Abstract
(1) Background: This systematic review explores the bioactive properties of Punica granatum (pomegranate) and its potential applications in the prevention and treatment of gingivitis, periodontitis, and other oral diseases. (2) Methods: A comprehensive literature search was conducted using PubMed and Google Scholar, focusing on pomegranate and oral diseases. Inclusion criteria included studies evaluating the effects of pomegranate on oral health, while exclusion criteria eliminated non-peer-reviewed and non-English articles. This review aims to assess the efficacy of pomegranate extracts as a natural alternative to synthetic pharmaceuticals in oral health care. A structured search strategy included key terms such as “pomegranate”, “oral health”, “gingivitis”, and “periodontitis”. A total of 125 relevant references were reviewed to identify the most pertinent findings. (3) Results: The results indicate that pomegranate extracts have demonstrated efficacy in reducing plaque, inhibiting harmful oral microorganisms, and promoting overall oral health. Furthermore, clinical studies highlight the potential of pomegranate-based products, such as mouthwashes and gels, as viable alternatives to conventional pharmaceuticals, particularly in resource-limited settings. However, the review also notes the need for further research, particularly in the form of clinical trials, to establish optimal formulations and long-term safety. (4) Conclusions: Pomegranate presents a promising, natural solution for preventing and treating gingivitis and periodontitis. Further studies should focus on long-term effects and clinical efficacy.
Keywords:
Punica granatum; pomegranate; gingivitis; periodontitis; oral health; antibacterial; antioxidant; inflammation 1. Introduction
Nutraceuticals, as defined by Dr. Stephen DeFelice, are food products that provide health benefits, including disease prevention or treatment. With growing concerns over antimicrobial resistance and side effects associated with synthetic treatments, natural alternatives such as pomegranate (Punica granatum) are becoming increasingly attractive for oral health care. Pomegranate has been traditionally used in Middle Eastern and Indian medicine, offering antibacterial, anti-inflammatory, and antioxidant properties. This review aims to evaluate the potential of pomegranate extracts in treating gingivitis and periodontitis [,]. These products encompass a broad range of categories, including herbal products, dietary supplements, processed foods, isolated nutrients, specific diets, and genetically modified “designer” foods [,].
Nutraceuticals have been studied extensively across various medical fields, with increasing interest in their application for dental and oral health issues [,]. They consist of biologically active molecules derived from food, which function similarly to both nutrients and pharmaceuticals [,]. Due to their diverse bioactive components, nutraceuticals offer significant medicinal value with minimal side effects []. In contrast, the overuse and misuse of synthetic microbial agents and antibiotics have led to antimicrobial resistance and the emergence of infections previously considered rare. Natural phytochemicals have demonstrated promise as effective alternatives to synthetic agents [].
Several natural plant extracts, such as Curcuma zedoaria, Calendula officinalis, Aloe vera, Origanum vulgare, and Magnolia officinalis, have been shown to be effective in the prevention or treatment of oral diseases [,,]. The ethno-medical history of pomegranates (Punica granatum) positions them as one of the most researched medicinal plants, with growing interest in their use in dental phytotherapy [].
Punica granatum, commonly known as pomegranate, belongs to the Punicaceae family []. The genus name, Punica, originates from the Roman name for Carthage, while the name “pomegranate” is derived from the Latin words “pomum” (apple) and “granatus” (seeded) []. A specific subspecies, Punica granatum var. pleniflora (also known as golnaar), is notable for being the male version of the plant, which produces flowers but no fruit [].
Some of the most well-known pomegranate varieties include Wonderful from the United States, Hicanzar from Turkey, Acco from Israel, Bagua from India, Valenciana and Mollar del Elche from Spain, as well as a dwarf variety, Punica granatum Nana, often used as an ornamental plant [].
Pomegranates come from a large shrub, reaching heights of 3–5 m, with many spiny branches and glossy, lance-shaped leaves. As the tree matures, its bark turns gray. The flowers are large and can be red, white, or variegated, with a tubular calyx that eventually develops into fruit. The ripe fruit is roughly five centimeters in diameter, with a deep red skin and a pointed calyx. It contains numerous seeds, each surrounded by tart, red juice and separated by a white, membranous pericarp [].
Native to Asia, various parts of the pomegranate shrub have been used traditionally for their anti-inflammatory, astringent, and hemostatic properties []. In the traditional medicine of the Middle East and India, pomegranate has been used for centuries as a medicinal fruit, a source of nutrition, and in cosmetics []. The tree was first domesticated in ancient Mediterranean regions characterized by mild winters and hot, dry summers, which are ideal for its growth []. Known as the “jewel of winter”, the pomegranate has been lauded for its disease-fighting abilities, largely attributed to its potent antioxidant properties []. It has a significant history in Persian medicine and is frequently referenced in historical medical and pharmaceutical literature [].
The goal of this review is to assess the efficacy of Punica granatum as a natural alternative to synthetic pharmaceuticals in oral health care. Hypothesis: Pomegranate-based products will demonstrate significant antibacterial and anti-inflammatory effects on oral diseases such as gingivitis and periodontitis. Null hypothesis: Pomegranate extracts will not show a significant difference in treating oral diseases compared to synthetic treatments.
2. Materials and Methods
Review Type: Systematic review based on a structured search strategy.
Search Strategy: A literature search was conducted in PubMed and Google Scholar using combinations of terms like “pomegranate”, “Punica granatum”, “oral diseases”, “gingivitis”, “periodontitis”, and “antibacterial effects”. Inclusion criteria were peer-reviewed studies focused on pomegranate and oral diseases, published in English, and involving in vitro, in vivo, or clinical trials. Exclusion criteria (Table 1) removed non-peer-reviewed studies, articles not related to oral health, and non-English publications.

Table 1.
Inclusion and exclusion criteria for study selection.
Data Extraction: Data on the antibacterial, anti-inflammatory, and antioxidant properties of Punica granatum, as well as its efficacy in treating gingivitis and periodontitis, were extracted.
Review Process: PRISMA guidelines were followed, and the search process is outlined in Figure 1.
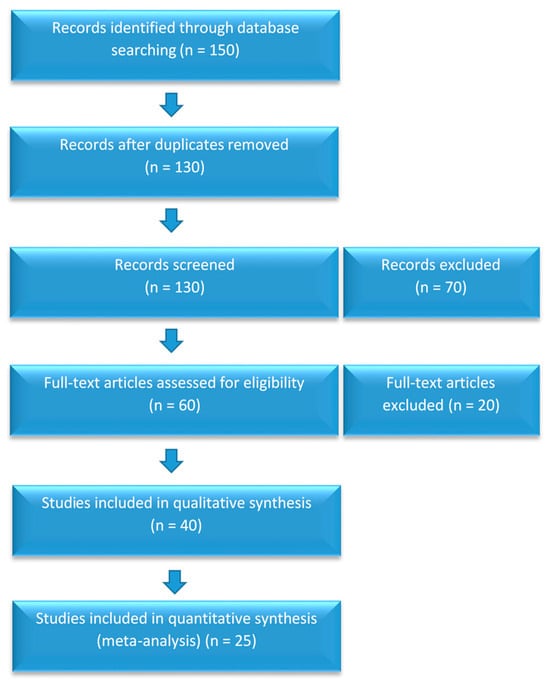
Figure 1.
