 | Methylchlorogenate | Hydroxycinnamic acid derivatives | [63,67,75] |
| 64 | Lycibarbarphenylpropanoid A | Phenylpropanoids | [63,67,75] |
| 65 | Lycibarbarphenylpropanoid B | Phenylpropanoids | [62,67] |
| 66 | Lycibarbarphenylpropanoid C | Phenylpropanoids | [62,67] |
| 67 | Lycibarbarphenylpropanoid D | Phenylpropanoids | [62,67] |
| 68 | Lycibarbarphenylpropanoid E | Phenylpropanoids | [62,67] |
| 69 | Lycibarbarphenylpropanoid F | Phenylpropanoids | [62,67] |
| 70 | Lycibarbarphenylpropanoid G | Phenylpropanoids | [62,67] |
| 71 | Lycibarbarphenylpropanoid H | Phenylpropanoids | [62,67] |
| 72 | Lycibarbarphenylpropanoid I | Phenylpropanoids | [62,67] |
| 73 | Lycibarbarphenylpropanoid M | Phenylpropanoids | [63,67,75] |
| 74 | Syringenin | Phenylpropanoids | [62,67] |
| 75 | Isoscopoletin | Phenylpropanoids | [62,67] |
| 76 | Ethyl-4-O-β-d-glucopyranosyl-E-ferulate | Phenylpropanoids | [62,67] |
| 77 | Phloretic acid | Phenolic acids | [63,67] |
| 78 | Dihydroferulic acid | Phenolic acids | [63,67] |
| 79 | Ethyl Dihydroferulate | Phenolic acid derivates | [63,67] |
| 80 | Arbutin | Phenolic acid derivates | [63,74] |
| 81 | p-Hydroxybenzaldehyde | Phenolic acid derivates | [63,67] |
| 82 | Protocatechuic aldehyde | Phenolic acid derivates | [63,73] |
| 83 | N-Trans-feruloyl tyramine | Phenolic acid amides | [63,73] |
| 84 | N-Trans-feruloyl 3-methoxyturamine | Phenolic acid amides | [63,73] |
| 85 | Lyciumide A | Phenolic acid amides | [63,71,76] |
| 86 | N-Trans-p-coumaroyl tyramine | Phenolic acid amides | [63,71,76] |
| 87 | N-Cis-p-coumaroyl tyramine | Phenolic acid amides | [63,71,76] |
| 88 | N-feruloyl agmatine | Phenolic acid amides | [63,71,76] |
References
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Einstein, G.P.; Tulp, O.L.; Sainvil, F.; Branly, R. Introduction to Traditional Medicine and Their Role in Prevention and Treatment of Emerging and Re-Emerging Diseases. Biomolecules 2022, 12, 1442. [Google Scholar] [CrossRef]
- Alves, R.R.; Rosa, I.M. Biodiversity, traditional medicine and public health: Where do they meet? J. Ethnobiol. Ethnomed. 2007, 3, 14. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Marino, P.; Pepe, G.; Basilicata, M.G.; Vestuto, V.; Marzocco, S.; Autore, G.; Procino, A.; Gomez-Monterrey, I.M.; Manfra, M.; Campiglia, P. Potential Role of Natural Antioxidant Products in Oncological Diseases. Antioxidants 2023, 12, 704. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I. Functional Foods as a New Therapeutic Strategy. Nutraceuticals 2023, 3, 231–233. [Google Scholar] [CrossRef]
- Novi, S.; Vestuto, V.; Campiglia, P.; Tecce, N.; Bertamino, A.; Tecce, M.F. Anti-Angiogenic Effects of Natural Compounds in Diet-Associated Hepatic Inflammation. Nutrients 2023, 15, 2748. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Basilicata, M.G.; Carrizzo, A.; Adesso, S.; Ostacolo, C.; Sala, M.; Sommella, E.; Ruocco, M.; Cascioferro, S.; Ambrosio, M.; et al. β-Lactoglobulin Heptapeptide Reduces Oxidative Stress in Intestinal Epithelial Cells and Angiotensin II-Induced Vasoconstriction on Mouse Mesenteric Arteries by Induction of Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Translocation. Oxid. Med. Cell. Longev. 2019, 1616239. [Google Scholar] [CrossRef]
- Sato, H.; Goto, W.; Yamamura, J.; Kurokawa, M.; Kageyama, S.; Takahara, T.; Watanabe, A.; Shiraki, K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antivir. Res. 1996, 30, 171–177. [Google Scholar] [CrossRef]
- Negi, A.S.; Kumar, J.K.; Luqman, S.; Shanker, K.; Gupta, M.M.; Khanuja, S.P. Recent advances in plant hepatoprotectives: A chemical and biological profile of some important leads. Med. Res. Rev. 2008, 28, 746–772. [Google Scholar] [CrossRef]
- Chung, M.-K.; Campbell, J.N. Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals 2016, 9, 66. [Google Scholar] [CrossRef]
- Tomaras, S.; Keyßer, G.; Feist, E. Curcumin: Useful add-on for Rheumatic Diseases? J. Clin. Med. 2022, 11, 2908. [Google Scholar] [CrossRef]
- Vestuto, V.; Amodio, G.; Pepe, G.; Basilicata, M.G.; Belvedere, R.; Napolitano, E.; Guarnieri, D.; Pagliara, V.; Paladino, S.; Rodriquez, M.; et al. Cocoa Extract Provides Protection against 6-OHDA Toxicity in SH-SY5Y Dopaminergic Neurons by Targeting PERK. Biomedicines 2022, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front. Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef]
