Effects of a Nutrition Education Programme in Stage IV Cardiac Rehabilitation Patients: A 3-Arm Randomised Controlled Trial
Abstract
:1. Introduction
1.1. Rationale
1.2. Aims
1.3. Hypotheses
2. Materials and Methods
2.1. Study Design and Setting
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Sample Size
2.5. Participants and Recruitment
2.6. Ethical Approval and Trial Registration
2.7. Intervention
2.7.1. Usual Care
2.7.2. Biggest Loser
2.7.3. Nutrition Education
2.8. Data Collection
2.8.1. Anthropometric Measurements
2.8.2. Haematological Testing
2.8.3. Blood Pressure
2.8.4. Questionnaires
2.9. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Loss to Follow-Up and Adverse Events
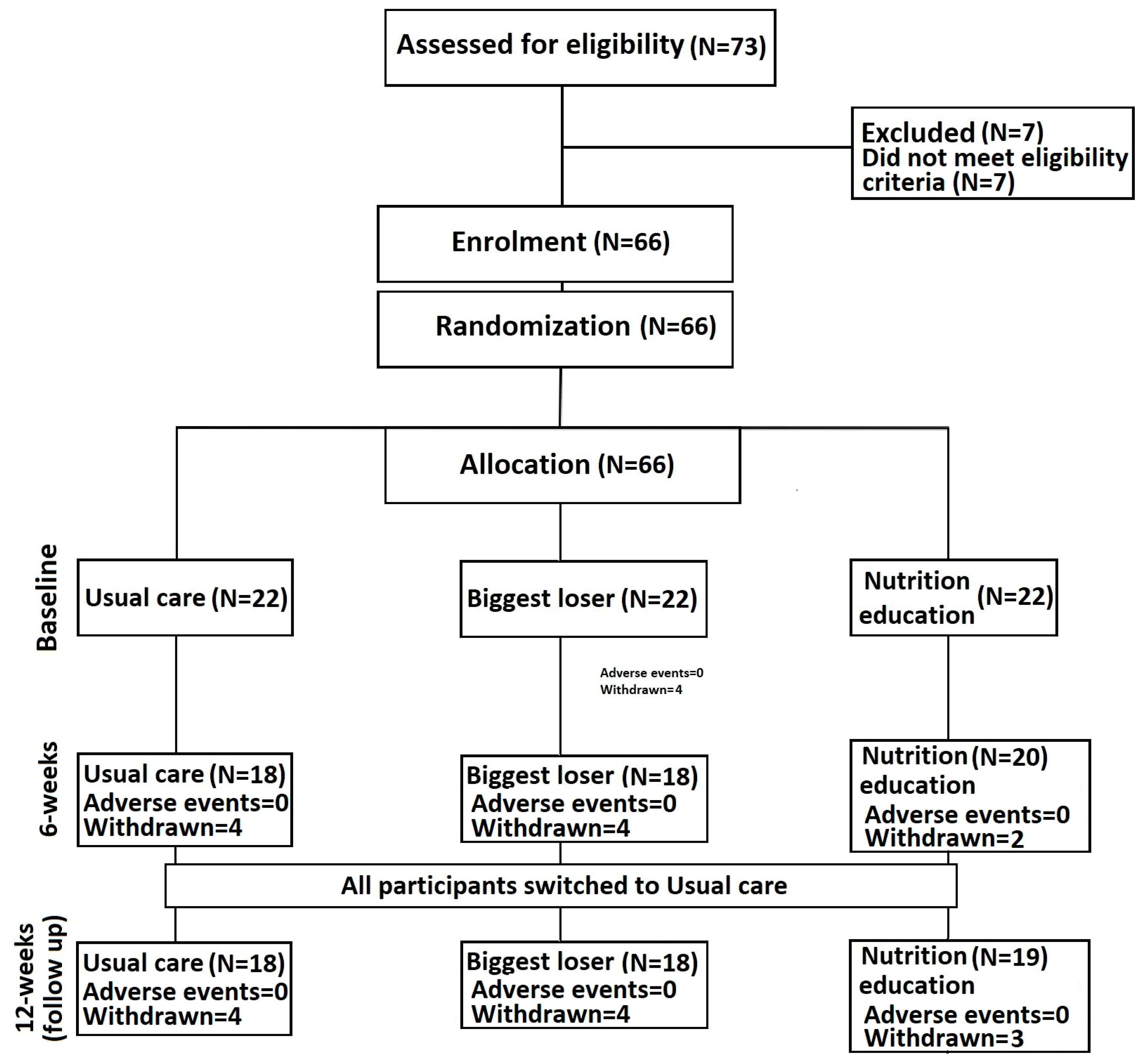
3.3. Anthropometric Measurements
3.4. Haematological
3.5. Blood Pressure
3.6. Nutrition Knowledge
3.7. Dietary Practices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Cardiovascular Diseases (CVD). 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 4 October 2023).