PRISMA flowchart of the study selection process.
2.1. Materials
Source of Nutraceuticals: Various parts of the pomegranate plant, including seeds, peels, and arils, contain significant bioactive components. The peel comprises 50% of the fruit’s weight and is rich in flavonoids, ellagitannins, proanthocyanidins, and minerals like potassium and iron []. The seeds are composed of water, sugars, vitamins (C, A, B), organic acids, and lipids [,].
2.2. Methods of Preparation and Consumption of Punica granatum
Pomegranate Juice: Known for its high vitamin C content, pomegranate juice contains phenolic compounds [], such as punicalagin [,], and significant amounts of potassium, calcium [], and antioxidants []. The juice is primarily composed of sugars, organic acids, polyphenols, and tannins [,], which contribute to its strong antioxidant properties [,].
Pomegranate Peels: Representing 43–60% of the fruit [,], the peels are rich in bioactive compounds like phenolics, flavonoids, and tannins. Traditionally used to treat ulcers and diarrhea [], pomegranate peels have also shown anti-cancer [,,] and anti-inflammatory effects [,,,,,].
Pomegranate Seeds: The seeds are known for their antimicrobial, anti-cancer, and antioxidant properties [,,], containing anthocyanins, tannins [,,,,], fatty acids, and phytochemical compounds like lignans and sterols [,,,].
Pomegranate Extract: The extract is obtained by processing the peels and seeds, yielding standardized components such as punicalagin, ellagic acid, and polyphenols [,]. These extracts are recognized for their high antioxidant capacity, exceeding that of green tea and red wine [].
3. Functional Components of Punica granatum
Each part of the pomegranate plant, including the bark, seeds, flowers, and peels, offers unique health benefits, firmly establishing it as a significant nutraceutical food. The most prominent product derived from pomegranate is the juice, which is rich in polyphenols, vitamins, and minerals, contributing to its high antioxidant capacity. Pomegranate peel extracts have traditionally been used to treat ulcers and diarrhea, and recent studies highlight their anti-cancer and anti-inflammatory properties. The content of polyphenols and tannins of Punica granatum is presented in Figure 2.
3.1. Polyphenols
Punica granatum has a high polyphenol content that contributes to its antioxidant activity, as demonstrated in vitro. However, the bioactive substances responsible for antioxidant activity in vivo may differ from those present in the whole fruit, as these compounds are metabolized during digestion, resulting in the production of ellagic acid and urolithins. The health benefits of pomegranate are also attributed to anthocyanins and the unique fatty acid profile of pomegranate seed oil [].
3.2. Flavonoids
Pomegranate is particularly rich in flavonoids, which are the primary polyphenols in the fruit [,,]. These compounds have anti-inflammatory properties that promote good oral health, particularly by helping to prevent gingivitis []. The pericarp (peel) contains flavones, flavanones, flavanols, and tannins (such as punicalagin) that exhibit anti-inflammatory, anti-mutagenic, and antifungal activity []. Punicalagin, in particular, is an antioxidant that is three times more potent than those found in red wine or green tea [].
3.3. Anthocyanins and Anthocyanidins
Anthocyanins are a class of flavonoids that serve as the main pigments in the plant kingdom. Their colors range from pink to red, purple, and blue, depending on the pH. Beyond their use as natural food colorants, anthocyanins have been widely studied for their potential health benefits, particularly in the prevention of cardiovascular, neurological, and other chronic diseases due to their antioxidant and anti-inflammatory activities [,,].
Anthocyanidins in red fruits contribute to antioxidant activity, giving pomegranate juice superior bioactivity compared to purified polyphenols []. Pigmented fruits and vegetables, such as pomegranates, dark grapes, berries, and eggplants, are rich in delphinidin, a powerful antioxidant []. Bertuglia et al. described the mechanism by which delphinidin prevents endothelial dysfunction induced by oxidative stress in vivo []. Additionally, cyanidin and its glycosides, another type of anthocyanin, are commonly consumed through red wine, fruits, and vegetables, indicating a substantial daily intake of these compounds []. Another flavonoid, pelargonidin, is found in large quantities in raspberries, currants, and blueberries [].
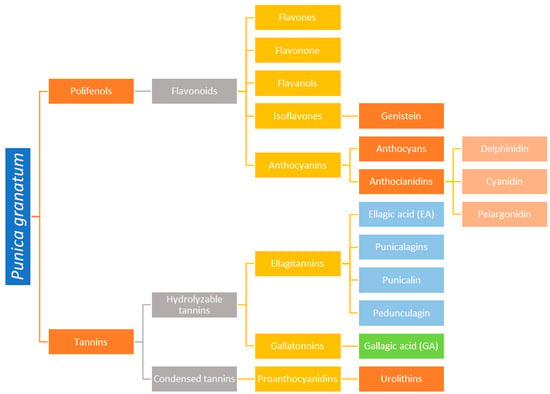
Figure 2.
The content of polyphenols and tannins of Punica granatum.
3.4. Fatty Acids
Pomegranate seeds contain a variety of fatty acids, comprising 12–20% of their total dry weight. Among these, alpha-linolenic acid (omega-3), linoleic acid (omega-6), and oleic acid (omega-9) are present in significant amounts, along with stearic acid, which can help lower cholesterol levels, and palmitic acid [] (Figure 3). The seeds are also rich in proteins, crude fibers, vitamins, minerals, pectins, sugars, polyphenols, isoflavones (such as genistein), coumestrol, and steroids (such as estrone) [,]. Due to these bioactive components, pomegranate seeds are used in the treatment of heart diseases, diabetes, obesity, cognitive imbalance, and cancer, as well as to improve male fertility [,,].
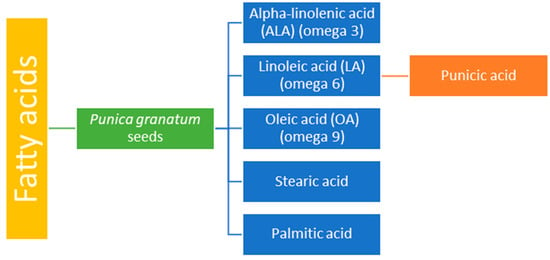
Figure 3.
Fatty acid content of Punica granatum seeds.
Pomegranate seed oil is primarily composed of punicic acid and sterols, which possess nephroprotective properties [,]. Punicic acid, the main fatty acid in pomegranate, is a type of conjugated linoleic acid that has biological effects such as supporting weight loss and controlling diabetes. It may also help reduce the risk of breast cancer and prostate cancer [].
3.5. Tannins
The tannins present in pomegranate are classified into two types: hydrolyzable tannins and condensed tannins [,,]. Hydrolyzable tannins can be further divided into gallotannins, which hydrolyze to produce sugar and gallic acid, and ellagitannins, which hydrolyze to produce ellagic acid in addition to sugar and gallic acid [,]. Hydrolyzable tannins such as punicalagin, pedunculagin, and punicalin, along with glucose esters of gallic acid and ellagic acid, contribute significantly to the antioxidant activity of the whole fruit [,].
