Evaluation of Peripheral Circulatory Changes Following Hydrotherapy and Controlled Physical Training in Patients with Atherosclerotic Lower Limb Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group Characteristics
2.2. Study Inclusion Criteria
- -
- Peripheral arterial disease due to atherosclerosis, confirmed by Doppler ultrasound examination;
- -
- Nonparticipation in other physical therapy treatments;
- -
- Informed consent of the patient;
- -
- Absence of contraindications for participation in this study [23].
2.3. Rehabilitation Program
2.4. Training Sessions
2.5. Assessment of Blood Flow in the Lower Extremities
2.6. The 6-Min Walk Test (6MWT)
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Groups Included in the Study
3.2. Analysis of Changes in Selected Local Flow Parameters
3.3. Analysis of Changes in Selected Parameters of the 6MWT
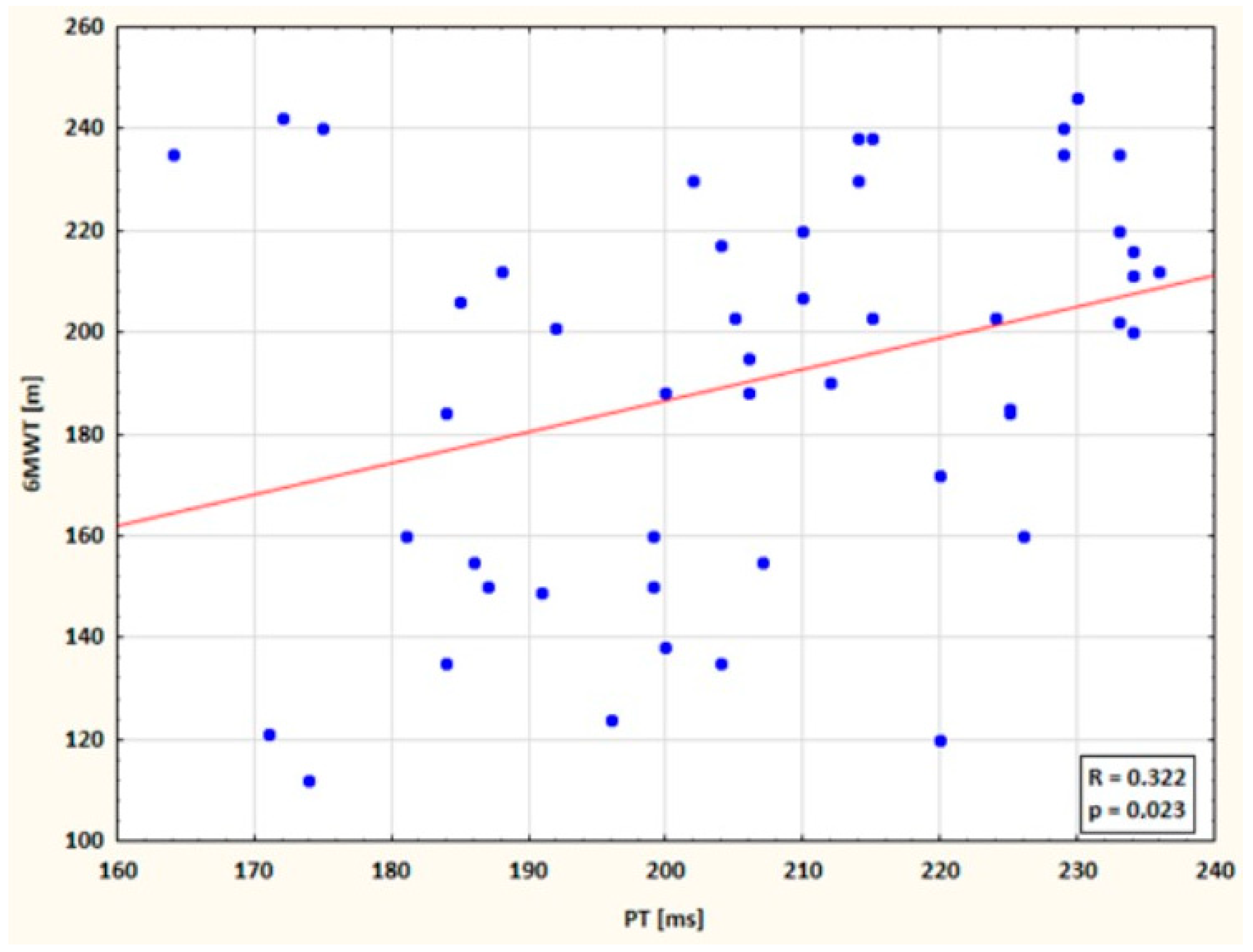
3.4. Correlations Between 6MWT Distance and Plethysmographic Parameters (PAmpl, PSlope, CT, PT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jawień, A. Arterial Surgery. Pract. Med. 2007, 1, 55. [Google Scholar]
- Novo, S. The patients with intermittent claudication. In Everyday Problems in Clinical Cardiology; Excerpta Medica: Amsterdam, The Netherlands, 1995; Volume 7, pp. 882–884. [Google Scholar]
- Chetter, I.C.; Dolan, P.; Spark, J.I.; Scott, D.J.; Kester, R.C. Correlating clinical indicators of lower limb ischaemia with quality of life. Cardiovasc. Surg. 1997, 5, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Seabrook, G.R.; Cambria, R.A.; Freischlag, J.A.; Towne, J.B. Health-related quality of life and functional outcome following arterial reconstruction for limb salvage. Cardiovasc. Surg. 1999, 7, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.C.; Fowkes, F.G. The Edinburgh Claudication Questionnaire: An improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J. Clin. Epidemiol. 