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Moraga, P.; GBD Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Ruan, Y.; Guo, Y.; Zheng, Y.; Huang, Z.; Sun, S.; Kowal, P.; Wu, F. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: Results from SAGE Wave 1. BMC Public Health 2018, 18, 404. [Google Scholar] [CrossRef] [PubMed]
- British Heart Foundation. Factsheet, U.K. British Heart Foundation. 2023. Available online: https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-uk-factsheet.pdf (accessed on 11 September 2023).
- British Heart Foundation. Analysis of European Cardiovascular Disease Statistics 2017, EHN. 2017. Available online: www.ehnheart.org/cvd-statistics/cvd-statistics-2017.html (accessed on 9 September 2023).
- British Heart Foundation. The National Audit of Cardiac Rehabilitation Quality and Outcomes Report 2020. 2020. Available online: https://www.bhf.org.uk/-/media/images/information-support/publications/hcp/nacr_quality_and_outcomes_report_2020.pdf?rev=b2d2789a242a452ea43b128e8f02c11d (accessed on 9 September 2023).
- Woolf-May, K.; Bird, S. Physical activity levels during phase IV cardiac rehabilitation in a group of male myocardial infarction patients. Br. J. Sports Med. 2005, 39, e12. [Google Scholar] [CrossRef] [PubMed]
- Sniehotta, F.F.; Gorski, C.; Araújo-Soares, V. Adoption of community-based cardiac rehabilitation programs and physical activity following phase III cardiac rehabilitation in Scotland: A prospective and predictive study. Psychol. Health 2020, 25, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Noites, A.; Freitas, C.P.; Pinto, J.; Melo, C.; Vieira, A.; Albuquerque, A.; Bastos, J.M. Effects of a phase IV home-based cardiac rehabilitation program on cardiorespiratory fitness and physical activity. Heart Lung Circ. 2017, 26, 455–462. [Google Scholar] [CrossRef]
- Atkins, S.; Holland, S.; Crossley, D.; Taylor, P.J.; Sinclair, J. Assessment of the effectiveness of a phase IV cardiac rehabilitation programme. Lancet 2017, 389, 23–24. [Google Scholar] [CrossRef]
- Thow, M.; Hinton, S.; Rafferty, D. A survey of phase IV cardiac rehabilitation provision in the, U.K. In Proceedings of the BACR Exercise Professionals Conference, Belfast, Northern Ireland, 29–30 September 2006. [Google Scholar]
- Sinclair, J.; Ageely, H.; Mahfouz, M.S.; Hummadi, A.A.; Darraj, H.; Solan, Y.; Bottoms, L. Effects of a Home-Based Physical Activity Program on Blood Biomarkers and Health-Related Quality of Life Indices in Saudi Arabian Type-2 Diabetes Mellitus Patients: A Randomized Controlled Trial. Life 2023, 13, 1413. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Recommendations on Physical Activity for Health; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 2004, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, G.T.; Buring, J.E.; Yusuf, S.; Goldhaber, S.Z.; Olmstead, E.M.; Paffenbarger, R.S., Jr.; Hennekens, C.H. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989, 80, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Oldridge, N.B.; Guyatt, G.H.; Fischer, M.E.; Rimm, A.A. Cardiac rehabilitation after myocardial infarction: Combined experience of randomized clinical trials. JAMA 1988, 260, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Willmer, K.; Waite, M.; Nurse, C.R.; Willmer, K.A. Long-term benefits of cardiac rehabilitation: A five-year follow-up of community-based phase 4 programmes. Br. J. Cardiol. 2009, 16, 5–8. [Google Scholar]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and cardiovascular health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [PubMed]
- Kocanda, L.; Schumacher, T.L.; Plotnikoff, R.C.; Whatnall, M.C.; Fenwick, M.; Brown, L.J.; Collins, C.E. Effectiveness and reporting of nutrition interventions in cardiac rehabilitation programmes: A systematic review. Eur. J. Cardiovasc. Nurs. 2023, 22, 1–12. [Google Scholar] [CrossRef]
- British Heart Foundation. National Audit of Cardiac Rehabilitation (NACR) Report 2019-BHF; 2019. [Google Scholar]
- Ma, Y.; Olendzki, B.C.; Pagoto, S.L.; Merriam, P.A.; Ockene, I.S. What are patients actually eating: The dietary practices of cardiovascular disease patients. Curr. Opin. Cardiol. 2010, 22, 518. [Google Scholar] [CrossRef]
- Melia, A.A.; Lowe, N.M.; Sinclair, J.K.; Dillon, S.A. Evaluation of nutritional knowledge, understand and practice of patients who attend a cardiac rehabilitation program in Preston. Proc. Nutr. Soc. 2016, 75, 157–158. [Google Scholar] [CrossRef]
- Chaiyasoot, K.; Sarasak, R.; Pheungruang, B.; Dawilai, S.; Pramyothin, P.; Boonyasiri, A.; Batterham, R.L. Evaluation of a 12-week lifestyle education intervention with or without partial meal replacement in Thai adults with obesity and metabolic syndrome: A randomised trial. Nutr. Diabetes 2018, 8, 23. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.; Bottoms, L.; Dillon, S.; Allan, R.; Shadwell, G.; Butters, B. Effects of montmorency tart cherry and blueberry juice on cardiometabolic and other health-related outcomes: A three-arm placebo randomized controlled trial. Int. J. Environ. Res. Public Health 2022, 19, 5317. [Google Scholar] [CrossRef] [PubMed]
- Makai, P.; IntHout, J.; Deinum, J.; Jenniskens, K.; Wilt, G.J.V.D. A network meta-analysis of clinical management strategies for treatment-resistant hypertension: Making optimal use of the evidence. J. Gen. Intern. Med. 2017, 32, 921–930. [Google Scholar] [CrossRef]
- British Heart Foundation. So You Want to Lose Weight for Good? 2012. Available online: http://www.bhf.org.uk/publications/view-publication.aspx?ps=1000807 (accessed on 10 July 2023).
- Lee, C.L.; Wang, J.S. Systolic blood pressure trajectory and cardiovascular outcomes: An analysis using data in the Systolic Blood Pressure Intervention Trial. Int. J. Clin. Pract. 2020, 74, e13450. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Kengne, A.P.; Huxley, R.R.; Batty, G.D.; De Galan, B.; Grobbee, D.; ADVANCE Collaborative Group. Comparison of waist-to-hip ratio other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: A prospective cohort study from, ADVANCE. Eur. J. Prev. Cardiol. 