Physical Performance of Geriatric Women and Its Impact on Fracture Risk and Bone Mineral Density Assessed with Radiofrequency Echographic Multispectrometry (REMS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. BMD and Fracture Risk
2.3. Physical Performance
2.4. Statistical Analysis
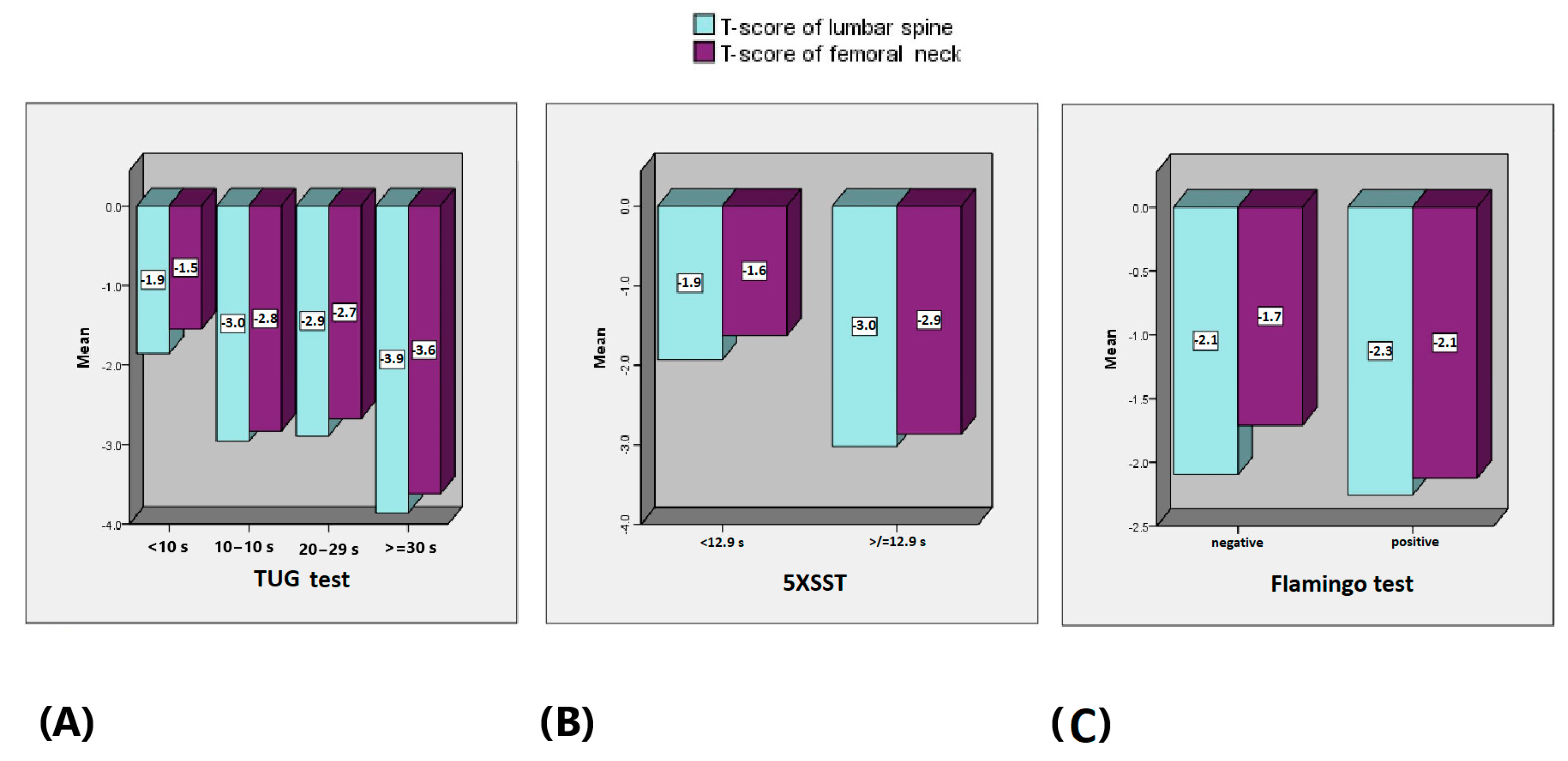
3. Results
4. Discussion
Strengths and Weaknesses of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keen, M.U.; Reddivari, A.K.R. Osteoporosis in Females. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559156/ (accessed on 12 June 2023).