1992, 45, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Piotrkowska, R.; Dobosz, M.; Ksiazek, J.; Halena, G. Quality of life in patients with peripheral atheromatosis—Literature review. Ann. Acad. Med. Gedan. 2011, 41, 89–95. [Google Scholar]
- Crowther, R.G.; Spinks, W.L.; Leicht, A.S.; Sangla, K.; Quigley, F.; Golledge, J. Effects of a long-term exercise program on lower limb mobility, physiological responses, walking performance, and physical activity levels in patients with peripheral arterial disease. J. Vasc. Surg. 2008, 47, 303–309. [Google Scholar] [CrossRef]
- Tsai, J.C.; Chan, P.; Wang, C.H.; Jeng, C.; Hsieh, M.H.; Kao, P.F.; Chen, Y.J.; Liu, J.C. The effects of exercise training on walking function and perception of health status in elderly patients with peripheral arterial occlusive disease. J. Intern. Med. 2002, 252, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Fiodorenko, Ż. Assessment of Quality of Life in Patients with Obliterative Atherosclerosis and Diabetic Macroangiopathy. Ph.D. Thesis, Wroclaw Medical Academy, Wroclaw, Poland, 2006; pp. 145–146. [Google Scholar]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Dormandy, J.A.; Rutherford, R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J. Vasc. Surg. 2000, 31, S1–S296. [Google Scholar] [PubMed]
- National Center for Health Promotion and Disease Prevention. Available online: https://www.prevention.va.gov/Healthy_Living/Limit_Alcohol.asp%5D (accessed on 7 October 2024).
