Phylogeographic Reconstruction to Trace the Source Population of Asian Giant Hornet Caught in Nanaimo in Canada and Blaine in the USA
Abstract
1. Introduction
2. Material and Methods
3. Results
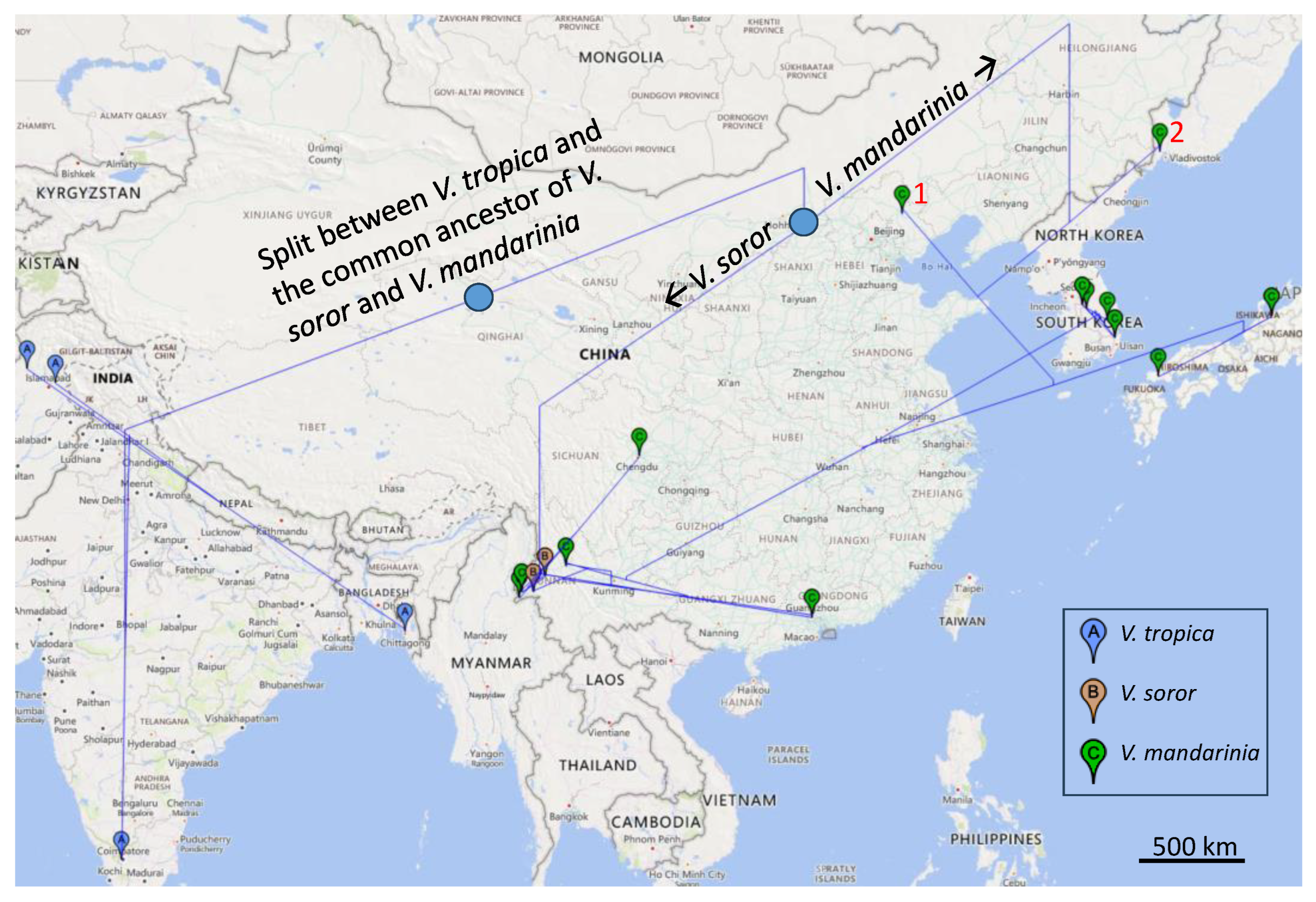
3.1. Identification of the Source Population for the Specimens from Canada and the USA
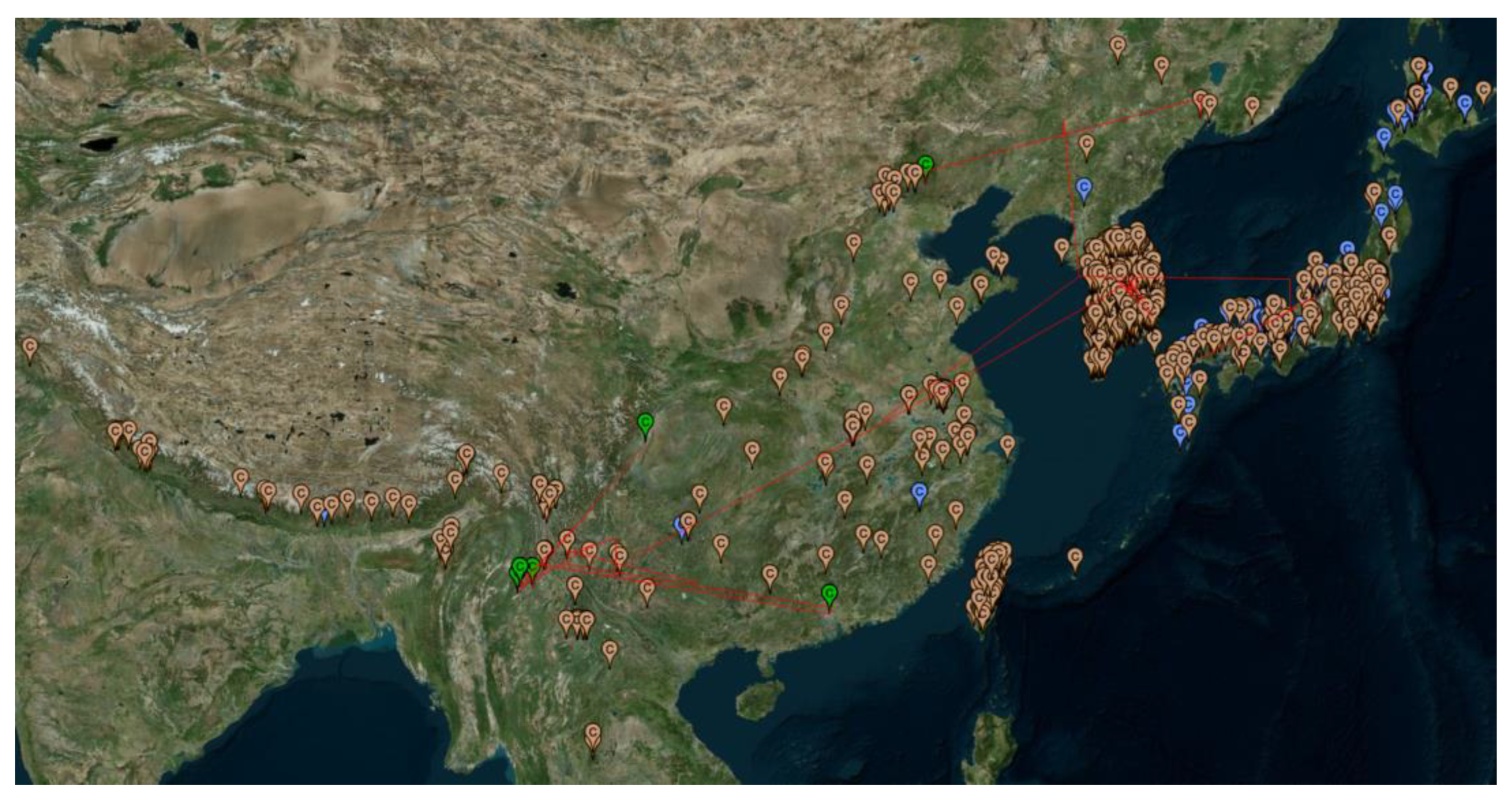
3.2. Phylogeographic Patterns of V. mandarinia and the Two Outgroup Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bass, A.; Needham, K.; Bennett, A.M.R. First record of Vespa crabro Linnaeus (Hymenoptera: Vespidae) in western North America with a review of recorded species of Vespa Linnaeus in Canada. Zootaxa 2022, 5154, 305–318. [Google Scholar] [CrossRef]
- Otis, G.W.; Taylor, B.A.; Mattila, H.R. Invasion potential of hornets (Hymenoptera: Vespidae: Vespa spp.). Front. Insect Sci. 2023, 3, 1145158. [Google Scholar] [CrossRef]
- Beggs, J.R.; Brockerhoff, E.G.; Corley, J.C.; Kenis, M.; Masciocchi, M.; Muller, F.; Rome, Q.; Villemant, C. Ecological effects and management of invasive alien Vespidae. BioControl 2011, 56, 505–526. [Google Scholar] [CrossRef]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2014, 87, 1–16. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Salles, J.-M.; Courchamp, F. The economic cost of control of the invasive yellow-legged Asian hornet. NeoBiota 2020, 55, 11–25. [Google Scholar] [CrossRef]