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium. The burden of musculoskeletal conditions at the start of the new millennium. World Health. Organ. Tech. Rep. Ser. 2003, 919, 1–218. [Google Scholar] [PubMed]
- Rodrigues, F.; Monteiro, A.M.; Forte, P.; Morouço, P. Effects of Muscle Strength, Agility, and Fear of Falling on Risk of Falling in Older Adults. Int. J. Environ. Res. Public Health 2023, 20, 4945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W. Osteoporosis and the burden of osteoporosis-related fractures. Am. J. Manag. Care 2011, 17 (Suppl. 6), S164–S169. [Google Scholar] [PubMed]
- Morri, M.; Ambrosi, E.; Chiari, P.; Orlandi Magli, A.; Gazineo, D.; D’Alessandro, F.; Forni, C. One-year mortality after hip fracture surgery and prognostic factors: A prospective cohort study. Sci. Rep. 2019, 9, 18718. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Whittier, D.E.; Bevers, M.S.A.M.; Geusens, P.P.M.M.; van den Bergh, J.P.; Gabel, L. Characterizing Bone Phenotypes Related to Skeletal Fragility Using Advanced Medical Imaging. Curr. Osteoporos. Rep. 2023, 21, 685–697. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Bennett, S.; Zou, J.; Xu, J.; Zhang, L. The Effects of Different Dietary Patterns on Bone Health. Nutrients 2024, 16, 2289. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone quality—The key to understanding fracture risk. Nat. Rev. Rheumatol. 2006, 2, 680–688. [Google Scholar] [CrossRef]
- Nyman, J.S.; Makowski, A.J. The contribution of the extracellular matrix to the fracture resistance of bone. Curr. Osteoporos. Rep. 2012, 10, 169–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosen, C.J. Bone Quality: The Overlooked Dimension of Bone Health. Nat. Rev. Endocrinol. 2015, 11, 698–709. [Google Scholar] [CrossRef]
- Papalia, G.F.; Papalia, R.; Diaz Balzani, L.A.; Torre, G.; Zampogna, B.; Vasta, S.; Fossati, C.; Alifano, A.M.; Denaro, V. The Effects of Physical Exercise on Balance and Prevention of Falls in Older People: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simpkins, C.; Yang, F. Muscle power is more important than strength in preventing falls in community-dwelling older adults. J. Biomech. 2022, 134, 111018. [Google Scholar] [CrossRef] [PubMed]