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [CrossRef]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef]
- Gardner, A.; Poehlman, E. Exercise rehabilitation programs for the treatment of claudication pain: A meta-analysis. JAMA 1995, 274, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.; Hiatt, W.; Regensteiner, J.; Hirsch, A. Exercise training for claudication. N. Engl. J. Med. 2002, 347, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Hoag, S.; Hamman, R.F. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation 1995, 91, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Erlinger, T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004, 110, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Szmidt, J.; Kużdżał, J. Fundamentals of Surgery, 2nd ed.; Practical Medicine: Kraków, Poland, 2009. [Google Scholar]
- Żyżniewska-Banaszak, E.; Mosiejczuk, H.; Cichocki, P. Physiotherapy and biological regeneration: For everyone? Ann. Acad. Medicae Stetin. 2010, 56, 113–120. [Google Scholar]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart. J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, J.; Irzmański, R. The Impact of Controlled Physical Training with Hydrotherapy on Changes in Swelling and Claudication Distance in Patients with Atherosclerotic Ischemia of the Lower Limbs. Int. J. Environ. Res. Public Health 2022, 19, 15715. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Fronek, A.; Barrett-Connor, E.; Klauber, M.R.; Gabriel, S.; Goodman, D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985, 71, 510–515. [Google Scholar] [CrossRef]