- Matsuura, M.; Sakagami, S.F. A Bionomic Sketch of the Giant Hornet, Vespa mandarinia, a Serious Pest for Japanese Apiculture. Jour. Faa. Sci. Hokkaido Univ. Ser. VI Zool. 1973, 19, 125–162. [Google Scholar]
- Matsuura, M.; Yamane, S. Biology of the Vespine Wasps; Springer: Berlin/Heidelberg, Germany, 1990; p. XIX, 323. [Google Scholar]
- Yanagawa, Y.; Morita, K.; Sugiura, T.; Okada, Y. Cutaneous hemorrhage or necrosis findings after Vespa mandarinia (wasp) stings may predict the occurrence of multiple organ injury: A case report and review of literature. Clin. Toxicol. 2007, 45, 803–807. [Google Scholar] [CrossRef]
- McClenaghan, B.; Schlaf, M.; Geddes, M.; Mazza, J.; Pitman, G.; McCallum, K.; Rawluk, S.; Hand, K.; Otis, G.W. Behavioral responses of honey bees, Apis cerana and Apis mellifera, to Vespa mandarinia marking and alarm pheromones. J. Apic. Res. 2019, 58, 141–148. [Google Scholar] [CrossRef]
- Wilson, T.M.; Takahashi, J.; Spichiger, S.-E.; Kim, I.; van Westendorp, P. First Reports of Vespa mandarinia (Hymenoptera: Vespidae) in North America Represent Two Separate Maternal Lineages in Washington State, United States, and British Columbia, Canada. Ann. Entomol. Soc. Am. 2020, 113, 468–472. [Google Scholar] [CrossRef]