The primary hydrolyzable tannin present in pomegranate is punicalagin, while other hydrolyzable tannins include ellagitannins and gallotannins [,,,]. Research has shown that hydrolyzable tannins and polyphenols in pomegranate extract, particularly punicalagin and gallic acid, are likely responsible for the fruit’s antibacterial activity []. The antimicrobial activity of tannins is believed to be linked to their molecular structure and toxicity to bacteria. Tannins can damage bacterial cell walls and membranes [,].
In pomegranate leaves, major constituents include tannins such as punicalin and punicafolin, as well as glycoside flavones like luteolin and apigenin []. The leaves are known for their excellent antioxidant properties [].
3.6. Triterpenoids
Pomegranate flowers contain triterpenoids such as ursolic acid, oleic acid, and asiatic acid (Figure 4), which possess antioxidant and hepatoprotective properties. These compounds are also used as a remedy for diabetes mellitus [,].
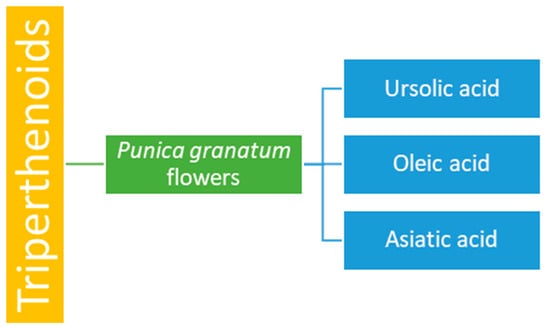
Figure 4.
Triterpenoid content of Punica granatum flowers.
Ellagitannins and piperidine alkaloids are present in the roots and bark of pomegranate shrubs. The bark exhibits molluscicidal properties [], and both the bark and roots are known for their anthelmintic and vermifuge properties []. The most beneficial components of pomegranate include ellagitannins, punicic acid, flavonoids, anthocyanidins, anthocyanins, and estrogenic flavones [,].
4. Major Active Substances of Punica granatum
The bioactive compounds responsible for Punica granatum’s health benefits include punicalagin, punicalin, ellagic acid (EA), and delphinidin, presented in Figure 5. These substances provide strong antioxidant, anti-inflammatory, and antimicrobial properties, making them crucial to the plant’s therapeutic potential. Punicalagin and punicalin are powerful polyphenolic compounds known for their strong antioxidant and anti-inflammatory properties. Ellagic acid is another potent antioxidant, recognized for its role in protecting cells from oxidative stress and reducing the risk of certain chronic diseases. Delphinidin, a type of anthocyanin, is responsible for the vibrant color of pomegranate and possesses antioxidant and anti-inflammatory effects, further enhancing the fruit’s therapeutic potential [,,,].
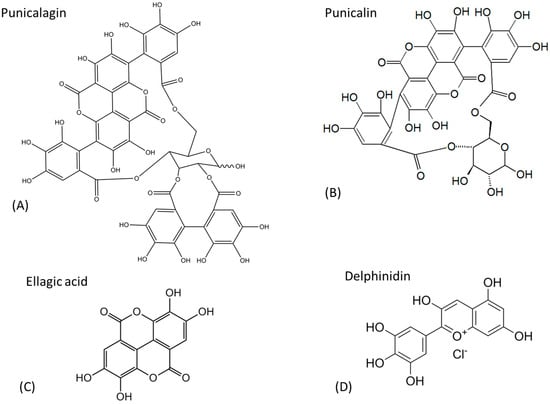
Figure 5.
Chemical structures of the major active substances in Punica granatum.
5. Therapeutic Effects of Punica granatum
5.1. Punica granatum in Diseases of the Oral Cavity
5.1.1. Antibacterial Effects of Punica granatum
In dentistry, the antibacterial effects of pomegranate against Streptococcus mutans and Streptococcus sanguinis have been studied in vitro [,]. El-Sharkawy et al. (2019) evaluated the antibacterial activity of pomegranate extract against Streptococcus mutans and demonstrated that mouthwash containing pomegranate peel and juice was an effective antimicrobial agent. It significantly reduced the total number of bacteria in the saliva of children included in the study, comparable to the effect of a standard antiseptic (chlorhexidine 0.2%) [].
DiSilvestro et al. (2009) showed that a pomegranate-based mouthwash reduced the number of microorganisms in bacterial dental plaque by approximately 84% []. In another study, the effectiveness of a pomegranate mouthwash was compared to a 0.2% chlorhexidine mouthwash in diabetic patients with gingivitis. After 21 days of use, both groups showed significant reductions in plaque and gingivitis indices, as well as probing bleeding []. According to Jacob et al. (2021), pomegranate extract mouthwash was less effective against Streptococcus mutans compared to chlorhexidine. However, it proved to be a natural and ecological alternative, effectively disrupting the activity of all tested microorganisms in their triple-blind randomized clinical trial focused on Lactobacilli and Veillonella [].
Other studies, such as those by Kote et al. (2011), have demonstrated the effectiveness of pomegranate-containing mouthwashes against plaque microorganisms by significantly reducing the colonies of Lactobacilli (46%) and streptococci (23%) []. During a 24-h incubation period, Punica granatum gel, equivalent to 0.234% punicalagin, inhibited S. mutans and S. sanguinis but not L. casei, suggesting its potential use in the prevention of dental caries, as indicated by the other results [].
Pomegranate juice has also been shown to be effective against bacterial dental plaque microorganisms, reducing colony-forming units (CFU). This was demonstrated by a significant reduction in the level of dental plaque microorganisms after rinsing with pomegranate juice []. The hydroalcoholic extract of the fruit similarly acts on bacterial dental plaque microorganisms, reducing the microbial load []. Another study examined the effects of rinsing the mouth with PomElla® pomegranate extract in young adults, finding that the treatment favorably modulated salivary values, thus supporting oral health and preventing gingivitis [].
Ghazi et al. (2020) evaluated the effect of pomegranate extract in mouthwash as a treatment for gingivitis in patients with type 2 diabetes. Both biochemical parameters (IL-1β, salivary AST) and clinical parameters (PI, GI, BOP) in the three groups included in the study were significantly reduced after 14 days of treatment. However, salivary IL-1β in the group that used chlorhexidine mouthwash (0.12%) did not show a significant reduction compared to the group that used pomegranate peel extract (6.25%) and the group with pomegranate peels and arils extract (12.5%) [].
However, the antibacterial effects of pomegranate against periodontogenic microbial species have not been conclusively demonstrated, according to Laleman et al. (2020) []. In a clinical trial by Kiany et al. (2016), pomegranate mouthwash was found to be as effective against plaque as Persica and Matrica mouthwashes, two herbal mouthwashes commonly used in Iran, made from Salvadora persica extract and Matricaria chamomilla, respectively [].
5.1.2. Punica granatum and Gingivitis
Several clinical trials suggest that pomegranate extract significantly reduces plaque and gingival inflammation. Punica granatum extract has shown significant improvements in patients with gingivitis, reducing plaque index and bleeding on probing. Clinical trials have shown comparable effectiveness to standard antiseptic mouthwashes. Pomegranate mouthwash improved gingival health, comparable to conventional mouthwashes like chlorhexidine. The results obtained by Pasupuleti et al. (2023) suggest that pomegranate fruit and pomegranate peel extract may serve as promising alternatives in the management of chronic gingivitis and oral ulcers as adjuncts to scaling and root planing (SRP) procedures. They have the potential to be competitive alternatives to modern pharmaceutical products []. Eltay et al. (2021) demonstrated the adjuvant effect of a pulsatile oral spray containing 5% pomegranate extract. The irrigation solution effectively reduced plaque index (PI), gingival index (GI), and interleukin-1β (IL-1β) values in patients with chronic gingivitis []. Pomegranate mouthwash has been shown to improve gingival health by reducing the PI (Tureskey–Gilmore–Glickman modification of the Quigley–Hein Index) and bleeding on probing (BOP) indices, with effects comparable to those of two commonly used herbal mouthwashes [].