- Olin, J.W.; Sealove, B.A. Peripheral artery disease: Current insight into the disease and its diagnosis and management. Mayo. Clin. Proc. 2010, 85, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Murabito, J.M.; Evans, J.C.; D’Agostino, R.B., Sr.; Wilson, P.W.; Kannel, W.B. Temporal trends in the incidence of intermittent claudication from 1950 to 1999. Am. J. Epidemiol. 2005, 162, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Schroll, M.; Munck, O. Estimation of peripheral arteriosclerotic disease by ankle blood pressure measurements in a population study of 60-year-old men and women. J. Chronic. Dis. 1981, 34, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Degischer, S.; Labs, K.H.; Hochstrasser, J.; Aschwanden, M.; Tschoepl, M.; Jaeger, K.A. Physical training for intermittent claudication: A comparison of structured rehabilitation versus home-based training. Vasc. Med. 2002, 7, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Prevost, A.; Lafitte, M.; Pucheu, Y.; Couffinhal, T.; On behalf the CEPTA educational team. Education and home based training for intermittent claudication: Functional effects and quality of life. Eur. J. Prev. Cardiol. 2015, 22, 373–379. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Liu, K.; Guralnik, J.M.; Criqui, M.H.; Spring, B.; Tian, L.; Domanchuk, K.; Ferrucci, L.; Lloyd-Jones, D.; Kibbe, M.; et al. Home-based walking exercise intervention in peripheral artery disease: A randomized clinical trial. JAMA 2013, 310, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.O.; Lee, D.Y.; Shin, W.S. The effects of a warm whirlpool bath on pain and stiffness of patients with chronic stroke induced knee osteoarthritis. J. Phys. Ther. Sci. 2013, 25, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Akerman, A.P.; Thomas, K.N.; van Rij, A.M.; Body, E.D.; Alfadhel, M.; Cotter, J.D. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: A 12-wk randomized, controlled trial. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1495–H1506. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kwak, Y.-S.; Pekas, E.J. Impacts of aquatic walking on arterial stiffness, exercise tolerance, and physical function in patients with peripheral artery disease: A randomized clinical trial. J. Appl. Physiol. 2019, 127, 940–949. [Google Scholar] [CrossRef]
- Rościszewski, P.; Koziarska-Rościszewska, M. Inability to work in patients with lower limbs ischemia. Med. Certif. 2009, 6, 48–66. [Google Scholar]
- Barcroft, H.; Dornhorst, A.C. The blood flow through the human calf during rhythmic exercise. J. Physiol. 1949, 109, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Reading, J.; Goodman, J.; Plyley, M.J.; McLaughlin, P.R.; Liu, P.P.; Floras, P.; Shephard, R.J. Design of a calf muscle ergometer for the study of local muscle blood flow. Can. J. Sport. Sci. 1992, 17, 91–93. [Google Scholar] [PubMed]
- Goodman, J.M.; Pallandi, D.V.; Reading, J.R.; Plyley, M.J.; Liu, P.P.; Kavanagh, T. Central and peripheral adaptations after 12 weeks of exercise training in post-coronary artery bypass surgery patients. J. Cardiopulm. Rehabil. 1999, 19, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.W.; Mac Gabhann, F.; Popel, A.S. Skeletal Muscle VEGF gradients in peripheral arterial disease: Simulations of rest and exercise. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3740–H3749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Blum, N.; Blum, A. Beneficial effects of sauna bathing for heart failure patients. Exp. Clin. Cardiol. 2007, 12, 29–32. [Google Scholar] [PubMed]
- Migdalski, A.; Ciecierski, M.; Jawień, A. Physiology and pathophysiology of venous outflow. Przew. Lek. 2004, 8, 33–35. [Google Scholar]