- Girish Kumar, P.; Srinivasan, G. Taxonomic Studies of Hornet Wasps (Hymenoptera: Vespidae) Vespa Linnaeus of India. Rec. Zool. Surv. India 2010, 110, 57–80. [Google Scholar] [CrossRef]
- Smith-Pardo, A.H.; Carpenter, J.M.; Kimsey, L. The Diversity of Hornets in the Genus Vespa (Hymenoptera: Vespidae; Vespinae), Their Importance and Interceptions in the United States. Insect Syst. Divers. 2020, 4, 2. [Google Scholar] [CrossRef]
- Carpenter, J.M.; Kojima, J. Checklist of the Species in the Subfamily Vespinae (Insecta: Hymenoptera: Vespidae). Nat. Hist. Bull. Ibaraki Univ. 1997, 1, 51–92. [Google Scholar]
- Alaniz, A.J.; Carvajal, M.A.; Vergara, P.M. Giants are coming? Predicting the potential spread and impacts of the giant Asian hornet (Vespa mandarinia, Hymenoptera: Vespidae) in the USA. Pest Manag. Sci. 2021, 77, 104–112. [Google Scholar] [CrossRef]
- Nuñez-Penichet, C.; Osorio-Olvera, L.; Gonzalez, V.H.; Cobos, M.E.; Jiménez, L.; DeRaad, D.A.; Alkishe, A.; Contreras-Díaz, R.G.; Nava-Bolaños, A.; Utsumi, K.; et al. Geographic potential of the world’s largest hornet, Vespa mandarinia Smith (Hymenoptera: Vespidae), worldwide and particularly in North America. PeerJ 2021, 9, e10690. [Google Scholar] [CrossRef]
- British Columbia Government. Asian Giant Hornet Nest Eradicated in Nanaimo. 2019. Available online: https://news.gov.bc.ca/releases/2019AGRI0106-001818 (accessed on 27 December 2023).