5.1.3. Punica granatum and Periodontitis
Pomegranate extracts used as adjuncts to scaling and root planing (SRP) were shown to reduce inflammatory markers and improve periodontal health. In patients with periodontitis, pomegranate extract combined with conventional scaling and root planing has demonstrated reductions in inflammatory markers and clinical symptoms. Sastravaha et al. (2003) demonstrated that clinical symptoms of chronic periodontitis were reduced in 20 patients following scaling and root planing, combined with the subgingival delivery of biodegradable chips containing Centella asiatica and Punica granatum []. Another study comparing adjunctive topical treatment with extracts of Centella asiatica and Punica granatum to standard supportive periodontal therapy revealed significant improvements in interleukin-1 levels and clinical parameters of chronic periodontitis in 15 patients []. In 2005, Sastravaha et al. presented the efficacy of a toothpaste containing pomegranate extracts as a complementary treatment to routine periodontal therapies, demonstrating in vitro that pomegranate flavonoids have antibacterial properties against microorganisms responsible for gingivitis [].
5.1.4. Punica granatum and Stomatitis
Pomegranate-based gels have shown accelerated healing in patients with aphthous stomatitis. In one study, pomegranate gel significantly reduced pain and healing time compared to placebo. Tavangar et al. (2019) clinically evaluated the effects of a mucoadhesive pomegranate gel administered to 60 patients with moderate aphthous stomatitis. They showed that, compared to the groups receiving Triadent product and placebo gel three times a day, local pain in stomatitis subsided more rapidly, and healing was accelerated []. Ghalayani et al. (2013) obtained similar results by administering a 10% pomegranate gel to patients with recurrent aphthous stomatitis (RAS), significantly reducing pain relief time, time to complete healing, and the “visual analog scale” score [].
Pomegranate peel gel, used in another clinical trial, had comparable effects in patients with RAS, reducing pain, ulcer size, and healing time []. Alcoholic and aqueous extracts of Punica granatum var. pleniflora also reduced the total duration of complete treatment []. An in vitro study conducted by Abdollahzadeh et al. (2011) showed that methanolic pomegranate extract (MEPGP) could be used to control common oral pathogens responsible for caries, stomatitis, and periodontal diseases. However, further phytochemical studies are needed to identify the specific antibacterial compounds in pomegranate involved in these effects [].
Several authors have investigated the effects of pomegranate gel in prosthetic stomatitis [,,]. César et al. (2003) observed a reduction in prosthetic stomatitis caused by candidiasis in 76.7% of patients treated with Punica granatum Linne gel three times a day for 15 days, highlighting its antifungal effects []. Periodontal gel with pomegranate peel extract appears to be as effective as miconazole gel, a topical antifungal agent [].
Vanconcelos et al. (2003) conducted an in vivo study using pomegranate gel as an antifungal agent against candidiasis associated with dental stomatitis and found that symptoms were resolved and overall oral health was improved []. In 2006, Vanconcelos et al. investigated the antimicrobial effect of pomegranate gel against Streptococcus mutans, Streptococcus mitis, and Candida albicans and found that pomegranate gel effectively inhibited microbial adhesion []. The findings suggest that pomegranate gel could be used to control the adhesion of various microorganisms in the oral cavity responsible for dental caries, stomatitis, and periodontal disease.
The pomegranate fruit, referred to by Jurenka et al. (2008) as “a pharmacy in itself”, exhibits numerous medicinal properties, including antifungal, antiviral, immunomodulatory, bactericidal, astringent, diuretic, and vermifuge effects []. Numerous studies have demonstrated that pomegranate extracts or the fruit itself can effectively manage inflammation []. Punica granatum has been widely used in the treatment of cardiovascular diseases (hypertension, atherosclerosis), metabolic disorders (diabetes, obesity, hyperlipidemia), respiratory conditions (asthma, bronchitis, cough), and dental issues (coagulation disorders, dental stomatitis) [,,,,].
The recent development of phytotherapy in dentistry has made it possible to use the medicinal properties of plants and herbs to treat a variety of oral conditions. This alternative, natural, safe, and affordable therapy offers an effective substitute for synthetic drugs [].
5.1.5. Punica granatum Mouthwash
Studies demonstrate that Punica granatum mouthwashes are highly effective in reducing bacterial load and plaque formation. For instance, pomegranate-based mouthwashes reduce bacterial plaque by 84%, similar to the effects of chlorhexidine, a standard antiseptic.
The long-term use of chemical and pharmaceutical preparations is known to pose health risks. Therefore, it is sensible to seek more plant-based alternatives. Pomegranate extract mouthwash is gaining popularity as a viable option, especially in rural areas and less developed countries, where access to conventional oral care products may be limited [,].
- -
- Punica granatum gels
- -
- Punica granatum toothpaste
- -
- Other pharmaceutical forms of Punica granatum
Recently, Gawor et al. (2023) evaluated the effectiveness of a pomegranate additive in water to limit the accumulation of bacterial plaque and tartar in dogs. Their results showed, for the first time, that this additive reduces bacterial deposits and improves gingival health, recommending the tested product for home use due to its time-saving and convenient properties []. A previous study by Silva et al. (2020), also conducted in dogs, formulated and evaluated a mucoadhesive oral ointment (orabase) containing pomegranate peel extract as an adjuvant for oral hygiene. The results suggest that the product derived from pomegranate peel extract is a viable option for improving oral hygiene, helping to reduce the bacterial component of dental plaque in experimental animals []. An overview of the studies reviewed is presented in Table 2.

Table 2.
Overview of studies investigating the effects of Punica granatum in various pharmaceutical forms for oral health.
5.1.6. Other Health Benefits of Punica granatum in Comorbidities
Beyond oral health, pomegranate has demonstrated potential benefits for cardiovascular health, diabetes management, and cancer prevention. Traditional uses of pomegranate bark in treating diarrhea, inflammation, and parasitic diseases are well documented. Pomegranate has been formulated into various products such as mouthwashes, gels, and toothpastes, which show effectiveness in maintaining oral hygiene and reducing microbial loads.
5.2. Antioxidant Effects of Punica granatum
Pomegranate contains powerful antioxidants, including polyphenols, hydrolyzable tannins, and anthocyanins, which neutralize free radicals and protect against oxidative stress, reducing the risk of chronic diseases like cancer and cardiovascular conditions []. Studies show that pomegranate juice has a higher antioxidant capacity than green tea or red wine, largely due to its rich content of flavonoids and anthocyanidins such as delphinidin, cyanidin, and pelargonidin [,]. The antioxidant activity of pomegranate extends to scavenging hydroxyl radicals and superoxide anions [,], and methanolic extracts from the peel have demonstrated broad antioxidant potential through various assays [,]. Regular consumption of pomegranate juice has been linked to reduced oxidative stress, lowered systolic blood pressure [], and enhanced overall heart health []. Additionally, the antioxidant properties are stronger when the juice is extracted from the whole fruit, as the peel contributes to antibacterial and astringent effects beneficial for oral health [].