2011, 18, 312–319. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E. European Society of Hypertension Working Group on Blood Pressure Monitoring: European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J. Hypertens. 2003, 21, 821–848. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; PREDIMED Study Investigators. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Covas, M.I. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. Br. Med. J. 2008, 11, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.; Stainton, P.; Dillon, S.; Taylor, P.J.; Richardson, C.; Bottoms, L.; Allan, R. The efficacy of a tart cherry drink for the treatment of patellofemoral pain in recreationally active individuals: A placebo randomized control trial. Sport. Sci. Health 2022, 18, 1491–1504. [Google Scholar] [CrossRef]
- Lucassen, D.A.; Willemsen, R.F.; Geelen, A.; Brouwer-Brolsma, E.M.; Feskens, E.J. The accuracy of portion size estimation using food images and textual descriptions of portion sizes: An evaluation study. J. Hum. Nutr. Diet. 2021, 34, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Qualter, P.; Sinclair, J.K.; Gupta, S.; Zaman, M. School Feeding to Improve Cognitive Performance in Disadvantaged Children: A 3-Arm Parallel Controlled Trial in Northwest Pakistan. Nutrients 2023, 15, 1768. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T., Jr.; Whelton, P.K.; Reboussin, D.M. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2016, 374, 2294. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Pennathur, S. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Talayero, B.G.; Sacks, F.M. The role of triglycerides in atherosclerosis. Curr. Cardiol. Rep. 2011, 13, 544–552. [Google Scholar] [CrossRef]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary guidelines for Americans, 2020–2025: Understanding the scientific process, guidelines, and key recommendations. Nutr. Today 2021, 56, 287. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Mosterd, A.; Bakker, E.A.; Braber, T.L.; Nathoe, H.M.; Sharma, S.; Eijsvogels, T.M. Exercise volume versus intensity and the progression of coronary atherosclerosis in middle-aged and older athletes: Findings from the MARC-2 study. Circulation 2023, 147, 993–1003. [Google Scholar] [CrossRef]
- Pena-Hernandez, C.; Nugent, K.; Tuncel, M. Twenty-four-hour ambulatory blood pressure monitoring. J. Prim. Care Community Health 2020, 11, 2150132720940519. [Google Scholar] [CrossRef]
| Usual Care | Biggest Loser | Nutrition Education | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 68.80 | 8.69 | 68.05 | 9.29 | 69.14 | 7.85 |
| Sex (Male/Female) | 16/4 | 17/5 | 12/10 | |||
| Stature (m) | 1.72 | 0.08 | 1.70 | 0.10 | 1.65 | 0.09 |
| Body mass (kg) | 86.21 | 14.02 | 88.28 | 23.00 | 83.49 | 24.08 |
| Body mass index (kg/m2) | 29.41 | 5.78 | 30.51 | 8.29 | 30.57 | 7.72 |