- Bérubé, C. Giant alien insect invasion averted. Am. Bee J. 2020, 160, 209–214. [Google Scholar]
- Zhu, G.; Gutierrez Illan, J.; Looney, C.; Crowder, D.W. Assessing the ecological niche and invasion potential of the Asian giant hornet. Proc. Natl. Acad. Sci. USA 2020, 117, 24646–24648. [Google Scholar] [CrossRef]
- Baker, C.S.; Palumbi, S.R. Which whales are hunted? A molecular genetic approach to monitoring whaling. Science 1994, 265, 1538–1539. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Nguyen, L.T.P.; Perrard, A.; Bain, M.; Otis, G.W. Biology of the southern giant hornet, Vespa soror: Nest architecture, morphological differences among castes, and the genetic structure of colonies. Front. Insect Sci. 2023, 3, 1136297. [Google Scholar] [CrossRef]
- Mohamadzade Namin, S.; Jung, C. Genetic diversity of genus Vespa including an invaded species of V. velutina (Hymenoptera: Vespidae) in Korea inferred from DNA barcoding data. J. Asia-Pac. Entomol. 2020, 23, 540–545. [Google Scholar] [CrossRef]
- Arca, M.; Mougel, F.; Guillemaud, T.; Dupas, S.; Rome, Q.; Perrard, A.; Muller, F.; Fossoud, A.; Capdevielle-Dulac, C.; Torres-Leguizamon, M.; et al. Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol. Invasions 2015, 17, 2357–2371. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Xia, X. DAMBE6: New Tools for Microbial Genomics, Phylogenetics, and Molecular Evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Perrard, A.; Pickett, K.; Villemant, C.; Kojima, J.-I.; Carpenter, J.M. Phylogeny of hornets: A total evidence approach (Hymenoptera, Vespidae, Vespinae, Vespa). J. Hymenopt. Res. 2013, 32, 1–15. [Google Scholar] [CrossRef]
- Urtgam, S.; Jongjitvimol, T. Genetic Evolution of Asian Predatory Wasp, Vespa velutina, in Northernof Thailand Based on Cytochrome Oxidase Subunit I DNA Barcoding. NU. Int. J. Sci. 2020, 17, 101–113. [Google Scholar]
- Chen, P.Y.; Wei, S.J.; Liu, J.X. The mitochondrial genome of the Vespa mandarinia Smith (Hymenoptera: Vespidae: Vespinae) and a phylogenetic analysis of the Vespoidea. Mitochondrial DNA Part A 2016, 27, 4414–4415. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Asimenos, G.; Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 2009, 537, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Statistical properties of the maximum likelihood method of phylogenetic estimation and comparison with distance matrix method. Syst. Biol. 1994, 43, 329–342. [Google Scholar] [CrossRef]
- Xia, X. A Mathematical Primer of Molecular Phylogenetics; CRC Press: New York, NY, USA, 2020; p. xiii, 380. [Google Scholar]
- Xia, X. Information-theoretic indices and an approximate significance test for testing the molecular clock hypothesis with genetic distances. Mol. Phylogenet. Evol. 2009, 52, 665–676. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Tavaré, S. Some Probabilistic and Statistical Problems in the Analysis of DNA Sequences; American Mathematical Society: Providence, RI, USA, 1986; Volume 17, pp. 57–86. [Google Scholar]
- Lanave, C.; Preparata, G.; Saccone, C.; Serio, G. A new method for calculating evolutionary substitution rates. J. Mol. Evol. 1984, 20, 86–93. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. PGT: Visualizing temporal and spatial biogeographic patterns. Glob. Ecol. Biogeogr. 2019, 28, 1195–1199. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Occurrence Download. 2024. Available online: https://www.gbif.org/occurrence/download/0042721-231120084113126 (accessed on 3 January 2024).