5.2.1. Antioxidant and Anti-Inflammatory Effects of Punica granatum
The high polyphenol and flavonoid content of pomegranate contributes to its potent antioxidant activity, which has been shown to neutralize harmful free radicals, reduce inflammation, and enhance oral tissue health. From the perspective of pomegranate, the exploration of its bioactive compounds through neural networks in otolaryngology opens up new possibilities for enhancing treatment outcomes, but challenges such as data standardization, extraction of relevant biomarkers, and integration of traditional knowledge with advanced machine learning techniques remain significant hurdles [].
5.2.2. Topical Anti-Inflammatory and Analgesic Effects of Punica granatum
In addition to its antibacterial and antioxidant properties, pomegranate extract has demonstrated topical anti-inflammatory and analgesic effects. It works by preventing leukocyte infiltration and modulating the levels of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α), according to research by Mo et al. (2013) [].
5.2.3. Anti-Hemorrhagic and Astringent Effects of Punica granatum
Kiany et al. (2016) also highlighted the astringent and anti-hemorrhagic properties of Punica granatum when used as a mouthwash. The reduction in gum bleeding was comparable to that achieved by other mouthwashes containing standard plant extracts [].
6. Discussion
In this review, we used PubMed and Google Scholar for the literature search. PubMed was selected for its comprehensive coverage of peer-reviewed biomedical studies, ensuring high-quality sources relevant to health and oral diseases. Google Scholar was included for its broader range, capturing gray literature and studies not indexed in PubMed.
While additional databases like Scopus or Web of Science could have been used, we focused on PubMed and Google Scholar to balance comprehensiveness and relevance. We acknowledge this may limit the scope, but these databases provide a strong foundation for the study’s objectives.
The review was conducted by analyzing the specialized literature on the bioactive properties of pomegranate-derived products and their effects on conditions of the oral cavity. To identify the most relevant articles, the search strategy employed a combination of keywords, including “Punica granatum” or “pomegranate”, and “oral diseases”, “gingivitis”, “periodontitis”, “stomatitis”, and “nutraceutical”. No time constraints were applied to the database search.
Compared to other natural extracts [,,], this review investigates the antibacterial, antioxidant, and revitaminizing effects of pomegranate. Periodontal diseases are correlated with numerous chronic diseases [,,] and can significantly affect the quality of life.
The findings from this review underscore the potential of Punica granatum as a viable natural alternative for treating oral diseases such as gingivitis and periodontitis. Pomegranate’s antibacterial, anti-inflammatory, and antioxidant properties offer substantial benefits for oral health, making it a promising candidate for use in dental products like mouthwashes and gels. However, the limitations of current research include a lack of standardized dosages and long-term safety data. More clinical trials are needed to determine the optimal formulations and assess potential adverse effects.
Strengths and Limitations: Despite the thorough approach, the review is constrained by its reliance on only two databases, which may have excluded relevant studies from other sources or unpublished data, potentially limiting the breadth of the findings.
This review has strengths in its comprehensive coverage of both clinical and experimental studies, but it is limited by its reliance on two databases and the exclusion of non-English studies, which may have led to missed relevant research. Future reviews should aim for a broader search strategy.
7. Conclusions
Extracts from different components of the medicinal plant Punica granatum have antibacterial, anti-inflammatory, antioxidant, and astringent properties, offering favorable potential in limiting the pathogenic oral microbiome and preventing or treating oral cavity conditions such as gingivitis, periodontitis, and stomatitis. These bioactive compounds, derived from pomegranate seeds, peels, and juice, have shown efficacy in reducing plaque, inhibiting the growth of harmful microorganisms, and promoting overall oral health. Further clinical trials and in vivo studies are needed to fully understand the optimal formulations, dosages, and long-term effects of Punica granatum in dental care. The integration of pomegranate-based products into oral hygiene practices could provide a natural, effective, and accessible alternative to synthetic pharmaceuticals, particularly in regions with limited access to conventional dental care.
Pomegranate extracts exhibit antibacterial, anti-inflammatory, antioxidant, and astringent properties, demonstrating potential in treating oral diseases such as gingivitis, periodontitis, and stomatitis. These natural bioactive compounds can effectively reduce plaque and harmful microorganisms, offering a promising alternative to synthetic pharmaceuticals. Further clinical trials are required to confirm the optimal formulations and long-term safety of pomegranate-based dental products.
Author Contributions
Conceptualization, G.I.P.C. and M.G.; methodology, L.G.V., G.C. and T.C.G.; software, N.C. and R.A.C.; validation, L.G.V. and G.C.; formal analysis, F.M.; investigation, G.I.P.C., M.G. and F.M.; resources, G.I.P.C., N.C. and R.A.C.; data curation, G.I.P.C., L.G.V. and G.C.; writing—original draft preparation, G.I.P.C. and T.C.G.; writing—review and editing, G.I.P.C., T.C.G. and F.M.; visualization, M.G.; supervision, L.G.V., G.C. and M.G.; project administration, G.I.P.C. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the University of Oradea, Oradea, Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Acknowledgments
The authors would like to thank the University of Oradea for supporting the payment of the invoice through an internal project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Kalra, E.K. Nutraceutical-definition and introduction. AAPS PharmSci 2003, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kumar, P.; Kaushik, N.; Singh, A. The potentials of nutraceuticals. Pharmainfo. Net 2008, 6, 151–183. [Google Scholar]
- Dureja, H.; Kaushik, D.; Kumar, V. Developments in nutraceuticals. Indian J. Pharmacol. 2003, 35, 363–372. [Google Scholar]
- Cenzato, N.; Khijmatgar, S.; Carloni, P.; Dongiovanni, P.; Meroni, M.; Del Fabbro, M.; Tartaglia, G.M. What is the use of nutraceuticals in dentistry? A scoping review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4899–4913. [Google Scholar] [CrossRef]