- Maiorana, A.; O’Driscoll, G.; Taylor, R.; Green, D. Exercise and the Nitric Oxide Vasodilator System. Sports Med. 2003, 33, 1013–1035. [Google Scholar] [CrossRef] [PubMed]
- Sarelius, I.; Pohl, U. Control of muscle blood flow during exercise: Local factors and integrative mechanisms. Acta Physiol. 2010, 199, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; de Cossart, L.; Edwards, P.R. Exercise training and peripheral vascular disease. Br. J. Surg. 2000, 87, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Frans, F.A.; Bipat, S.; Reekers, J.A.; Legemate, D.A.; Koelemay, M.J. Systematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudication. Br. J. Surg. 2012, 99, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Mazari, F.A.; Khan, J.A.; Carradice, D.; Samuel, N.; Abdul Rahman, M.N.; Gulati, S.; Lee, H.L.; Mehta, T.A.; McCollum, P.T.; Chetter, I.C. Randomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial disease. Br. J. Surg. 2012, 99, 39–48. [Google Scholar] [CrossRef] [PubMed]


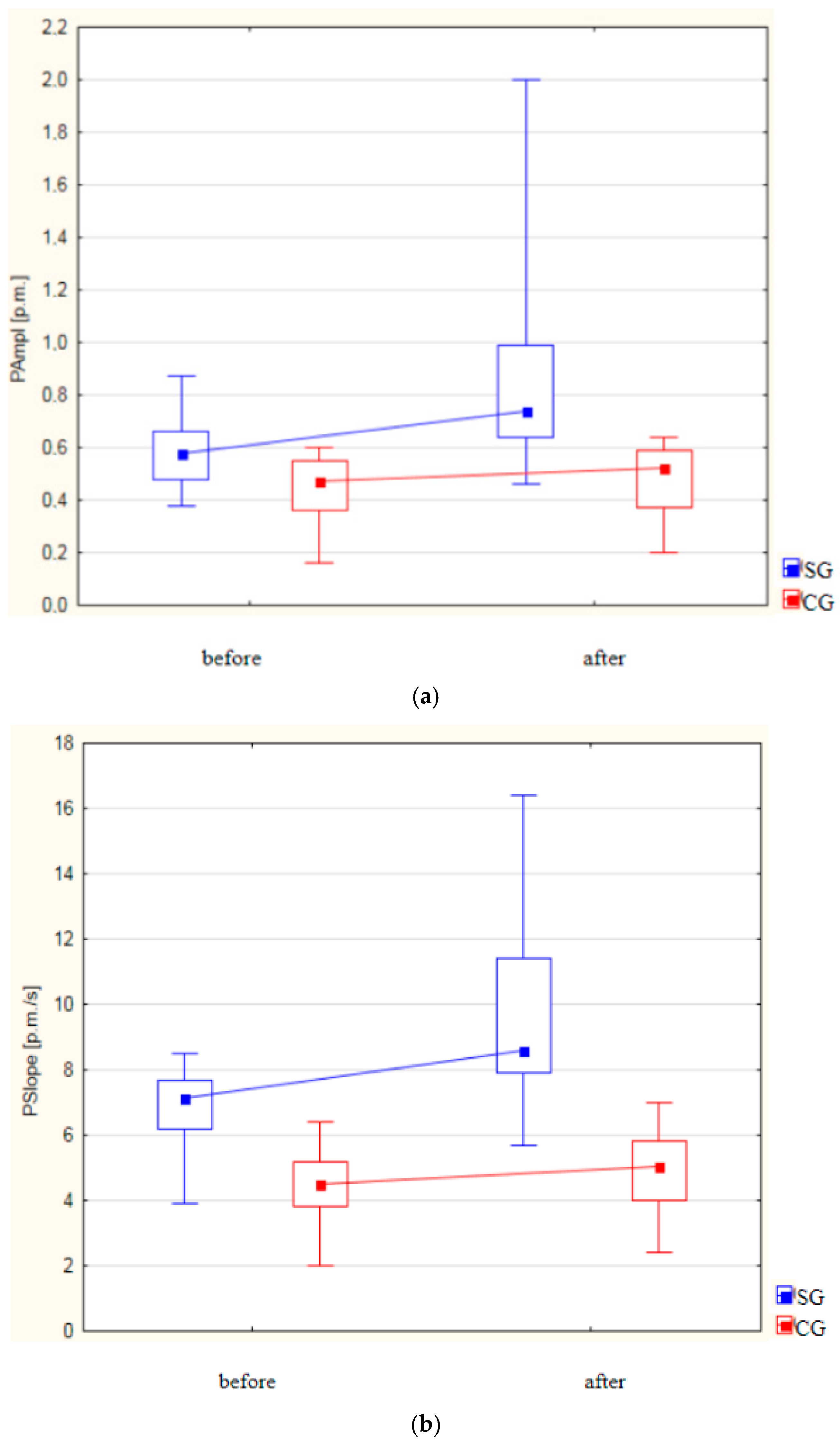
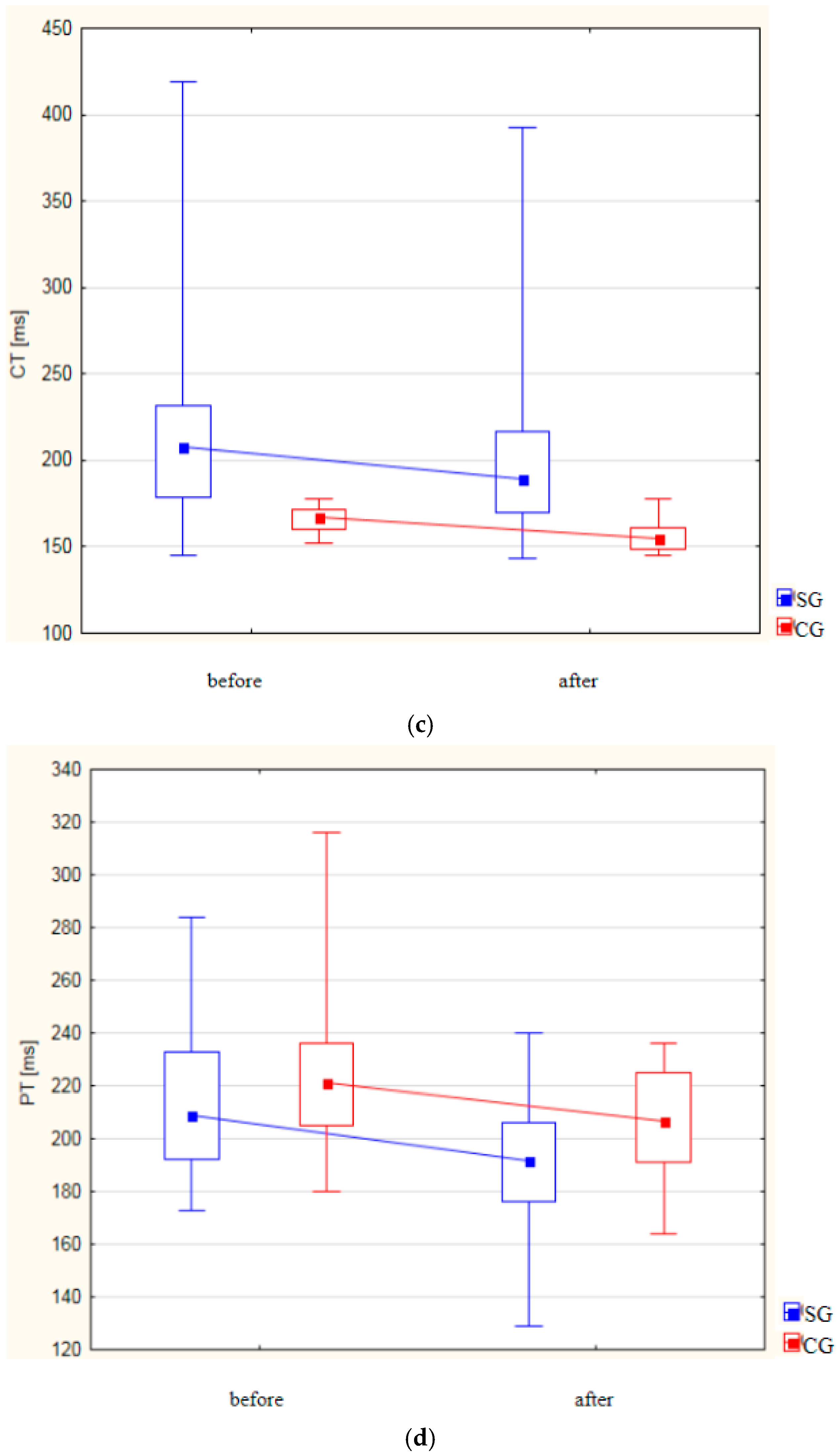
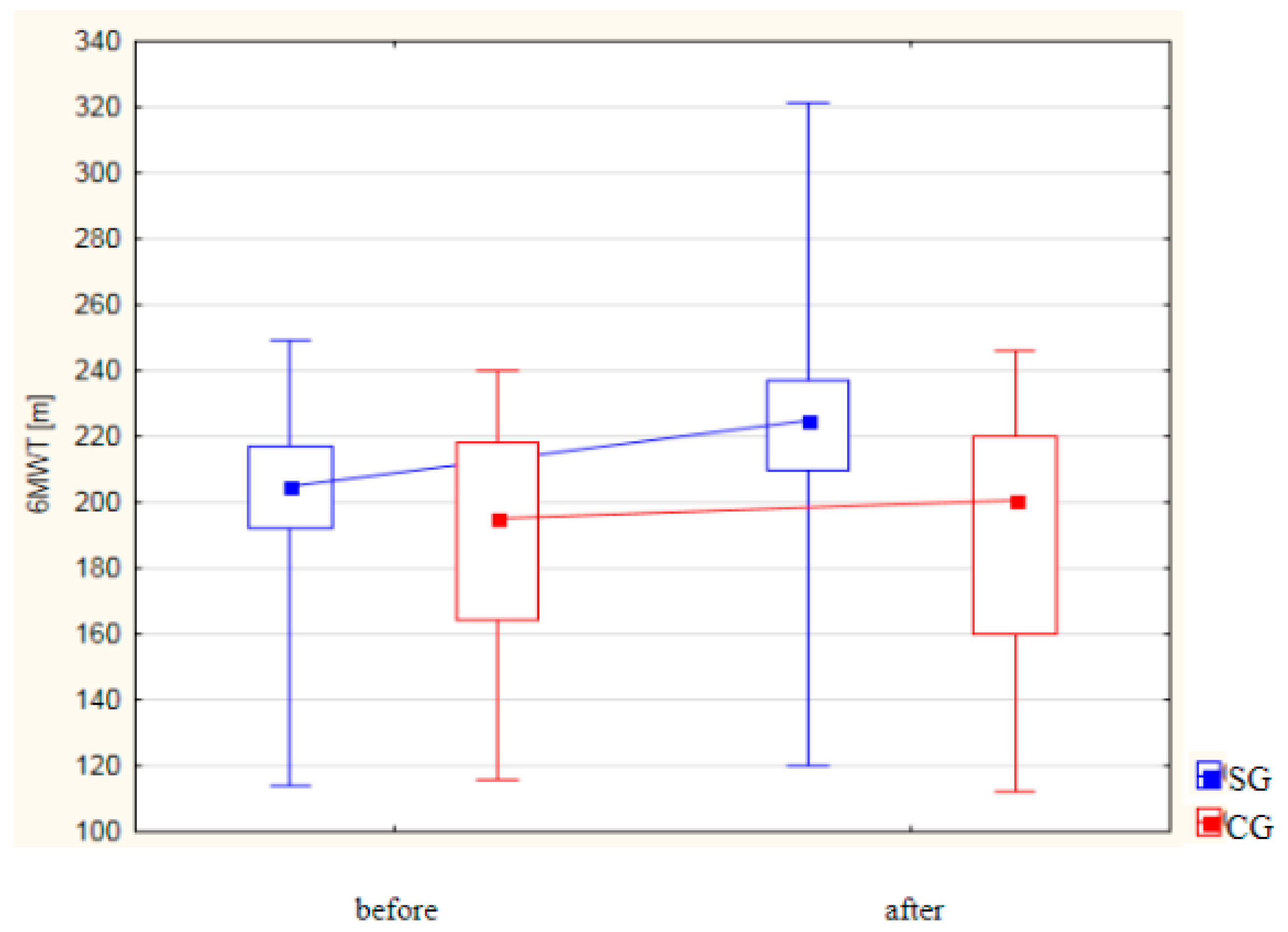
| Variable | Group I | p-Value | Group II | p-Value | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| PAmpl [‰] | ||||||
| Mean (±SD) | 0.57 ± 0.11 | 0.85 ± 0.32 | p < 0.001 | 0.44 ± 0.13 | 0.470 ± 0.14 | p = 0.005 |
| Median Me (IQR) | 0.58 (0.48–0.66) | 0.74 (0.64–0.99) | 0.47 (0.36–0.55) | 0.53 (0.37–0.59) | ||
| PSlope [‰/sek] | ||||||
| Mean (±SD) | 6.86 ± 1.05 | 9.84 ± 2.67 | p < 0.001 | 4.42 ± 1.05 | 4.99 ± 1.13 | p < 0.001 |
| Median Me (IQR) | 7.15 (6.20–7.70) | 8.60 (7.90–11.40) | 4.50 (3.80–5.20) | 5.05 (4.00–5.80) | ||
| CT [ms] | ||||||
| Mean (±SD) | 221.64 ± 61.35 | 203.08 ± 54.44 | p < 0.001 | 166.22 ± 6.88 | 156.76 ± 8.68 | p < 0.001 |