| Usual Care | Biggest Loser | Nutrition Education | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-Weeks | 12-Weeks | Baseline | 6-Weeks | 12-Weeks | Baseline | 6-Weeks | 12-Weeks | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body mass (kg) | 86.21 | 14.02 | 83.23 | 8.23 | 82.69 | 8.70 | 88.28 | 23.00 | 85.54 | 24.88 | 87.04 | 24.30 | 83.49 | 24.08 | 82.25 | 25.46 | 82.05 | 26.37 |
| Body mass index (kg/m2) | 29.41 | 5.78 | 28.04 | 3.84 | 27.96 | 4.00 | 30.51 | 8.29 | 30.12 | 9.22 | 30.41 | 8.84 | 30.57 | 7.72 | 29.82 | 7.99 | 29.73 | 8.25 |
| Waist circumference (cm) | 103.35 | 10.92 | 99.94 | 9.05 | 100.88 | 7.48 | 104.86 | 15.29 | 100.88 | 14.79 | 103.78 | 14.55 | 103.23 | 19.65 | 101.00 | 19.15 | 99.79 | 20.51 |
| Waist to hip ratio | 0.96 | 0.06 | 0.94 | 0.06 | 0.95 | 0.05 | 0.97 | 0.08 | 0.95 | 0.09 | 0.98 | 0.10 | 0.93 | 0.08 | 0.95 | 0.07 | 0.93 | 0.09 |
| Systolic blood pressure (mm/Hg) | 130.45 | 11.10 | 127.83 | 18.50 | 126.35 | 15.58 | 133.50 | 21.55 | 123.06 | 18.99 | 135.22 | 23.36 | 135.23 | 20.16 | 129.20 | 28.82 | 126.26 | 18.98 |
| Diastolic blood pressure (mm/Hg) | 74.40 | 9.72 | 72.17 | 7.58 | 72.12 | 11.70 | 78.27 | 10.28 | 72.65 | 10.78 | 78.22 | 12.50 | 72.05 | 12.00 | 70.30 | 11.91 | 71.84 | 13.42 |
| Total cholesterol (mmol/L) | 4.20 | 0.71 | 4.50 | 0.70 | 4.49 | 0.61 | 4.39 | 0.95 | 4.67 | 0.95 | 4.28 | 1.01 | 4.47 | 0.70 | 5.03 | 0.69 | 4.59 | 0.45 |
| Glucose (mmol/L) | 7.27 | 2.64 | 6.91 | 2.31 | 6.71 | 2.37 | 7.55 | 1.93 | 7.37 | 2.79 | 7.23 | 1.88 | 6.83 | 1.64 | 7.51 | 3.52 | 7.10 | 2.39 |
| Triglycerides (mmol/L) | 1.61 | 1.10 | 1.43 | 0.67 | 1.54 | 1.08 | 1.90 | 1.20 | 1.86 | 1.23 | 1.92 | 0.96 | 1.70 | 0.78 | 2.21 | 0.99 | 1.84 | 0.74 |
| Triglyceride glucose index | 4.80 | 0.36 | 4.72 | 0.27 | 4.75 | 0.37 | 4.91 | 0.37 | 4.89 | 0.36 | 4.93 | 0.32 | 4.84 | 0.28 | 5.00 | 0.32 | 4.90 | 0.27 |
| Mediterranean Diet score | 7.71 | 2.37 | 8.00 | 1.87 | 8.00 | 2.39 | 7.71 | 2.35 | 8.73 | 2.49 | 8.36 | 2.46 | 8.48 | 2.20 | 9.77 | 1.59 | 10.07 | 1.73 |
| Usual Care | Biggest Loser | Nutrition Education | |||||
|---|---|---|---|---|---|---|---|
| Baseline–6 Weeks | Baseline–12 Weeks | Baseline–6 Weeks | Baseline–12 Weeks | Baseline–6 Weeks | Baseline–12 Weeks | ||
| Body mass (kg) | −2.98 | −3.52 | −2.74 | −1.24 | −1.24 | −1.44 | |
| Body mass index (kg/m2) | −1.37 | −1.45 | −0.39 | −0.1 | −0.75 | −0.84 | |
| Waist circumference (cm) | −3.41 | −2.47 | −3.98 | −1.08 | −2.23 | −3.44 | |
| Waist to hip ratio | −0.02 | −0.01 | −0.02 | 0.01 | 0.02 | 0.00 | |
| Systolic blood pressure (mm/Hg) | −2.62 | −4.1 | −10.44 | 1.72 | −6.03 | −8.97 | |
| Diastolic blood pressure (mm/Hg) | −2.23 | −2.28 | −5.62 | −0.05 | −1.75 | −0.21 | |
| Total cholesterol (mmol/L) | 0.30 | 0.29 | 0.28 | −0.11 | 0.56 | 0.12 | |
| Glucose (mmol/L) | −0.36 | −0.56 | −0.18 | −0.32 | 0.68 | 0.27 | |
| Triglycerides (mmol/L) | −0.18 | −0.07 | −0.04 | 0.02 | 0.51 | 0.14 | A |
| Triglyceride glucose index | −0.08 | −0.05 | −0.02 | 0.02 | 0.16 | 0.06 | |
| Mediterranean Diet score | 0.29 | 0.29 | 1.02 | 0.65 | 1.29 | 1.59 | B, C, D |