- Smulders, E.; van Lankveld, W.; Laan, R.; Duysens, J.; Weerdesteyn, V. Does osteoporosis predispose falls? a study on obstacle avoidance and balance confidence. BMC Musculoskelet. Disord. 2011, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Abdel Gader, A.M. The effect of exercise and nutrition on bone health. J. Musculoskelet. Surg. Res. 2018, 2, 142–147. [Google Scholar] [CrossRef]
- Charde, S.H.; Joshi, A.; Raut, J. A Comprehensive Review on Postmenopausal Osteoporosis in Women. Cureus 2023, 15, e48582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narula, R.; Tauseef, M.; Ahmad, I.A.; Agarwal, K.; Ashok, A.; Anjana, A. Vitamin d deficiency among postmenopausal women with osteoporosis. J. Clin. Diagn. Res. 2013, 7, 336–338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vandenput, L.; Johansson, H.; McCloskey, E.; Liu, E.; Akesson, K.; Anderson, F.; Azagra-Ledesma, R.; Bager, C.; Beaudart, C.; Bischoff-Ferrari, H.; et al. Update of the fracture risk prediction tool FRAX: A systematic review of potential cohorts and analysis plan. Osteoporos. Int. 2022, 33, 2103–2136. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M. The Innovative REMS Technology for Early Osteoporosis Diagnosis and Fracture Risk Prediction. Остеoпoрoз И Остеoпатии 2020, 23, 215. [Google Scholar]
- Cortet, B.; Dennison, E.; Diez-Perez, A.; Locquet, M.; Muratore, M.; Nogués, X.; Crespo, D.O.; Quarta, E.; Brandi, M.L. Radiofrequency Echographic Multi Spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone 2021, 143, 115786. [Google Scholar] [CrossRef]