| Median Me (IQR) | 208.00 (179.00–232.00) | 189.50 (170.00–217.00) | 167.00 (160.00–172.00) | 154.50 (149.00–161.00) | ||
| PT [ms] | ||||||
| Mean (±SD) | 213.66 ± 26.77 | 192.92 ± 20.96 | p < 0.001 | 223.88 ± 24.89 | 207.02 ± 20.19 | p < 0.001 |
| Median Me (IQR) | 209.00 (192.000–233.00) | 191.50 (176.00–206.00) | 221.00 (205.00–236.00) | 206.50 (191.00–225.00) | ||
| 6MWT [m] | ||||||
| Mean (±SD) | 201.76 ± 26.39 | 224.42 ± 37.01 | p < 0.001 | 188.42 ± 35.32 | 191.04 ± 37.83 | p = 0.980 |
| Median Me (IQR) | 205.00 (192.00–217.00) | 225.00 (210.00–237.00) | 195.00 (164.00–218.00) | 200.50 (160.00–220.00) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusta, J.; Kapusta, A.; Babicki, M.; Irzmański, R. Evaluation of Peripheral Circulatory Changes Following Hydrotherapy and Controlled Physical Training in Patients with Atherosclerotic Lower Limb Ischemia. Life 2024, 14, 1578. https://doi.org/10.3390/life14121578
Kapusta J, Kapusta A, Babicki M, Irzmański R. Evaluation of Peripheral Circulatory Changes Following Hydrotherapy and Controlled Physical Training in Patients with Atherosclerotic Lower Limb Ischemia. Life. 2024; 14(12):1578. https://doi.org/10.3390/life14121578
Chicago/Turabian StyleKapusta, Joanna, Anna Kapusta, Mateusz Babicki, and Robert Irzmański. 2024. "Evaluation of Peripheral Circulatory Changes Following Hydrotherapy and Controlled Physical Training in Patients with Atherosclerotic Lower Limb Ischemia" Life 14, no. 12: 1578. https://doi.org/10.3390/life14121578
APA StyleKapusta, J., Kapusta, A., Babicki, M., & Irzmański, R. (2024). Evaluation of Peripheral Circulatory Changes Following Hydrotherapy and Controlled Physical Training in Patients with Atherosclerotic Lower Limb Ischemia. Life, 14(12), 1578. https://doi.org/10.3390/life14121578