| Usual Care | Biggest Loser | Nutrition Education | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Weeks | 12 Weeks | Baseline | 6 Weeks | 12 Weeks | Baseline | 6 Weeks | 12 Weeks | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Energy intake (Kcal) | 1761.69 | 420.88 | 1656.06 | 373.09 | 1714.73 | 512.96 | 1676.79 | 441.06 | 1596.94 | 585.22 | 1603.10 | 465.56 | 1516.00 | 387.23 | 1362.83 | 285.15 | 1573.00 | 412.81 |
| Fat (g) | 62.21 | 19.83 | 61.52 | 16.25 | 68.76 | 29.18 | 58.85 | 25.80 | 56.49 | 25.57 | 56.45 | 19.02 | 56.56 | 19.26 | 52.92 | 13.98 | 59.36 | 17.07 |
| Saturated fatty acids (g) | 20.26 | 5.46 | 21.34 | 6.34 | 23.37 | 10.33 | 22.52 | 9.62 | 19.72 | 8.64 | 19.39 | 7.53 | 19.25 | 7.58 | 16.78 | 3.82 | 20.75 | 6.16 |
| Protein (g) | 82.48 | 19.16 | 74.16 | 14.15 | 81.21 | 19.97 | 72.19 | 14.97 | 71.22 | 15.02 | 69.07 | 15.91 | 73.19 | 16.55 | 75.94 | 19.75 | 75.68 | 18.62 |
| Carbohydrate (g) | 219.33 | 77.50 | 193.55 | 60.95 | 192.55 | 63.03 | 205.65 | 61.63 | 189.24 | 87.81 | 195.66 | 93.90 | 175.57 | 49.52 | 142.89 | 57.48 | 183.83 | 58.45 |
| Free sugars (g) | 33.61 | 20.91 | 30.41 | 18.97 | 36.14 | 19.25 | 40.95 | 18.85 | 33.78 | 18.96 | 27.86 | 16.01 | 29.16 | 15.58 | 23.13 | 14.06 | 23.14 | 12.85 |
| Fibre (g) | 19.51 | 5.63 | 18.45 | 5.25 | 16.37 | 7.83 | 16.44 | 5.55 | 16.16 | 7.36 | 17.53 | 5.75 | 15.27 | 4.73 | 16.78 | 4.65 | 17.24 | 3.61 |
| Alcohol (mL) | 6.88 | 9.69 | 8.90 | 12.78 | 7.56 | 10.80 | 12.52 | 14.74 | 10.74 | 9.91 | 14.50 | 18.24 | 7.97 | 9.14 | 4.47 | 4.83 | 5.18 | 11.14 |
| Vitamin A (ug) | 575.19 | 270.44 | 578.94 | 462.32 | 515.47 | 206.97 | 513.58 | 243.24 | 498.38 | 301.00 | 548.80 | 321.36 | 469.23 | 231.40 | 567.17 | 331.97 | 490.40 | 243.01 |
| Thiamine (mg) | 1.47 | 0.34 | 12.50 | 44.97 | 1.43 | 0.53 | 1.56 | 0.64 | 1.53 | 0.63 | 1.50 | 0.58 | 1.47 | 0.49 | 1.36 | 0.42 | 1.43 | 0.27 |
| Riboflavin (mg) | 1.81 | 0.61 | 1.79 | 0.69 | 1.70 | 0.58 | 1.74 | 0.81 | 1.72 | 0.81 | 1.59 | 0.83 | 1.52 | 0.38 | 1.40 | 0.37 | 1.47 | 0.39 |
| Niacin (mg) | 32.96 | 6.52 | 31.29 | 8.01 | 31.32 | 9.62 | 32.23 | 9.06 | 33.67 | 10.09 | 29.83 | 7.02 | 29.96 | 7.73 | 30.33 | 9.95 | 29.41 | 10.07 |
| Vitamin B6 (mg) | 1.54 | 0.29 | 1.70 | 0.52 | 1.55 | 0.46 | 1.59 | 0.64 | 1.61 | 0.79 | 1.59 | 0.39 | 1.41 | 0.40 | 1.38 | 0.39 | 1.37 | 0.32 |
| Vitamin B12 (mg) | 4.41 | 1.69 | 4.93 | 3.24 | 4.45 | 1.55 | 4.04 | 1.84 | 4.60 | 2.73 | 4.08 | 2.38 | 4.51 | 1.94 | 5.09 | 2.34 | 4.09 | 2.05 |
| Folate (ug) | 232.44 | 51.80 | 237.47 | 86.14 | 227.80 | 85.02 | 235.84 | 111.35 | 221.56 | 88.43 | 228.10 | 88.99 | 208.82 | 66.84 | 226.58 | 43.25 | 216.00 | 32.02 |
| Vitamin C (mg) | 83.99 | 65.58 | 75.75 | 34.90 | 70.12 | 39.72 | 78.53 | 50.85 | 78.14 | 46.89 | 85.63 | 38.71 | 81.17 | 58.11 | 86.35 | 19.01 | 88.61 | 43.38 |
| Calcium (mg) | 942.81 | 688.96 | 764.47 | 188.83 | 728.87 | 148.33 | 859.47 | 314.02 | 717.75 | 266.18 | 742.70 | 303.60 | 785.91 | 203.75 | 676.58 | 185.86 | 743.47 | 255.10 |
| Salt (g) | 4.87 | 1.93 | 4.28 | 1.20 | 4.53 | 1.31 | 4.75 | 1.23 | 3.83 | 1.07 | 4.78 | 3.72 | 4.34 | 1.39 | 3.94 | 1.23 | 4.63 | 1.80 |