- Al Refaie, A.; Baldassini, L.; Mondillo, C.; Giglio, E.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S.; Caffarelli, C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture. Diagnostics 2023, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Conversano, F.; Muratore, M.; Adami, G.; Brandi, M.L.; Caffarelli, C.; Casciaro, E.; Di Paola, M.; Franchini, R.; Gatti, D.; et al. Fragility Score: A REMS-based indicator for the prediction of incident fragility fractures at 5 years. Aging Clin. Exp. Res. 2023, 35, 763–773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuggle, N.R.; Reginster, J.Y.; Al-Daghri, N.; Bruyere, O.; Burlet, N.; Campusano, C.; Cooper, C.; Perez, A.D.; Halbout, P.; Ghi, T.; et al. Radiofrequency echographic multi spectrometry (REMS) in the diagnosis and management of osteoporosis: State of the art. Aging Clin. Exp. Res. 2024, 36, 135. [Google Scholar] [CrossRef]
- Sakai, T.; Hirao, M.; Takashina, Y.; Kitagawa, R.; Oishi, T. Radiofrequency echographic multi-spectrometry-based measurement of bone mineral density in patients with severe motor and intellectual disability: An opportunity for patients with severe scoliosis and hip dislocation. Bone Rep. 2024, 22, 101781. [Google Scholar] [CrossRef]
- Caffarelli, C.; Al Refaie, A.; Mondillo, C.; Versienti, A.; Baldassini, L.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S. Radiofrequency Echographic Multispectrometry (REMS): A New Option in the Assessment Bone Status in Adults with Osteogenesis Imperfecta. J. Imaging 2023, 9, 210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evenson, K.R.; Buchner, D.M.; Morland, K.B. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev. Chronic Dis. 2012, 9, E26. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.D.O.; Ostolin, T.L.V.D.P.; Ferreira, M.; Sperandio, E.F.; Dourado, V.Z. Test timed up and go and its correlation with age and functional exercise capacity in asymptomatic women. Fisioter. Em Mov. 2017, 30, 463–471. Available online: https://api.semanticscholar.org/CorpusID:55997328 (accessed on 12 June 2023). [CrossRef]
- Skelton, D.A.; Greig, C.A.; Davies, J.M.; Young, A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 1994, 23, 371–377. [Google Scholar] [CrossRef]