- Trifan, D.F.; Tirla, A.G.; Mos, C.; Danciu, A.; Bodog, F.; Manole, F.; Ghitea, T.C. Involvement of Vitamin D3 in the Aging Process According to Sex. Cosmetics 2023, 10, 114. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Trifan, D.F.; Tirla, A.G.; Moldovan, A.F.; Moș, C.; Bodog, F.; Maghiar, T.T.; Manole, F.; Ghitea, T.C. Can Vitamin D Levels Alter the Effectiveness of Short-Term Facelift Interventions? Healthcare 2023, 11, 1490. [Google Scholar] [CrossRef]
- Sangeetha, J.; Vijayalakshmi, K. Antimicrobial Activity of Rind Extracts of Punica granatum Linn. Bioscan 2011, 6, 119–124. [Google Scholar]
- Abdollahzadeh, S.; Mashouf, R.; Mortazavi, H.; Moghaddam, M.; Roozbahani, N.; Vahedi, M. Antibacterial and antifungal activities of Punica granatum peel extracts against oral pathogens. J. Dent. 2011, 8, 1–6. [Google Scholar]
- Somu, C.A.; Ravindra, S.; Ajith, S.; Ahamed, M.G. Efficacy of a herbal extract gel in the treatment of gingivitis: A clinical study. J. Ayurveda Integr. Med. 2012, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Cicalău, G.I.P.; Babes, P.A.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.M.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef] [PubMed]
- Iova, G.M.; Calniceanu, H.; Popa, A.; Szuhanek, C.A.; Marcu, O.; Ciavoi, G.; Scrobota, I. The antioxidant effect of curcumin and rutin on oxidative stress biomarkers in experimentally induced periodontitis in hyperglycemic Wistar rats. Molecules 2021, 26, 1332. [Google Scholar] [CrossRef]
- Eltay, E.G.; Gismalla, B.G.; Mukhtar, M.M.; Awadelkarim, M.O. Punica granatum peel extract as adjunct irrigation to nonsurgical treatment of chronic gingivitis. Complement. Ther. Clin. Pract. 2021, 43, 101383. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.D.; Maia, J.L.; Pereira, S.L.; de Lemos, T.L.; Mota, O.M. Antiplaque and antigingivitis effects of a gel containing Punica granatum Linn extract: A double-blind clinical study in humans. J. Appl. Oral Sci. 2006, 14, 162–166. [Google Scholar] [CrossRef][Green Version]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Sedigh-Rahimabadi, M.; Fani, M.; Rostami-Chijan, M.; Zarshenas, M.M.; Shams, M. A traditional mouthwash (Punica granatum var pleniflora) for controlling gingivitis of diabetic patients: A double-blind randomized controlled clinical trial. J. Evid.-Based Complement. Altern. Med. 2017, 22, 59–67. [Google Scholar] [CrossRef]
- Caruso, A.; Barbarossa, A.; Tassone, A.; Ceramella, J.; Carocci, A.; Catalano, A.; Basile, G.; Fazio, A.; Iacopetta, D.; Franchini, C.; et al. Pomegranate: Nutraceutical with Promising Benefits on Human Health. Appl. Sci. 2020, 10, 6915. [Google Scholar] [CrossRef]
- Kote, S.; Kote, S.; Nagesh, L. Effect of pomegranate juice on dental plaque microorganisms (streptococci and lactobacilli). Anc. Sci. Life 2011, 31, 49–51. [Google Scholar]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Almatroodi, S.A. Active constituents of pomegranates (Punica granatum) as potential candidates in the management of health through modulation of biological activities. Pharmacogn. J. 2017, 9, 689–695. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernandez-Lopez, J.; Perez-Alvarez, J. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Duo, L.; Wang, J.; GegenZhula; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Jiménez-Cabrera, T.; Urrutia-Hernández, T.A.; Chehue-Romero, A.; Olvera-Hernández, E.G.; Bautista, M. Punica protopunica Balf., the Forgotten Sister of the Common Pomegranate (Punica granatum L.): Features and Medicinal Properties—A Review. Plants 2020, 9, 1214. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement. Altern. Med. 2016, 16, 358. [Google Scholar] [CrossRef]
- Karimi, M.; Sadeghi, R.; Kokini, J. Pomegranate as a promising opportunity in medicine and nanotechnology. Trends Food Sci. Technol. 2017, 69, 59–73. [Google Scholar] [CrossRef]
- Jaiswal, V.; DerMarderosian, A.; Porter, J.R. Anthocyanins and polyphenol oxidase from dried arils of pomegranate (Punica granatum L.). Food Chem. 2010, 118, 11–16. [Google Scholar] [CrossRef]
- Melgarejo, P.; Salazar, D.M.; Artes, F. Organic acids and sugars composition of harvested pomegranate fruits. Eur. Food Res. Technol. 2000, 211, 185–190. [Google Scholar] [CrossRef]
- Tezcan, F.; Gültekin-Özgüven, M.; Diken, T.; Özçelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Ghosh, M. Khabaar: An Immigrant Journey of Food, Memory, and Family; University of Iowa Press: Iowa City, IA, USA, 2022. [Google Scholar]
- Bhandari, P.R. Pomegranate (Punica granatum L). Ancient seeds for modern cure? Review of potential therapeutic applications. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 171–184. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and therapeutic properties of Punica granatum phytochemicals: Possible roles in breast cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- Esther Lydia, D.; Khusro, A.; Immanuel, P.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Photo-activated synthesis and characterization of gold nanoparticles from Punica granatum L. seed oil: An assessment on antioxidant and anticancer properties for functional yoghurt nutraceuticals. J. Photochem. Photobiol. B Biol. 2020, 206, 111868. [Google Scholar] [CrossRef]
- Wong, T.L.; Strandberg, K.R.; Croley, C.R.; Fraser, S.E.; Nagulapalli Venkata, K.C.; Fimognari, C.; Sethi, G.; Bishayee, A. Pomegranate bioactive constituents target multiple oncogenic and oncosuppressive signaling for cancer prevention and intervention. Semin. Cancer Biol. 2021, 73, 265–293. [Google Scholar] [CrossRef]
- Rafiqul Islam, A.T.M.; Ferdousi, J.; Shahinozzaman, M. Previously published ethno-pharmacological reports reveal the potentiality of plants and plant-derived products used as traditional home remedies by Bangladeshi COVID-19 patients to combat SARS-CoV-2. Saudi J. Biol. Sci. 2021, 28, 6653–6673. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 351682. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Adams, J.D. Does the World Need Plant Medicines? Medicines 2018, 5, 39. [Google Scholar] [CrossRef]
- Setiadhi, R.; Sufiawati, I.; Zakiawati, D.; Nuraeny, N.; Hidayat, W.; Firman, D. Evaluation of antibacterial activity and acute toxicity of pomegranate (Punica granatum L.) seed ethanolic extracts in swiss webster mice. J. Dentomaxillofacial Sci. 2017, 2, 119–123. [Google Scholar] [CrossRef]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Al Juhaimi, F.; Özcan, M.; Ghafoor, K. Characterization of pomegranate (Punica granatum L.) seed and oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1700074. [Google Scholar] [CrossRef]
- Dheeraj Singh, D.S.; Vijay Sethi, V.S. Screening of pomegranate genotypes for the preparation of quality grade Anardana. J. Food Sci. Technol. 2003, 40, 236–238. [Google Scholar]