- Rambaut, A. FigTree. Version 1.4.4. 2020. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 16 September 2019).
- Archer, M.E.; Penney, D. Vespine Wasps of the World: Behaviour, Ecology & Taxonomy of the Vespinae; Siri Scientific Press: Manchester, UK, 2012. [Google Scholar]
- Barss, P. Renal failure and death after multiple stings in Papua New Guinea. Ecology, prevention and management of attacks by vespid wasps. Med. J. Aust. 1989, 151, 659–663. [Google Scholar] [CrossRef]
- Bessa, A.S.; Carvalho, J.; Gomes, A.; Santarém, F. Climate and land-use drivers of invasion: Predicting the expansion of Vespa velutina nigrithorax into the Iberian Peninsula. Insect Conserv. Divers. 2016, 9, 27–37. [Google Scholar] [CrossRef]
- Takahashi, R.; Okuyama, H.; Minoshima, Y.N.; Takahashi, J.I. Complete mitochondrial DNA sequence of the alien hornet Vespa velutina (Insecta: Hymenoptera) invading Kyushu Island, Japan. Mitochondrial DNA Part B 2018, 3, 179–181. [Google Scholar] [CrossRef]
- Power, K.; Martano, M.; Ragusa, E.; Altamura, G.; Maiolino, P. Detection of honey bee viruses in larvae of Vespa orientalis. Front. Cell. Infect. Microbiol. 2023, 13, 1207319. [Google Scholar] [CrossRef]
- Ball, S.L.; Armstrong, K.F. DNA barcodes for insect pest identification: A test case with tussock moths (Lepidoptera: Lymantriidae). Can. J. For. Res. 2006, 36, 337–350. [Google Scholar] [CrossRef]
- Madden, M.J.L.; Young, R.G.; Brown, J.W.; Miller, S.E.; Frewin, A.J.; Hanner, R.H. Using DNA barcoding to improve invasive pest identification at U.S. ports-of-entry. PLoS ONE 2019, 14, e0222291. [Google Scholar] [CrossRef]
- Rakesh, M.; Aris-Brosou, S.; Xia, X. Testing alternative hypotheses on the origin and speciation of Hawaiian katydids. BMC Ecol. Evol. 2022, 22, 83. [Google Scholar] [CrossRef]

| ACCN/Sample ID | Species | Latitude | Longitude | LCOX1 | GC% |
|---|---|---|---|---|---|
| AB851894 | V. mandarinia | 36.77 | 137.47 | 655 | 30.84 |
| BIOUG26171-B05 | V. mandarinia | 43.35 | 131.57 | 588 | 29.76 |
| KR059904 | V. mandarinia | 40.94 | 117.99 | 1533 | 29.88 |
| LC541727 | V. mandarinia | 34.18 | 131.47 | 1536 | 29.88 |
| LC541728 | V. mandarinia | 37.06 | 127.70 | 1536 | 30.08 |
| LC541729 | V. mandarinia | 48.99 | −122.75 | 1536 | 30.01 |
| LC541730 | V. mandarinia | 49.18 | −123.94 | 1536 | 29.88 |
| MN716824 | V. mandarinia | 36.60 | 128.78 | 658 | 31.16 |
| MN716825 | V. mandarinia | 35.84 | 129.22 | 658 | 31.31 |
| MN716826 | V. mandarinia | 35.84 | 129.22 | 658 | 31.16 |
| MN716827 | V. mandarinia | 37.22 | 127.48 | 658 | 31.16 |
| MN716828 | V. mandarinia | 37.22 | 127.48 | 658 | 31.00 |
| MZ165590 | V. mandarinia | 25.11 | 99.17 | 766 | 31.59 |
| MZ165591 | V. mandarinia | 25.11 | 99.17 | 777 | 31.40 |
| MZ165592 | V. mandarinia | 25.11 | 99.17 | 778 | 31.36 |
| MZ165593 | V. mandarinia | 24.37 | 97.96 | 818 | 30.81 |