- Granacher, U.; Gruber, M.; Gollhofer, A. Force production capacity and functional reflex activity in young and elderly men. Aging Clin. Exp. Res. 2010, 22, 374–382. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Yang, G.-Y.; Jin, K. Age-Related Dysfunction in Balance: A Comprehensive Review of Causes, Consequences, and Interventions. Aging Disease 2024, 16. online ahead of print. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Hu, F.; Chen, S.; Liu, N. Association of body mass index and waist circumference with falls in Chinese older adults. Geriatr. Nurs. 2022, 44, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ogliari, G.; Ryg, J.; Andersen-Ranberg, K.; Scheel-Hincke, L.L.; Masud, T. Association between body mass index and falls in community-dwelling men and women: A prospective, multinational study in the Survey of Health, Ageing and Retirement in Europe (SHARE). Eur. Geriatr. Med. 2021, 12, 837–849. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.S.; Sim, S.; Park, B.; Choi, H.G. Association Between Obesity and Falls Among Korean Adults: A Population-Based Cross-Sectional Study. Medicine 2016, 95, e3130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lu, S.F.; Zhou, Y.; Zhang, B.; Copeland, L.; Gurwitz, J.H. Body Mass Index, Falls, and Hip Fractures Among Nursing Home Residents. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Hermenegildo-Lopez, Y.; Sandoval-Insausti, H.; Donat-Vargas, C.; Banegas, J.R.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. General and central obesity operate differently as predictors of falls requiring medical care in older women: A population-based cohort study in Spain. Age Ageing 2021, 50, 213–219. [Google Scholar] [CrossRef]
- Houston, D.K.; Ding, J.; Nicklas, B.J.; Harris, T.B.; Lee, J.S.; Nevitt, M.C.; Rubin, S.M.; Tylavsky, F.A.; Kritchevsky, S.B. Health ABC Study. Overweight and obesity over the adult life course and incident mobility limitation in older adults: The health, aging and body composition study. Am. J. Epidemiol. 