| Iron (mg) | 12.25 | 6.66 | 9.68 | 3.31 | 10.10 | 3.83 | 10.46 | 5.14 | 10.64 | 6.33 | 10.75 | 5.06 | 9.02 | 2.53 | 8.44 | 1.63 | 8.61 | 1.64 |
| Zinc (mg) | 9.19 | 2.03 | 7.89 | 2.06 | 8.18 | 2.39 | 7.91 | 1.76 | 7.81 | 2.01 | 7.82 | 2.17 | 10.26 | 10.46 | 8.15 | 1.77 | 7.67 | 1.62 |
| Selenium (mg) | 58.50 | 22.63 | 48.59 | 19.21 | 49.60 | 18.26 | 41.89 | 12.88 | 48.56 | 28.20 | 43.30 | 13.65 | 49.95 | 14.92 | 55.50 | 19.36 | 48.20 | 20.51 |
| Usual Care | Biggest Loser | Nutrition Education | |||||
|---|---|---|---|---|---|---|---|
| Baseline–6 Weeks | Baseline–12 Weeks | Baseline–6 Weeks | Baseline–12 Weeks | Baseline–6 Weeks | Baseline–12 Weeks | ||
| Energy intake (Kcal) | 105.63 | 46.96 | 79.85 | 73.69 | 153.17 | −57.00 | |
| Fat (g) | 0.69 | −6.55 | 2.36 | 2.40 | 3.64 | −2.80 | |
| Saturated fatty acids (g) | −1.08 | −3.11 | 2.80 | 3.13 | 2.47 | −1.50 | B |
| Protein (g) | 8.32 | 1.27 | 0.97 | 3.12 | −2.75 | −2.49 | |
| Carbohydrate (g) | 25.78 | 26.78 | 16.41 | 9.99 | 32.68 | −8.26 | |
| Free sugars (g) | 3.20 | −2.53 | 7.17 | 13.09 | 6.03 | 6.02 | B |
| Fibre (g) | 1.06 | 3.14 | 0.28 | −1.09 | −1.51 | −1.97 | |
| Alcohol (mL) | −2.02 | −0.68 | 1.78 | −1.98 | 3.50 | 2.79 | |
| Vitamin A (ug) | −3.75 | 59.72 | 15.20 | −35.22 | −97.94 | −21.17 | |
| Thiamine (mg) | −11.03 | 0.04 | 0.03 | 0.06 | 0.11 | 0.04 | |
| Riboflavin (mg) | 0.02 | 0.11 | 0.02 | 0.15 | 0.12 | 0.05 | |
| Niacin (mg) | 1.67 | 1.64 | −1.44 | 2.40 | −0.37 | 0.55 | |
| Vitamin B6 (mg) | −0.16 | −0.01 | −0.02 | 0.00 | 0.03 | 0.04 | |
| Vitamin B12 (mg) | −0.52 | −0.04 | −0.56 | −0.04 | −0.58 | 0.42 | |
| Folate (ug) | −5.03 | 4.64 | 14.28 | 7.74 | −17.76 | −7.18 | |
| Vitamin C (mg) | 8.24 | 13.87 | 0.39 | −7.10 | −5.18 | −7.44 | |
| Calcium (mg) | 178.34 | 213.94 | 141.72 | 116.77 | 109.33 | 42.44 | |
| Salt (g) | 0.59 | 0.34 | 0.92 | −0.03 | 0.40 | −0.29 | E |
| Iron (mg) | 2.57 | 2.15 | −0.18 | −0.29 | 0.58 | 0.41 | |
| Zinc (mg) | 1.30 | 1.01 | 0.10 | 0.09 | 2.11 | 2.59 | |
| Selenium (mg) | 9.91 | 8.90 | −6.67 | −1.41 | −5.55 | 1.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinclair, J.; Dillon, S.; Lowe, N.M.; Melia, A. Effects of a Nutrition Education Programme in Stage IV Cardiac Rehabilitation Patients: A 3-Arm Randomised Controlled Trial. Life 2024, 14, 63. https://doi.org/10.3390/life14010063
Sinclair J, Dillon S, Lowe NM, Melia A. Effects of a Nutrition Education Programme in Stage IV Cardiac Rehabilitation Patients: A 3-Arm Randomised Controlled Trial. Life. 2024; 14(1):63. https://doi.org/10.3390/life14010063
Chicago/Turabian StyleSinclair, Jonathan, Stephanie Dillon, Nicola M. Lowe, and April Melia. 2024. "Effects of a Nutrition Education Programme in Stage IV Cardiac Rehabilitation Patients: A 3-Arm Randomised Controlled Trial" Life 14, no. 1: 63. https://doi.org/10.3390/life14010063
APA StyleSinclair, J., Dillon, S., Lowe, N. M., & Melia, A. (2024). Effects of a Nutrition Education Programme in Stage IV Cardiac Rehabilitation Patients: A 3-Arm Randomised Controlled Trial. Life, 14(1), 63. https://doi.org/10.3390/life14010063