- El-Nemr, S.; Ismail, I.; Ragab, M. Chemical composition of juice and seeds of pomegranate fruit. Food/Nahrung 1990, 34, 601–606. [Google Scholar] [CrossRef]
- Syed, D.N.; Afaq, F.; Mukhtar, H. Pomegranate derived products for cancer chemoprevention. Semin. Cancer Biol. 2007, 17, 377–385. [Google Scholar] [CrossRef]
- Sandhya, S.; Khamrui, K.; Prasad, W.; Kumar, C. Preparation of pomegranate peel extract powder and evaluation of its effect on functional properties and shelf life of curd. LWT 2018, 92, 416–421. [Google Scholar] [CrossRef]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A review study on Punica granatum L. J. Evid.-Based Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef]
- Mohiuddin, K.; Khizer, R. “Pomegranate”—One More Herbal Agent Against Dental Plaque/Biofilm. J. PEARLDENT 2014, 5, 18–22. [Google Scholar] [CrossRef]
- Johanningsmeier, S.D.; Harris, G.K. Pomegranate as a functional food and nutraceutical source. Annu. Rev. Food Sci. Technol. 2011, 2, 181–201. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Van Elswijk, D.A.; Schobel, U.P.; Lansky, E.P.; Irth, H.; van der Greef, J. Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry 2004, 65, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Jahfar, M.; Vijayan, K.; Azadi, P. Studies on a polysaccharide from the fruit rind of Punica granatum. Res. J. Chem. Environ. 2003, 7, 43–50. [Google Scholar]
- Speranța, A. Ghid Terapeutic Naturist; Elefant Online: Bucharest, Romania, 2016. [Google Scholar]
- Mathon, C.; Chater, J.M.; Green, A.; Merhaut, D.J.; Mauk, P.A.; Preece, J.E.; Larive, C.K. Quantification of punicalagins in commercial preparations and pomegranate cultivars, by liquid chromatography-mass spectrometry. J. Sci. Food Agric. 2019, 99, 4036–4042. [Google Scholar] [CrossRef]
- Gözlekçi, Ş.; Saraçoğlu, O.; Onursal, E.; Özgen, M. Total phenolic distribution of juice, peel, and seed extracts of four pomegranate cultivars. Pharmacogn. Mag. 2011, 7, 161–164. [Google Scholar] [CrossRef]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Gironi, G.; Servili, M. Chemical composition, antioxidant activity, and sensory characterization of commercial pomegranate juices. Antioxidants 2021, 10, 1381. [Google Scholar] [CrossRef]
- Bertuglia, S.; Malandrino, S.; Colantuoni, A. Effects of the natural flavonoid delphinidin on diabetic microangiopathy. Arzneimittel-Forschung 1995, 45, 481–485. [Google Scholar]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef]
- Mazza, G. Compositional and functional properties of saskatoon berry and blueberry. Int. J. Fruit Sci. 2005, 5, 101–120. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Nicoară, N.D.; Marian, P.; Petriș, A.O.; Delcea, C.; Manole, F. A review of the role of cognitive-behavioral therapy on anxiety disorders of children and adolescents. Pharmacophore 2023, 14, 35–39. [Google Scholar] [CrossRef]
- Sevilla, A.J.; Signes Pastor, A.; Carbonell-Barrachina, A. Pomegranate and pomegranate juices. Aliment. Equiposy Tecnol. 2008, 234, 36–39. [Google Scholar]
- Lamy, E.; Rawel, H.; Schweigert, F.J.; Capela e Silva, F.; Ferreira, A.; Costa, A.R.; Antunes, C.; Almeida, A.M.; Coelho, A.V.; Sales-Baptista, E. The effect of tannins on Mediterranean ruminant ingestive behavior: The role of the oral cavity. Molecules 2011, 16, 2766–2784. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Qu, W.; Breksa Iii, A.P.; Pan, Z.; Ma, H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012, 132, 1585–1591. [Google Scholar] [CrossRef]
- Fischer, U.; Dettmann, J.; Carle, R.; Kammerer, D. Impact of processing and storage on the phenolic profiles and contents of pomegranate (Punica granatum L.) juices. Eur. Food Res. Technol. 2011, 233, 797–816. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; Sampaio, F.C.; Sampaio, M.C.; Pereira Mdo, S.; Higino, J.S.; Peixoto, M.H. Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz. Dent. J. 2006, 17, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, L.C.; Sampaio, M.C.; Sampaio, F.C.; Higino, J.S. Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses 2003, 46, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Ng, T.P.; Yong, D.; Fong, N.P.; Gerald, K. Total direct cost, length of hospital stay, institutional discharges and their determinants from rehabilitation settings in stroke patients. Acta Neurol. Scand. 2006, 114, 307–314. [Google Scholar] [CrossRef]
- Naqvi, S.; Khan, M.; Vohora, S. Anti-bacterial, anti-fungal and anthelmintic investigations on Indian medicinal plants. Fitoterapia 1991, 62, 221–228. [Google Scholar]
- Prasad, D.; Kunnaiah, R. Punica granatum: A review on its potential role in treating periodontal disease. J. Indian Soc. Periodontol. 2014, 18, 428–432. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A pharmacological update of ellagic acid. Planta Medica 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Sharma, A.; Choi, H.-K.; Kim, Y.-K.; Lee, H.-J. Delphinidin and its glycosides’ war on cancer: Preclinical perspectives. Int. J. Mol. Sci. 2021, 22, 11500. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yin, P.; Yao, H.; Chen, L.; Chang, X.; Li, H.; Hou, X. Punicalin ameliorates cell pyroptosis induced by LPS/ATP through suppression of ROS/NLRP3 pathway. J. Inflamm. Res. 2021, 14, 711–718. [Google Scholar] [CrossRef]
- Venusova, E.; Kolesarova, A.; Horky, P.; Slama, P. Physiological and immune functions of punicalagin. Nutrients 2021, 13, 2150. [Google Scholar] [CrossRef]
- Hajifattahi, F.; Moravej-Salehi, E.; Taheri, M.; Mahboubi, A.; Kamalinejad, M. Antibacterial effect of hydroalcoholic extract of Punica granatum Linn. Petal on common oral microorganisms. Int. J. Biomater. 2016, 2016, 8098943. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; Malt, m.; Mostafa, M. Evaluation of the Antimicrobial Effect of Pomegranate Extract on Streptococcus Mutans. Al-Azhar Dent. J. Girls 2019, 6, 467–473. [Google Scholar] [CrossRef]
- DiSilvestro, R.A.; DiSilvestro, D.J.; DiSilvestro, D.J. Pomegranate extract mouth rinsing effects on saliva measures relevant to gingivitis risk. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Product. Deriv. 2009, 23, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Malli Sureshbabu, N.; Ranjan, M.; Ranganath, A.; Siddique, R. The Antimicrobial Effect of Pomegranate Peel Extract versus Chlorhexidine in High Caries Risk Individuals Using Quantitative Real-Time Polymerase Chain Reaction: A Randomized Triple-Blind Controlled Clinical Trial. Int. J. Dent. 2021, 2021, 5563945. [Google Scholar] [CrossRef]
- Millo, G.; Juntavee, A.; Ratanathongkam, A.; Nualkaew, N.; Peerapattana, J.; Chatchiwiwattana, S. Antibacterial Inhibitory Effects of Punica granatum Gel on Cariogenic Bacteria: An in vitro Study. Int. J. Clin. Pediatr. Dent. 2017, 10, 152–157. [Google Scholar] [CrossRef]