2009, 169, 927–936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valentina, C.; Chiara, M.; Carlo, Z. Are body circumferences able to predict strength, muscle mass and bone characteristics in obesity? A preliminary study in women. Int. J. Med. Sci. 2020, 17, 881–891. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016, 17, 467–483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoo, M.C.; Won, C.W.; Soh, Y. Association of high body mass index, waist circumference, and body fat percentage with sarcopenia in older women. BMC Geriatr. 2022, 22, 937. [Google Scholar] [CrossRef]
- Kıskaç, M.; Soysal, P.; Smith, L.; Capar, E.; Zorlu, M. What is the Optimal Body Mass. Index. Range for Older Adults? Ann. Geriatr. Med. Res. 2022, 26, 49–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, H.; Chen, S.; Wang, X.; Jin, J.; Li, X.; Li, Z. Association between muscle strength and mass and bone mineral density in the US general population: Data from NHANES 1999-2002. J Orthop. Surg. Res. 2023, 18, 397. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Jiao, W. Correlation of muscle mass and bone mineral density in the NHANES US general population, 2017–2018. Medicine 2022, 101, e30735. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fu, L.; Jia, L.; Han, P.; Kang, L.; Yu, H.; Chen, X.; Yu, X.; Hou, L.; Wang, L.; et al. Muscle strength rather than muscle mass is associated with osteoporosis in older Chinese adults. J. Formos. Med. Assoc. 2018, 117, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.M.; Rasheedy, D.; El-Sorady, K.E.; Mortagy, A.K. Beyond mobility assessment: Timed up and go test and its relationship to osteoporosis and fracture risk. J. Clin. Gerontol. Geriatr. 2016, 7, 48–52. [Google Scholar] [CrossRef][Green Version]
- Zhu, K.; Devine, A.; Lewis, J.R.; Dhaliwal, S.S.; Prince, R.L. Timed Up and Go Test and Bone Mineral Density Measurement for Fracture Prediction. Arch. Intern. Med. 2011, 171, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Shin, D.W.; Han, K.; Jung, J.H.; Chun, S.; Jung, H.W.; Son, K.Y. Timed up-and-go test is a useful predictor of fracture incidence. Bone 2019, 127, 474–481. [Google Scholar] [CrossRef]