- Paesa, M.; Nogueira, D.P.; Velderrain-Rodríguez, G.; Esparza, I.; Jiménez-Moreno, N.; Mendoza, G.; Osada, J.; Martin-Belloso, O.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Valorization of Onion Waste by Obtaining Extracts Rich in Phenolic Compounds and Feasibility of Its Therapeutic Use on Colon Cancer. Antioxidants 2022, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Meyers, K.J.; van der Heide, J.; Liu, R.H. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Aquino, G.; Basilicata, M.G.; Crescenzi, C.; Vestuto, V.; Salviati, E.; Cerrato, M.; Ciaglia, T.; Sansone, F.; Pepe, G.; Campiglia, P. Optimization of microwave-assisted extraction of antioxidant compounds from spring onion leaves using Box-Behnken design. Sci. Rep. 2023, 13, 14923. [Google Scholar] [CrossRef] [PubMed]
- Moremane, M.M.; Abrahams, B.; Tiloke, C. Moringa oleifera: A Review on the Antiproliferative Potential in Breast Cancer Cells. Curr. Issues Mol. Biol. 2023, 45, 6880–6902. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Basilicata, M.G.; Pepe, G.; De Anseris, R.; Di Mauro, A.; Scognamiglio, G.; Palma, G.; Vestuto, V.; Buccolo, S.; Luciano, A.; et al. Combination of Spirulina platensis, Ganoderma lucidum and Moringa oleifera Improves Cardiac Functions and Reduces Pro-Inflammatory Biomarkers in Preclinical Models of Short-Term Doxorubicin-Mediated Cardiotoxicity: New Frontiers in Cardioncology? J. Cardiovasc. Dev. Dis. 2022, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Di Sarno, V.; Giovannelli, P.; Medina-Peris, A.; Ciaglia, T.; Di Donato, M.; Musella, S.; Lauro, G.; Vestuto, V.; Smaldone, G.; Di Matteo, F.; et al. New TRPM8 blockers exert anticancer activity over castration-resistant prostate cancer models. Eur. J. Med. Chem. 2022, 238, 114435. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, T.; Vestuto, V.; Bertamino, A.; González-Muñiz, R.; Gómez-Monterrey, I. On the modulation of TRPM channels: Current perspectives and anticancer therapeutic implications. Front. Oncol. 2023, 12, 1065935. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Raskin, I.; Ripoll, C. Can an apple a day keep the doctor away? Curr. Pharm. Des. 2004, 10, 3419–3429. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Tang, W.M.; Chan, E.; Kwok, C.Y.; Lee, Y.K.; Wu, J.H.; Wan, C.W.; Chan, R.Y.; Yu, P.H.; Chan, S.W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef]
- Pehlİvan KarakaŞ, F.; CoŞkun, H.; SoytÜrk, H.; Bozat, B.G. Anxiolytic, antioxidant, and neuroprotective effects of goji berry polysaccharides in ovariectomized rats: Experimental evidence from behavioral, biochemical, and immunohistochemical analyses. Turk. J. Biol. 2020, 44, 238–251. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Yu, M.S.; Leung, S.K.; Lai, S.W.; Che, C.M.; Zee, S.Y.; So, K.F.; Yuen, W.H.; Chang, R.C. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against beta-amyloid peptide neurotoxicity. Exp. Gerontol. 2005, 40, 716–727. [Google Scholar] [CrossRef]
- Qiang, X.; Xia, T.; Geng, B.; Zhao, M.; Li, X.; Zheng, Y.; Wang, M. Bioactive Components of Lycium barbarum and Deep-Processing Fermentation Products. Molecules 2023, 28, 8044. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Halasa, M.; Okla, K. Chapter 35—Lycium barbarum (goji berry), human breast cancer, and antioxidant profile. In Cancer, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 399–406. [Google Scholar] [CrossRef]
- Witt, B.L.; Tollefsbol, T.O. Molecular, Cellular, and Technical Aspects of Breast Cancer Cell Lines as a Foundational Tool in Cancer Research. Life 2023, 13, 2311. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Xu, A.; Ma, X.; Yao, Y.; Zhao, Y.; Wang, C.; Chen, C. Research progress of Claudin-low breast cancer. Front. Oncol. 2023, 11, 1226118. [Google Scholar] [CrossRef] [PubMed]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Brenton, J.D.; Carey, L.A.; Ahmed, A.A.; Caldas, C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J. Clin. Oncol. 2005, 23, 7350–7360. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheon, S.; Jung, M.K.; Song, S.B.; Kim, D.; Kim, H.J.; Park, H.; Bang, S.I.; Cho, D. Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2015, 459, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.-A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Y.; Wu, M.; Liu, X.; Shen, X.; Liu, C.; Wang, Y. Identification and validation of reference genes for quantitative real-time PCR normalization and its applications in lyceum. PLoS ONE 2014, 9, e97039. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, J.; Chen, X.; Xu, Z.; Wang, Y.; Sun, W.; Song, J.; Yao, H. Complete chloroplast genome and comparative analysis of three Lycium (Solanaceae) species with medicinal and edible properties. Gene Rep. 2019, 17, 100464. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Nea, F.; Makemteu, J.; Ngandeu Neubi, G.M.; Mabou, F.D.; Noumedem Kenfack, J.A.; Djeussi, D.E.; Sun, G. Comparative study of polyphenols quantification, total phenolic content, and antioxidant activities of the fruits of three plants of the family of Solanaceae: Lycium ruthenicum, Lycium barbarum, and Lycium chinense. Investig. Med. Chem. Pharmacol. 2022, 5, 2. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Amodio, M.L.; De Chiara, M.L.V.; Mastrandrea, L.; Colelli, G. Characterization and postharvest behavior of goji berry (Lycium barbarum L.) during ripening. Postharvest Biol. Technol. 2022, 19, 111975. [Google Scholar] [CrossRef]
- Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar, D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018, 115, 414–424. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Ortega-Barrales, P.; Ruiz-Medina, A. Characterization and comparison of the chemical composition of exotic superfoods. Microchem. J. 2013, 110, 444–451. [Google Scholar] [CrossRef]
- Li, X.M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007, 40, 461–465. [Google Scholar] [CrossRef]
- Lu, S.-P.; Zhao, P.-T. Chemical characterization of Lycium barbarum polysaccharides and their reducing myocardial injury in ischemia/reperfusion of rat heart. Int. J. Biol. Macromol. 2010, 47, 681–684. [Google Scholar] [CrossRef]
- Nardi, G.M.; Farias Januario, A.G.; Freire, C.G.; Megiolaro, F.; Schneider, K.; Perazzoli, M.R.; Do Nascimento, S.R.; Gon, A.C.; Mariano, L.N.; Wagner, G.; et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacogn. Res. 2016, 8, S42–S49. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical Characterization, Antioxidant and Antimicrobial Properties of Goji Berries Cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef]
- Zhao, W.H.; Shi, Y.P. Comprehensive analysis of phenolic compounds in four varieties of goji berries at different ripening stages by UPLC–MS/MS. J. Food Compost. Anal. 2022, 106, 104279. [Google Scholar] [CrossRef]
- Ma, R.H.; Zhang, X.X.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Research progress of Lycium barbarum L. as functional food: Phytochemical composition and health benefits. Curr. Opin. Food Sci. 2022, 47, 100871. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar] [CrossRef]
- Souto, E.B.; Sampaio, A.C.; Campos, J.R.; Martins-Gomes, C.; Aires, A.; Silva, A.M. Polyphenols for skin cancer: Chemical properties, structure-related mechanisms of action and new delivery systems. Stud. Nat. Prod. Chem. 2019, 64, 21–42. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic Review of Chemical Constituents in the Genus Lycium (Solanaceae). Molecules 2017, 22, 911. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods 2021, 77, 104340. [Google Scholar] [CrossRef]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crișan, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) Fruit European Cultivars at Different Maturation Steps: Extraction, HPLC-DAD Analyses, and Biological Evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, S.; Zhou, W.