| MZ165594 | V. mandarinia | 24.37 | 97.96 | 791 | 31.23 |
| MZ165595 | V. mandarinia | 24.08 | 97.82 | 854 | 31.26 |
| MZ165597 | V. mandarinia | 25.11 | 99.17 | 762 | 31.36 |
| MZ165598 | V. mandarinia | 24.36 | 98.57 | 766 | 31.59 |
| MZ165599 | V. mandarinia | 24.37 | 97.96 | 790 | 31.27 |
| MZ165600 | V. mandarinia | 25.11 | 99.17 | 793 | 31.40 |
| MZ165601 | V. mandarinia | 24.36 | 98.57 | 772 | 31.48 |
| OQ836202 | V. mandarinia | 25.61 | 100.27 | 1536 | 29.82 |
| OQ836204 | V. mandarinia | 25.61 | 100.27 | 1536 | 29.95 |
| OQ909418 | V. mandarinia | 23.17 | 113.27 | 666 | 31.23 |
| OQ909419 | V. mandarinia | 23.17 | 113.27 | 658 | 31.16 |
| OQ909420 | V. mandarinia | 30.67 | 104.14 | 657 | 31.35 |
| NC_050197 | V. mandarinia | 49.18 | −123.94 | 1536 | 29.88 |
| MZ191819 | V. soror | 25.11 | 99.17 | 783 | 30.40 |
| MZ191820 | V. soror | 24.36 | 98.57 | 834 | 29.98 |
| MZ191821 | V. soror | 24.36 | 98.57 | 779 | 30.42 |
| NIBGE HYM-01001 | V. tropica | 34.51 | 71.91 | 658 | 31.00 |
| NIBGE HYM-01572 | V. tropica | 33.91 | 73.39 | 658 | 31.00 |
| BIOUG24885-F10 | V. tropica | 22.47 | 91.78 | 591 | 30.29 |
| MN893829 | V. tropica | 11.07 | 76.86 | 586 | 30.55 |
| Model | lnL (1) | k (2) | AIC |
|---|---|---|---|
| HKY | −3092.5571 | 74 | 6333.114 |
| HKY + Γ | −3054.0615 | 75 | 6258.123 |
| TN93 | −3083.4433 | 75 | 6316.887 |
| TN93 + Γ | −3052.3701 | 76 | 6256.740 |
| GTR | −3074.7981 | 78 | 6305.596 |
| GTR + Γ | −3047.1831 | 79 | 6252.366 |
| MSpecial (1) | MGeneral (1) | 2∆lnL (2) | DF (3) | p |
|---|---|---|---|---|
| HKY | TN93 | 18.22766 | 1 | 0.00002 |
| HKY | GTR | 35.51802 | 4 | 0.00000 |
| TN93 | GTR | 17.29036 | 3 | 0.00062 |
| HKY | HKY + G | 76.99118 | 1 | 0.00000 |
| TN93 | TN93 + G | 62.1463 | 1 | 0.00000 |
| GTR | GTR + G | 55.23006 | 1 | 0.00000 |
| HKY + Γ | TN93 + G | 3.38278 | 1 | 0.06588 |
| HKY + Γ | GTR + G | 13.7569 | 4 | 0.00811 |
| TN93 + Γ | GTR + G | 10.37412 | 3 | 0.01564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeman, A.; Xia, X. Phylogeographic Reconstruction to Trace the Source Population of Asian Giant Hornet Caught in Nanaimo in Canada and Blaine in the USA. Life 2024, 14, 283. https://doi.org/10.3390/life14030283
Freeman A, Xia X. Phylogeographic Reconstruction to Trace the Source Population of Asian Giant Hornet Caught in Nanaimo in Canada and Blaine in the USA. Life. 2024; 14(3):283. https://doi.org/10.3390/life14030283
Chicago/Turabian StyleFreeman, Alexa, and Xuhua Xia. 2024. "Phylogeographic Reconstruction to Trace the Source Population of Asian Giant Hornet Caught in Nanaimo in Canada and Blaine in the USA" Life 14, no. 3: 283. https://doi.org/10.3390/life14030283
APA StyleFreeman, A., & Xia, X. (2024). Phylogeographic Reconstruction to Trace the Source Population of Asian Giant Hornet Caught in Nanaimo in Canada and Blaine in the USA. Life, 14(3), 283. https://doi.org/10.3390/life14030283