- Larsson, B.A.M.; Johansson, L.; Johansson, H.; Axelsson, K.F.; Harvey, N.; Vandenput, L.; Magnusson, P.; McCloskey, E.; Liu, E.; Kanis, J.A.; et al. The timed up and go test predicts fracture risk in older women independently of clinical risk factors and bone mineral density. Osteoporos. Int. 2021, 32, 75–84. [Google Scholar] [CrossRef]
- Toshimitsu, I.; Yasumichi, A.; Motoko, F.; Michiyo, T.; Yukiko, A.; Keiko, A.; Yuji, N.; Toru, T.; Takashi, I.; Kazuo, K.; et al. Maximum Occlusal Force and Physical Performance in the Oldest Old: The Tokyo Oldest Old Survey on Total Health. J. Am. Geriatr. Soc. 2011, 60, 68–76. [Google Scholar] [CrossRef]
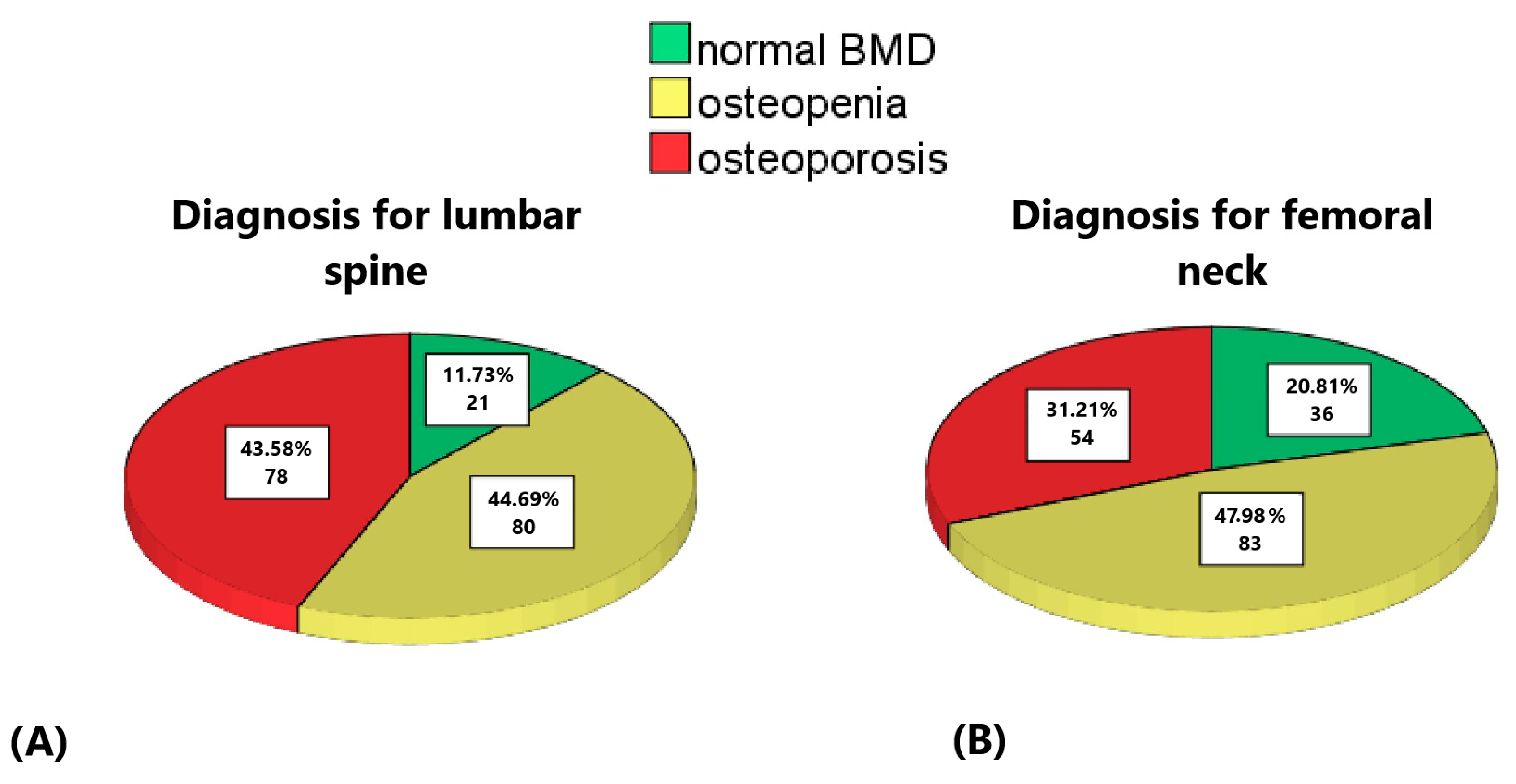
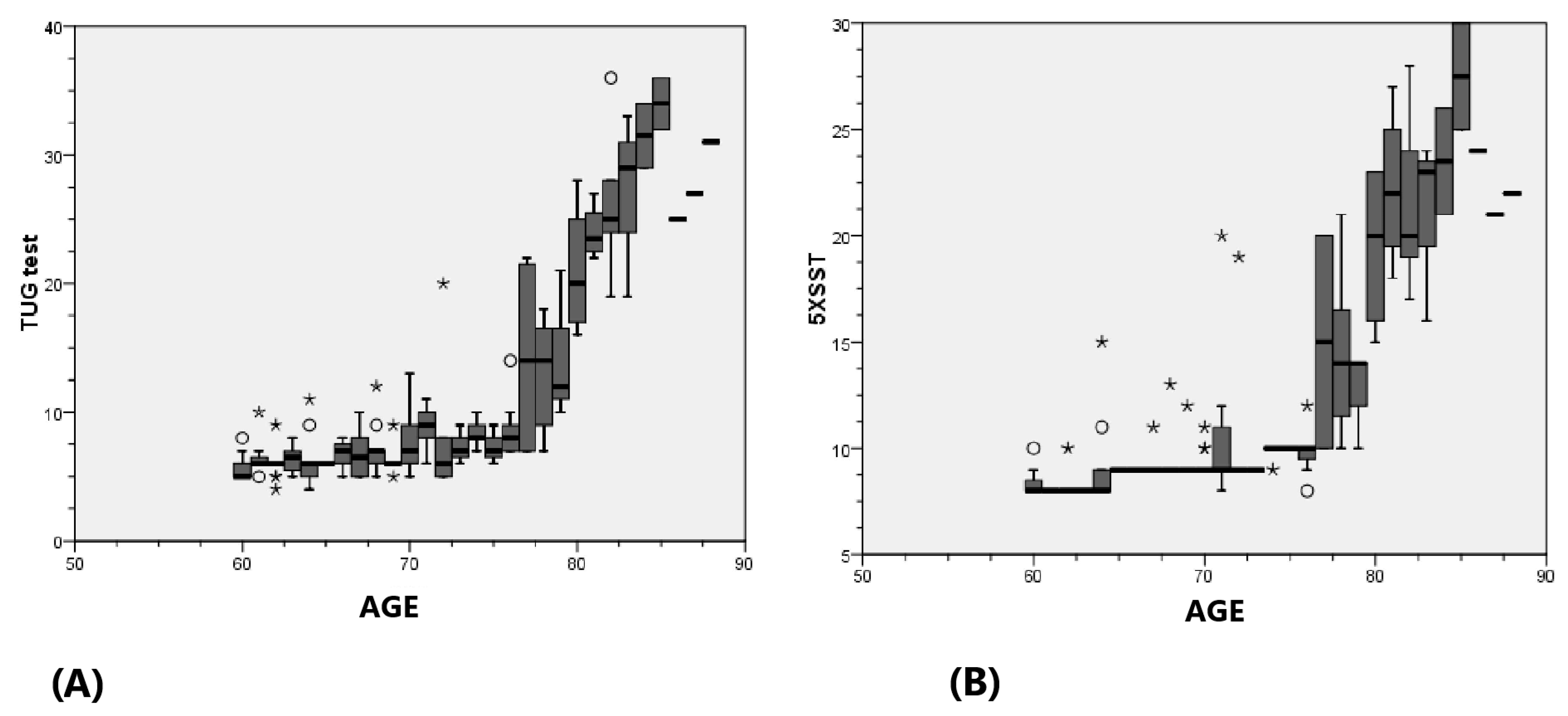
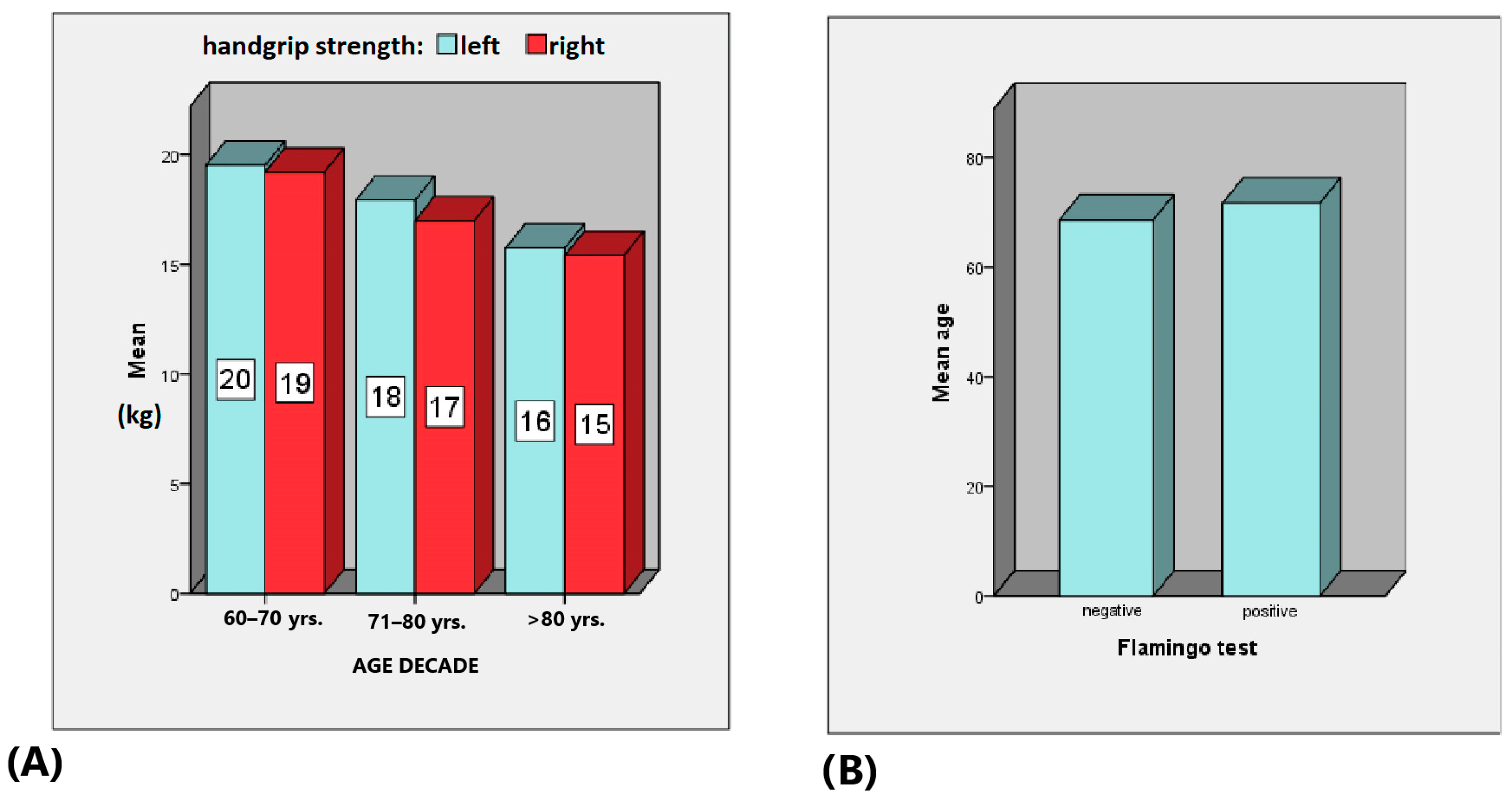

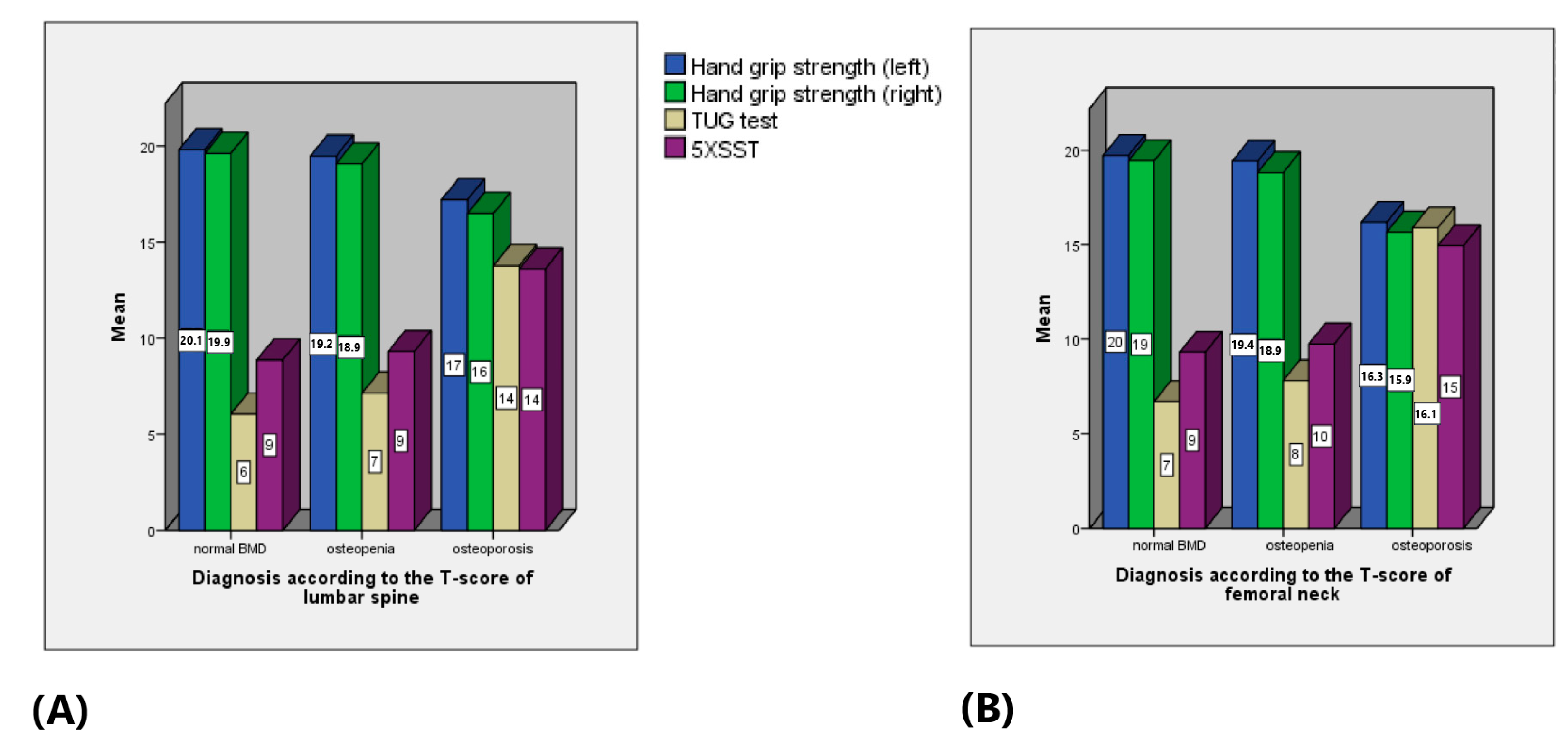

| Mean | Median | Minimum | Maximum | Standard Deviation | Standard Error of the Mean | |
|---|---|---|---|---|---|---|
| Age | 70 | 69 | 60 | 88 | 7 | 1 |
| Weight | 71.0 | 69.0 | 40.0 | 120.0 | 14.6 | 1.1 |
| Height | 154.5 | 155.0 | 100.0 | 182.0 | 8.3 | 0.6 |
| BMI | 29.6 | 29.3 | 17.3 | 47.5 | 5.6 | 0.4 |
| REMS-based BMD L1 | 0.712 | 0.697 | 0.427 | 1.134 | 0.127 | 0.01 |
| REMS-based BMD L2 | 0.788 | 0.778 | 0.567 | 1.251 | 0.123 | 0.01 |
| REMS-based BMD L3 | 0.844 | 0.829 | 0.578 | 1.284 | 0.121 | 0.01 |
| REMS-based BMD L4 | 0.868 | 0.859 | 0.632 | 1.315 | 0.117 | 0.01 |
| Total REMS-based BMD | 0.810 | 0.798 | 0.585 | 1.258 | 0.117 | 0.01 |
| Total REMS-based T-score | −2.2 | −2.3 | −4.2 | 3.0 | 1.1 | 0.1 |
| Total REMS-based Z-score | −0.1 | −0.3 | −1.5 | 2.8 | 0.8 | 0.1 |
| FRAX for major osteoporotic | 17.01 | 15.13 | 0.67 | 56.76 | 9.17 | 0.69 |
| FRAX for hip fracture | 5.05 | 3.16 | 0.60 | 35.53 | 5.11 | 0.38 |
| REMS-based BMD of femoral neck | 0.643 | 0.637 | 0.347 | 1.060 | 0.126 | 0.01 |