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Rapid qualitative and quantitative analyses of eighteen phenolic compounds from Lycium ruthenicum Murray by UPLC-Q-Orbitrap MS and their antioxidant activity. Food Chem. 2018, 269, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.R.; Feng, X.L.; Kurihara, H.; So, K.F.; Yao, X.S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Jarouche, M.; Suresh, H.; Hennell, J.; Sullivan, S.; Lee, S.; Singh, S.; Power, D.; Xu, C.; Khoo, C. The Quality Assessment of Commercial Lycium Berries Using LC-ESI-MS/MS and Chemometrics. Plants 2019, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Ibáñez, E.; Cifuentes, A.; Gilbert-López, B.; Fanali, C. Optimization of pressurized liquid extraction by response surface methodology of Goji berry (Lycium barbarum L.) phenolic bioactive compounds. Electrophoresis 2018, 39, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Ma, D.; Cheng, Y.; Tian, X.; Lu, Y.; Du, X.; Tang, H.; Chen, J. Three New Dimers and Two Monomers of Phenolic Amides from the Fruits of Lycium barbarum and Their Antioxidant Activities. J. Agric. Food Chem. 2015, 63, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Guo, S.; Yan, H.; Lu, Y.Y.; Zhang, F.; Qian, D.W.; Wang, H.Q.; Duan, J.A. Analysis of phenolic acids and flavonoids in leaves of Lycium barbarum from different habitats by ultra-high-performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4552. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.; Kao, T.H.; Chen, B.H. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC-DAD-ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef]
- Li, Q.W.; Zhang, R.; Zhou, Z.Q.; Sun, W.Y.; Fan, H.X.; Wang, Y.; Xiao, J.; So, K.F.; Yao, X.S.; Gao, H. Phenylpropanoid glycosides from the fruit of Lycium barbarum L. and their bioactivity. Phytochemistry 2019, 164, 60–66. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Xia, Y.; Qiao, Z.; Feng, F.; Bian, J.; Liu, W.; Qu, W. Comparison of the origin and phenolic contents of Lycium ruthenicum Murr. by high-performance liquid chromatography fingerprinting combined with quadrupole time-of-flight mass spectrometry and chemometrics. J. Sep. Sci. 2017, 40, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Pontieri, P.; Pepe, G.; Campiglia, P.; Merciai, F.; Basilicata, M.G.; Smolensky, D.; Calcagnile, M.; Troisi, J.; Romano, R.; Del Giudice, F.; et al. Comparison of Content in Phenolic Compounds and Antioxidant Capacity in Grains of White, Red, and Black Sorghum Varieties Grown in the Mediterranean Area. ACS Food Sci. 2021, 1, 1109–1119. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Lycium barbarum Berries (Solanaceae) as Source of Bioactive Compounds for Healthy Purposes: A Review. Int. J. Mol. Sci. 2023, 24, 4777. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, V.; Silva, A.R.; Silva, B.; Zhang, X.; Dias, A.C.P. Comparative studies on the anti-neuroinflammatory and antioxidant activities of black and red goji berries. J. Funct. Foods. 2022, 92, 105038. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Z.J.; Song, C.B.; Ma, W.P.; Cao, S.Q.; Wei, Z.J. Hydrogen sulfide treatment improves quality attributes via regulating the antioxidant system in goji berry (Lycium barbarum L.). Food Chem. 2023, 405, 134858. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Chen, P.Y.; Shih, T.H.; Chang, K.C.; Wang, J.S.; Yang, C.M.; Chang, Y.S. Potential of galled leaves of Goji (Lycium chinense) as functional food. BMC Nutr. 2020, 6, 26. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Vestuto, V.; Di Sarno, V.; Musella, S.; Di Dona, G.; Moltedo, O.; Gomez-Monterrey, I.M.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Ciaglia, T. New Frontiers on ER Stress Modulation: Are TRP Channels the Leading Actors? Int. J. Mol. Sci. 2022, 24, 185. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; An, M.-Y.; Hwang, H.-J.; Yoon, J.-G.; Cho, J.A. Antioxidant Effect of Lycium barbarum Leaf through Inflammatory and Endoplasmic Reticulum Stress Mechanism. Antioxidants 2021, 10, 20. [Google Scholar] [CrossRef]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Lin, S.C.; Chu, C.H.; Chang, Y.K.; Zhang, X.; Lin, C.C.; Tung, Y.T. The Gastroprotective Effect of Naringenin against Ethanol-Induced Gastric Ulcers in Mice through Inhibiting Oxidative and Inflammatory Responses. Int. J. Mol. Sci. 2021, 22, 11985. [Google Scholar] [CrossRef] [PubMed]
- Frangie, C.; Daher, J. Role of myeloperoxidase in inflammation and atherosclerosis (Review). Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Laparra-Llopis, J.M.; Schneider, C.; Espín, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7937. [Google Scholar] [CrossRef]