- Menezes, S.M.; Cordeiro, L.N.; Viana, G.S. Punica granatum (pomegranate) extract is active against dental plaque. J. Herb. Pharmacother. 2006, 6, 79–92. [Google Scholar] [CrossRef]
- Laleman, I.; Teughels, W. Novel natural product-based oral topical rinses and toothpastes to prevent periodontal diseases. Periodontology 2000 2020, 84, 102–123. [Google Scholar] [CrossRef]
- Kiany, F.; Niknahad, H.; Niknahad, M. Assessing the effect of pomegranate fruit seed extract mouthwash on dental plaque and gingival inflammation. J. Dent. Res. Rev. 2016, 3, 117–123. [Google Scholar] [CrossRef]
- Pasupuleti, M.K.; Nagate, R.R.; Alqahtani, S.M.; Penmetsa, G.S.; Gottumukkala, S.N.; Ramesh, K. Role of medicinal herbs in periodontal therapy: A systematic review. J. Int. Soc. Prev. Community Dent. 2023, 13, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sastravaha, G.; Yotnuengnit, P.; Booncong, P.; Sangtherapitikul, P. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts. A preliminary study. J. Int. Acad. Periodontol. 2003, 5, 106–115. [Google Scholar]
- Sastravaha, G.; Gassmann, G.; Sangtherapitikul, P.; Grimm, W.D. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts in supportive periodontal therapy. J. Int. Acad. Periodontol. 2005, 7, 70–79. [Google Scholar]
- Tavangar, A.; Aslani, A.; Nikbakht, N. Comparative Study of Punica granatum Gel and Triadent Oral Paste Effect on Recurrent Aphthous Stomatitis, a Double Blind Clinical Trial. J. Dent. 2019, 20, 184–189. [Google Scholar] [CrossRef]
- Ghalayani, P.; Zolfaghary, B.; Farhad, A.R.; Tavangar, A.; Soleymani, B. The efficacy of Punica granatum extract in the management of recurrent aphthous stomatitis. J. Res. Pharm. Pract. 2013, 2, 88–92. [Google Scholar] [CrossRef]
- Darakhshan, S.; Malmir, M.; Bagheri, F.; Safaei, M.; Sharifi, R.; Sadeghi, M.; Hatami, M.; Mozaffari, H.R.; Tahvilian, R. The effects of pomegranate peel extract on recurrent aphthous stomatitis. Curr. Issues Pharm. Med. Sci. 2019, 32, 115–120. [Google Scholar] [CrossRef]
- Gavanji, S.; Larki, B.; Bakhtari, A. The effect of extract of Punica granatum var. pleniflora for treatment of minor recurrent aphthous stomatitis. Integr. Med. Res. 2014, 3, 83–90. [Google Scholar] [CrossRef]
- Rajan, S.; Ravi, J.; Suresh, A.; Guru, S. Hidden secrets of ‘Punica granatum’ use and its effects on oral health: A short review. J. Orofac. Res. 2013, 3, 38–41. [Google Scholar] [CrossRef]
- Prakash, C.V.S.; Prakash, I. Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel-a review. Int. J. Res. Chem. Environ. 2011, 1, 1–18. [Google Scholar]
- Miguel, M.G.; Neves, M.A.; Antunes, M.D. Pomegranate (Punica granatum L.): A medicinal plant with myriad biological properties-A short review. J. Med. Plants Res. 2010, 4, 2836–2847. [Google Scholar]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid.-Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef]
- Narayan, T.; Deshpande, S.; Jha, A.; Vasthare, R. Punica granatum (Pomegranate) fruit and its relevance in Oral Hygiene. IOSR J. Dent. Med. Sci. 2014, 13, 29–34. [Google Scholar] [CrossRef]
- Gagari, E.; Kabani, S. Adverse effects of mouthwash use. A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 432–439. [Google Scholar] [CrossRef]
- Gawor, J.P.; Ziemann, D.; Nicolas, C.S. A water additive with pomegranate can reduce dental plaque and calculus accumulation in dogs. Front. Vet. Sci. 2023, 10, 1241197. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Bolzan, T.C.A.; Zanini, M.S.; Alencar, T.; Rodrigues, W.D.; Bastos, K.A.; Severi, J.A.; Resende, J.A.; Villanova, J.C.O. Development and Evaluation of a Novel Oral Mucoadhesive Ointment Containing Pomegranate Peel Extract as an Adjuvant for Oral Hygiene of Dogs. J. Vet. Dent. 2020, 37, 133–140. [Google Scholar] [CrossRef]
- Potra-Cicalău, G.; Ciavoi, G.; Todor, L.; Iurcov, R.; Iova, G.; Ganea, M.; Scrobotă, I. The benefits of Calendula officinalis extract as therapeutic agent in oral healthcare. Med. Evol. 2022, XXVIII, 316–323. [Google Scholar]
- Seeram, N.P.; Zhang, Y.; McKeever, R.; Henning, S.M.; Lee, R.-p.; Suchard, M.A.; Li, Z.; Chen, S.; Thames, G.; Zerlin, A. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J. Med. Food 2008, 11, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Hong, M.Y.; Seeram, N.P.; Heber, D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J. Nutr. Biochem. 2008, 19, 848–855. [Google Scholar] [CrossRef]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Pomegranate. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–216. [Google Scholar]
- Taciuc, I.-A.; Dumitru, M.; Vrinceanu, D.; Gherghe, M.; Manole, F.; Marinescu, A.; Serboiu, C.; Neagos, A.; Costache, A. Applications and challenges of neural networks in otolaryngology. Biomed. Rep. 2024, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Topical anti-inflammatory and analgesic activities of standardized pomegranate rind extract in comparison with its marker compound ellagic acid in vivo. J. Ethnopharmacol. 2013, 148, 901–908. [Google Scholar] [CrossRef]
- Ghitea, T.C.; El-Kharoubi, A.; Ganea, M.; Bimbo-Szuhai, E.; Nemeth, T.S.; Ciavoi, G.; Foghis, M.; Dobjanschi, L.; Pallag, A.; Micle, O. The Antimicrobial Activity of Origanum vulgare L. Correlated with the Gastrointestinal Perturbation in Patients with Metabolic Syndrome. Molecules 2021, 26, 283. [Google Scholar] [CrossRef]
- Gavra, D.I.; Endres, L.; Pető, Á.; Józsa, L.; Fehér, P.; Ujhelyi, Z.; Pallag, A.; Marian, E.; Vicas, L.G.; Ghitea, T.C.; et al. In Vitro and Human Pilot Studies of Different Topical Formulations Containing Rosa Species for the Treatment of Psoriasis. Molecules 2022, 27, 5499. [Google Scholar] [CrossRef]
- Potra Cicalău, G.I.; Ciavoi, G.; Scrobotă, I.; Marcu, A.O.; Romanul, I.; Marian, E.; Vicaș, L.G.; Ganea, M. Assessing the Antioxidant Benefits of Topical Carvacrol and Magnolol Periodontal Hydrogel Therapy in Periodontitis Associated with Diabetes in Wistar Rats. Dent. J. 2023, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Ghitea, T.C. Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome. Biomedicines 2021, 9, 1709. [Google Scholar] [CrossRef] [PubMed]
- Rat, L.A.; Moldovan, A.F.; Trifan, D.F.; Matiș, L.; Murvai, G.F.; Maris, L.; Ghitea, T.C.; Maghiar, M.A. Can the Correlation of Periodontopathies with Gastrointestinal Diseases Be Used as Indicators in Severe Colorectal Diseases? Biomedicines 2023, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Potra Cicalău, G.I.; Marcu, O.A.; Ghitea, T.C.; Ciavoi, G.; Iurcov, R.C.; Beiusanu, C.; Trifan, D.F.; Vicaș, L.G.; Ganea, M. Study of Periodontal Bacteria in Diabetic Wistar Rats: Assessing the Anti-Inflammatory Effects of Carvacrol and Magnolol Hydrogels. Biomedicines 2024, 12, 1445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).