| REMS-based T-score of femoral neck | −1.9 | −1.9 | −4.5 | 1.2 | 1.1 | 0.1 |
| REMS-based Z-score of femoral neck | −0.1 | −0.2 | −2.1 | 3.0 | 0.9 | 0.1 |
| REMS-based BMD of trochanter | 0.803 | 0.800 | 0.456 | 1.214 | 0.135 | 0.01 |
| REMS-based T-score of trochanter | −0.1 | −1.0 | −3.6 | 2.1 | 0.9 | 0.07 |
| REMS-based Z-score of trochanter | 0.2 | 0.2 | −1.8 | 3.4 | 0.9 | 0.1 |
| REMS-based total BMD of hip | 0.787 | 0.783 | 0.442 | 1.266 | 0.141 | 0.01 |
| REMS-based total T-score of hip | −1.3 | −1.3 | −4.1 | 1.9 | 1.1 | 0.1 |
| REMS-based total Z-score of hip | 0.2 | 0.1 | −2.0 | 3.4 | 0.9 | 0.1 |
| Hand grip strength (left) | 18.4 | 20 | 8 | 20 | 3 | 0 |
| Hand grip strength (right) | 18.4 | 20 | 6 | 20 | 3 | 0 |
| TUG test | 10 | 7 | 4 | 36 | 7 | 1 |
| 5XSST | 11 | 9 | 8 | 30 | 5 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischoff, E.; Popova-Belova, S.; Bischoff, F.; Kirilov, N. Physical Performance of Geriatric Women and Its Impact on Fracture Risk and Bone Mineral Density Assessed with Radiofrequency Echographic Multispectrometry (REMS). Life 2024, 14, 1579. https://doi.org/10.3390/life14121579
Bischoff E, Popova-Belova S, Bischoff F, Kirilov N. Physical Performance of Geriatric Women and Its Impact on Fracture Risk and Bone Mineral Density Assessed with Radiofrequency Echographic Multispectrometry (REMS). Life. 2024; 14(12):1579. https://doi.org/10.3390/life14121579
Chicago/Turabian StyleBischoff, Elena, Stanislava Popova-Belova, Fabian Bischoff, and Nikola Kirilov. 2024. "Physical Performance of Geriatric Women and Its Impact on Fracture Risk and Bone Mineral Density Assessed with Radiofrequency Echographic Multispectrometry (REMS)" Life 14, no. 12: 1579. https://doi.org/10.3390/life14121579
APA StyleBischoff, E., Popova-Belova, S., Bischoff, F., & Kirilov, N. (2024). Physical Performance of Geriatric Women and Its Impact on Fracture Risk and Bone Mineral Density Assessed with Radiofrequency Echographic Multispectrometry (REMS). Life, 14(12), 1579. https://doi.org/10.3390/life14121579