- Cerqua, I.; Musella, S.; Peltner, L.K.; D’Avino, D.; Di Sarno, V.; Granato, E.; Vestuto, V.; Di Matteo, R.; Pace, S.; Ciaglia, T.; et al. Discovery and Optimization of Indoline-Based Compounds as Dual 5-LOX/sEH Inhibitors: In Vitro and In Vivo Anti-Inflammatory Characterization. J. Med. Chem. 2022, 65, 14456–14480. [Google Scholar] [CrossRef]
- Musella, S.; D’Avino, D.; Peltner, L.K.; Di Sarno, V.; Cerqua, I.; Merciai, F.; Vestuto, V.; Ciaglia, T.; Smaldone, G.; Di Matteo, F.; et al. Design, Synthesis, and Pharmacological Characterization of a Potent Soluble Epoxide Hydrolase Inhibitor for the Treatment of Acute Pancreatitis. J. Med. Chem. 2023, 66, 9201–9222. [Google Scholar] [CrossRef]
- Colarusso, E.; Potenza, M.; Lauro, G.; Chini, M.G.; Sepe, V.; Zampella, A.; Fischer, K.; Hofstetter, R.K.; Werz, O.; Bifulco, G. Thiazolidin-4-one-based compounds interfere with the eicosanoid biosynthesis pathways by mPGES-1/sEH/5-LO multi-target inhibition. Eur. J. Med. Chem. Rep. 2022, 5, 100046. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hilz, Y.Y.; Boerboom, A.M.; Westphal, A.H.; Berkel, W.J.; Aarts, J.M.; Rietjens, I.M. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 2006, 19, 1499–14505. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.; Breithaupt, D.E. Identification and quantification of zeaxanthin esters in plants using liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2003, 51, 7044–7049. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, C.; Li, Y.; Leung, K.S.Y.; Jiang, Z.H.; Zhao, Z. Quantification of zeaxanthin dipalmitate and total carotenoids in Lycium fruits (Fructus lycii). Plant Foods Hum. Nutr. 2005, 60, 161–164. [Google Scholar] [CrossRef]
- Molnar, P.; Pfander, H.; Olah, P.; Deli, J.; Toth, G. Carotenoid composition of Lycium barbarum L. seeds of Chinese and Hungarian origin. Olaj Szappan Kozmet. 2003, 522, 50–55. [Google Scholar]
- Long, J.T.; Fan, H.X.; Zhou, Z.Q.; Sun, W.Y.; Li, Q.W.; Wang, Y.; Ma, M.; Gao, H.; Zhi, H. The major zeaxanthin dipalmitate derivatives from wolfberry. J. Asian Nat. Prod. Res. 2020, 22, 746–753. [Google Scholar] [CrossRef]
- Bahaji Azami, N.L.; Sun, M. Zeaxanthin Dipalmitate in the Treatment of Liver Disease. Evid. Based Complement. Altern. Med. 2019, 2019, 1475163. [Google Scholar] [CrossRef]
- Seo, Y.Y.; Cho, Y.K.; Bae, J.C. Tumor necrosis factor-α as a predictor for the development of nonalcoholic fatty liver disease: a 4-year follow-up study. Endocrinol. Metab. 2013, 28, 41–45. [Google Scholar] [CrossRef]
- Li, J.J.; Gao, H.; Lv, Y. Zeaxanthin dipalmitate alleviates hepatic injury induced by superimposed chronic hepatitis B and non-alcoholic steatohepatitis in non-obese mice. J. Asian Nat. Prod. Res. 2017, 19, 910–923. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, X.; Luo, L.; Zhang, Q.; Huang, X.; Liu, Y.; Wang, K.; Zhang, Y. Advances in oral absorption of polysaccharides: Mechanism, affecting factors, and improvement strategies. Carbohydr. Polym. 2022, 282, 119110. [Google Scholar] [CrossRef]
- Kwok, S.S.; Bu, Y.; Lo, A.C.; Chan, T.C.; So, K.F.; Lai, J.S.; Shih, K.C. A Systematic Review of Potential Therapeutic Use of Lycium Barbarum Polysaccharides in Disease. BioMed Res. Int. 2019, 2019, 4615745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, Q.; Qiu, P.; Di, D. Polysaccharides isolated from Lycium barbarum L. by integrated tandem hybrid membrane technology exert antioxidant activities in mitochondria. Ind. Crops Prod. 2021, 168, 113547. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ni, Z.J.; Zhang, F.; Thakur, K.; Zhang, J.G.; Khan, M.R.; Busquets, R.; Wei, Z.J. Physicochemical and antioxidant properties of Lycium barbarum seed dreg polysaccharides prepared by continuous extraction. Food Chem. X 2022, 14, 100282. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, X.; Luo, S.; Zheng, Y.; Luo, X.; Zhou, L. Antitumor activity of Lycium barbarum polysaccharides with different molecular weights: An in vitro and in vivo study. Food Nutr. Res. 2017, 61, 1399770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, X.; Wang, F. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Dang, T.; Deng, Y.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.; Wang, Z. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Cheng, J.; Liu, G.; Qi, C.; Zhou, W.; Zhang, Y. Immune activities comparison of polysaccharide and polysaccharide-protein complex from Lycium barbarum L. Int. J. Biol. Macromol. 2014, 65, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.; Qi, D.; Wang, D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress, and inflammation in pulmonary endothelial cells. Free Radic. Res. 2018, 52, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Du, J.; Hei, Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017, 14, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Uğur, Y. Investigation of melatonin content and antioxidant capacity in grape berries. İnönü Üniversitesi Sağlık Hizmetleri Mesl. Yüksek Okulu Derg. 2021, 9, 820–830. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef]
- Sheng, Y.N.; Luo, Y.H.; Liu, S.B.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Wang, C.Y.; et al. Zeaxanthin Induces Apoptosis via ROS-Regulated MAPK and AKT Signaling Pathway in Human Gastric Cancer Cells. Onco Targets Ther. 2020, 13, 10995–11006. [Google Scholar] [CrossRef]
- Yang, H.; Yamazaki, T.; Pietrocola, F.; Zhou, H.; Zitvogel, L.; Ma, Y.; Kroemer, G. STAT3 Inhibition Enhances the Therapeutic Efficacy of Immunogenic Chemotherapy by Stimulating Type 1 Interferon Production by Cancer Cells. Cancer Res. 2015, 75, 3812–3822. [Google Scholar] [CrossRef]
- Yan, B.; Lu, M.S.; Wang, L.; Mo, X.F.; Luo, W.P.; Du, Y.F.; Zhang, C.X. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: A case-control study. Br. J. Nutr. 2016, 115, 129–137. [Google Scholar] [CrossRef]
- Cenariu, D.; Fischer-Fodor, E.; Țigu, A.B.; Bunea, A.; Virág, P.; Perde-Schrepler, M.; Toma, V.-A.; Mocan, A.; Berindan-Neagoe, I.; Pintea, A.; et al. Zeaxanthin-Rich Extract from Superfood Lycium barbarum Selectively Modulates the Cellular Adhesion and MAPK Signaling in Melanoma versus Normal Skin Cells In Vitro. Molecules 2021, 26, 333. [Google Scholar] [CrossRef]
- Buonocore, M.; Grimaldi, M.; Santoro, A.; Covelli, V.; Marino, C.; Napolitano, E.; Novi, S.; Tecce, M.F.; Ciaglia, E.; Montella, F.; et al. Exploiting the Features of Short Peptides to Recognize Specific Cell Surface Markers. Int. J. Mol. Sci. 2023, 24, 15610. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, B.; Zhang, H.; Li, H. Human epithelial ovarian cancer cells expressing CD105, CD44 and CD106 surface markers exhibit increased invasive capacity and drug resistance. Oncol. Lett. 2019, 17, 5351–5360. [Google Scholar] [CrossRef]
- Kwaśnik, P.; Lemieszek, M.K.; Rzeski, W. Impact of phytochemicals and plant extracts on viability and proliferation of NK cell line NK-92—A closer look at immunomodulatory properties of goji berries extract in human colon cancer cells. Ann. Agric. Environ. Med. 2021, 28, 291–299. [Google Scholar] [CrossRef]
- Gong, G.; Liu, Q.; Deng, Y.; Dang, T.; Dai, W.; Liu, T.; Liu, Y.; Sun, J.; Wang, L.; Liu, Y.; et al. Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2020, 149, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, H.; Huang, J.; Li, Z.; Zhu, C.; Zhang, S. Effect of Lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, Z.; Yan, J.; Zhu, F.; Xu, R.J.; Cai, Y.Z. Lycium barbarum polysaccharides induce apoptosis in human prostate cancer cells and inhibits prostate cancer growth in a xenograft mouse model of human prostate cancer. J. Med. Food 2009, 12, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Yu, H.Y.; Cai, Y.J.; Ke, M. Lycium barbarum polysaccharides inhibit proliferation and migration of bladder cancer cell lines BIU87 by suppressing Pi3K/AKT pathway. Oncotarget 2017, 8, 5936–5942. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yuan, Y.; Zheng, Y.; Sheng, R.; Liu, L.; Xie, F.; Tan, J. Anti-cerebral ischemia reperfusion injury of polysaccharides: A review of the mechanisms. Biomed. Pharmacother. 2021, 137, 111303. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Duan, G.; Fan, G.; Peng, N. Effect of Lycium barbarum polysaccharides on cell signal transduction pathways. Biomed. Pharmacother. 2022, 147, 112620. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Uddin, M.J.; Hossen, M.J.; Rahman, M.A.; Mohibbullah, M.; Hannan, M.A.; Choi, J.-S. Dendritic Cells (DCs)-Based Cancer Immunotherapy: A Review on the Prospects of Medicinal Plants and Their Phytochemicals as Potential Pharmacological Modulators. Appl. Sci. 2022, 12, 9452. [Google Scholar] [CrossRef]
- Georgiev, K.D.; Slavov, I.J.; Iliev, I.A. Antioxidant Activity and Antiproliferative Effects of Lycium barbarum’s (Goji berry) Fractions on Breast Cancer Cell Lines. Folia Med. 2019, 61, 104–112. [Google Scholar] [CrossRef]
- Wawruszak, A.; Czerwonka, A.; Okla, K.; Rzeski, W. Anticancer effect of ethanol Lycium barbarum (Goji berry) extract on human breast cancer T47D cell line. Nat. Prod. Res. 2016, 30, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Cumaoglu, A.; Bekci, H.; Ozturk, E.; Yerer, M.B.; Baldemir, A.; Bishayee, A. Goji Berry Fruit Extracts Suppress Proliferation of Triple-Negative Breast Cancer Cells by Inhibiting EGFR-Mediated ERK/MAPK and PI3K/Akt Signaling Pathways. Nat. Prod. Commun. 2018, 13, 1934578X1801300613. [Google Scholar] [CrossRef]
- Shen, L.; Du, G. Lycium barbarum polysaccharide stimulates proliferation of MCF-7 cells by the ERK pathway. Life Sci. 2012, 91, 353–357. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef]
- Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Telang, N.T.; Wong, G.Y. Lycium barbarum inhibits growth of estrogen receptor positive human breast cancer cells by favorably altering estradiol metabolism. Nutr. Cancer 2009, 61, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Bartucci, M.; Morelli, C.; Mauro, L.; Andò, S.; Surmacz, E. Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. 2001, 61, 6747–6754. [Google Scholar]
- Huang, X.; Zhang, Q.Y.; Jiang, Q.Y.; Kang, X.M.; Zhao, L. Polysaccharides derived from Lycium barbarum suppress IGF-1-induced angiogenesis via PI3K/HIF-1α/VEGF signalling pathways in MCF-7 cells. Food Chem. 2012, 13, 1479–1484. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Lei, G.; Mao, C.; Yan, Y.; Zhuang, L.; Gan, B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell. 2021, 12, 836–857. [Google Scholar] [CrossRef]
- DU, X.; Zhang, J.; Liu, L.; Xu, B.; Han, H.; Dai, W.; Pei, X.; Fu, X.; Hou, S. A novel anticancer property of Lycium barbarum polysaccharide in triggering ferroptosis of breast cancer cells. J. Zhejiang Univ. Sci. B 2022, 23, 286–299. [Google Scholar] [CrossRef]
- Miao, Y.; Xiao, B.X.; Jiang, Z.; Guo, Y.; Mao, F.; Zhao, J.; Huang, X.; Guo, J. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med. Oncol. 2010, 27, 785–790. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Xin, Y.F.; Zhou, G.L.; Deng, Z.Y.; Chen, Y.X.; Wu, Y.G.; Xu, P.S. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother. Res. 2007, 21, 1020–1024. [Google Scholar] [CrossRef]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y. Fraction from Lycium barbarum Polysaccharides Reduces Immunotoxicity and Enhances Antitumor Activity of Doxorubicin in Mice. Integr. Cancer Ther. 2018, 17, 860–866. [Google Scholar] [CrossRef]
- Green, P.S.; Leeuwenburgh, C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim. Biophys. Acta 2002, 1588, 94–101. [Google Scholar] [CrossRef]
- Childs, A.C.; Phaneuf, S.L.; Dirks, A.J.; Phillips, T.; Leeuwenburgh, C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002, 62, 4592–4598. [Google Scholar] [PubMed]
- Hou, Y.M.; Wang, J.; Zhang, X.Z. Lycium barbarum polysaccharide exhibits cardioprotection in an experimental model of ischemia-reperfusion damage. Mol. Med. Rep. 2017, 15, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, K.D.; Slavov, I.J.; Iliev, I.A. Synergistic Growth Inhibitory Effects of Lycium barbarum (Goji berry) Extract with Doxorubicin against Human Breast Cancer Cells. J. Pharm. Pharmacol. Res. 2019, 3, 051–058. [Google Scholar] [CrossRef]
- Chao, J.C.; Chiang, S.W.; Wang, C.C.; Tsai, Y.H.; Wu, M.S. Hot water-extracted Lycium barbarum and Rehmannia glutinosa inhibit proliferation and induce apoptosis of hepatocellular carcinoma cells. World J. Gastroenterol. 2006, 12, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int. Immunopharmacol. 2014, 4, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Hvarchanova, N.; Stoeva, S.; Radeva-Ilieva, M.; Zhelev, I.; Georgieva, M.; Dzhenkov, D.; Georgiev, K.D. Cardio- and nephroprotective effects of fractions isolated from Lycium barbarum (goji berry) in models of cardio- and nephrotoxicity in rats. Biotechnol. Biotechnol. Equip. 2023, 37, 64–73. [Google Scholar] [CrossRef]
- Wang, M. Emerging Multifunctional NIR Photothermal Therapy Systems Based on Polypyrrole Nanoparticles. Polymers 2016, 8, 373. [Google Scholar] [CrossRef]
- Sun, L.; Zuo, C.; Liu, X.; Guo, Y.; Wang, X.; Dong, Z.; Han, M. Combined Photothermal Therapy and Lycium barbarum Polysaccharide for Topical Administration to Improve the Efficacy of Doxorubicin in the Treatment of Breast Cancer. Pharmaceutics 2022, 14, 2677. [Google Scholar] [CrossRef]
- Cao, G.; Yang, W.; Du, P. Observation of the effects of LAK/IL-2 Therapy combining with Lycium barbarium polysaccharides in the treatment of 75 cancer patients. Chin. J. Oncol. 1994, 16, 428–431. [Google Scholar]
- Hu, Z.; Ning, M.; Qin, S.; Yu, Z. Exploring the molecular mechanism of Lycium barbarum L. against breast cancer based on network pharmacology. J. Funct. Foods 2023, 105, 105545. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, X.; Zhang, D.Y.; Hu, Y.J.; Li, S. Traditional Chinese medicine network pharmacology: Development in new era under guidance of network pharmacology evaluation method guidance. Zhongguo Zhong Yao Za Zhi 2022, 47, 7–17. [Google Scholar] [PubMed]

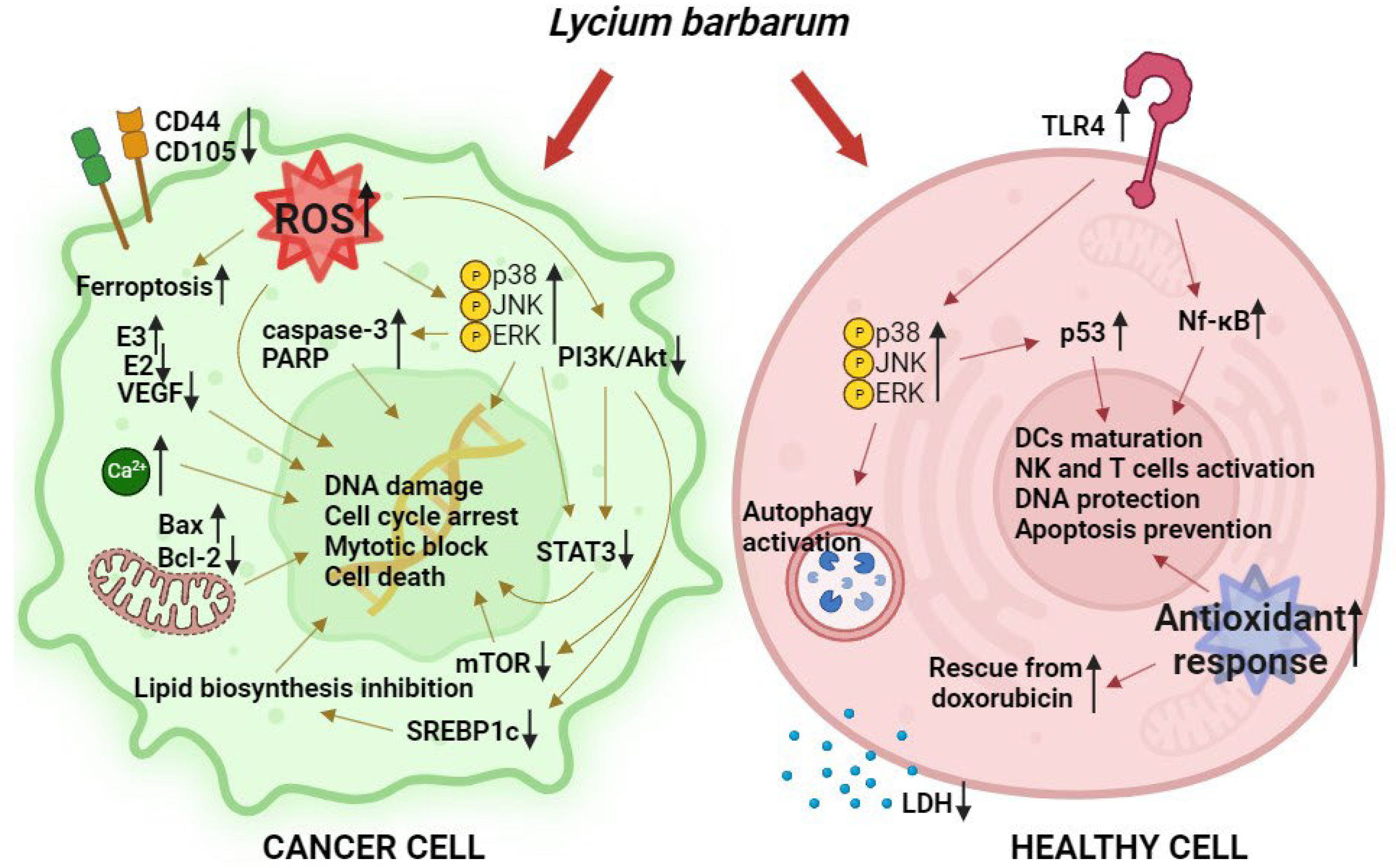
| N. | Phytochemicals | Bioactivity | Disease | Refs. |
|---|---|---|---|---|
| 1 | Dairy-derived β-lactoglobulin peptide | ROS reduction, Nrf2 activation and expression of cytoprotective enzymes such as NADPH oxidase. | Intestinal inflammation. | [11] |
| 2 | Chitosan | Reduction in total cholesterol, triglycerides, LDL, and VLDL. | Hyperlipidemias. | [8] |
| 3 | Glycyrrhizin | Alteration of intracellular transport of viral antigens and suppresses sialylation of the surface antigen (HBsAg) of hepatitis B virus (HBV). | Chronic hepatitis B. | [12,13] |
| 4 | Capsaicin | Selective activation of the Ca2+-permeable ion channel, TRPV1. | Acute pain, neuropathic pain, and inflammatory pain. | [14] https://www.sciencedirect.com/science/article/pii/S1756464620305648-b0580 (accessed on 22 February 2024) |
| 5 | Menthol | Agonism of the Ca2+-permeable ion channel TRPM8, and the k-opioid receptor, OPRK1. | Acute pain, neuropathic pain, and inflammatory pain. | [23,24] |
| 6 | Curcumin | Inhibition of MAPK and Nf-κB signaling pathways. | Inflammatory pathologies, cardiovascular disorders. | [15] |
| 7 | Catechins | PPAR-γ agonists. Powerful antioxidant effect due to the chelating capacity of metal ions. | Atherosclerosis, cardiac hypertrophy, neurodegenerative disorders. | [16,17] |
| 8 | Sulphur-containing compounds from onions and scallions | Stimulation of the production of detoxification enzymes, such as GSTs. | Hepatic dysfunction. | [18,20] |
| 9 | Coffee silver skin melanoidins | Reduction in intracellular ROS and inhibition of MMPs. | Skin aging and related diseases. | [25] |
| 10 | Spirulina platensis, Moringa oleifera, Ganoderma lucidum bioactive compounds | Reduction in NLRP3 expression and Nf-kB levels in the myocardium. | Heart failure and fibrosis. | [21,22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, M.R.; Vestuto, V.; Amodio, G.; Manfra, M.; Pepe, G.; Campiglia, P. Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential. Life 2024, 14, 420. https://doi.org/10.3390/life14030420
Miranda MR, Vestuto V, Amodio G, Manfra M, Pepe G, Campiglia P. Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential. Life. 2024; 14(3):420. https://doi.org/10.3390/life14030420
Chicago/Turabian StyleMiranda, Maria Rosaria, Vincenzo Vestuto, Giuseppina Amodio, Michele Manfra, Giacomo Pepe, and Pietro Campiglia. 2024. "Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential" Life 14, no. 3: 420. https://doi.org/10.3390/life14030420
APA StyleMiranda, M. R., Vestuto, V., Amodio, G., Manfra, M., Pepe, G., & Campiglia, P. (2024). Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential. Life, 14(3), 420. https://doi.org/10.3390/life14030420











