Abstract
A total of 160 Ross 308 male chickens were used in a 2 × 2 factorial design to examine the effects of anticoccidial vaccination (ACV; lack or 1× dose recommended by the manufacturer) and dietary supplementation with Taraxacum officinale (dandelion) extract (DE; with or without) on growth performance, immunity, biochemical parameters, and intestinal morphology in broiler chickens challenged with Eimeria spp. At 20 days of age, all birds were challenged with a 25× dose of ACV, including Eimeria acervulina, E. maxima, E. mitis, and E. tenella. No interaction between ACV and DE was observed in terms of growth performance. Vaccinated birds showed increased feed intake (FI) and feed conversion ratio (FCR) during the 11–20 day period. Meanwhile, DE supplementation led to decreased FI and body weight gain (BWG) during the 1–10 day period. ACV effectively induced immunity against Eimeria, as evidenced by reduced oocyst shedding and less intestinal lesions, decreased levels of pro-inflammatory interleukin-6, and improved BWG during both the post infection (PI) period (21–35 days) and the entire growth period. DE supplementation lowered FCR and increased BWG during the 35–42 day period, increased the concentration of butyric acid in the cecal digesta, and lowered oocyst shedding PI. In vaccinated birds, DE elevated levels of plasma total protein and immunoglobulin M, and influenced tight junction proteins zonula occludens-1 and claudin-3, indicating a more robust epithelial barrier. DE also lowered alanine aminotransferase activity in unvaccinated birds. Both ACV and DE independently improved intestinal morphology in the jejunum, decreasing crypt depth and increasing the villus height-to-crypt ratio. These findings suggest that both ACV and DE could be effective strategies for managing coccidiosis in broiler chickens.
1. Introduction
Coccidiosis, caused by Eimeria protozoan parasites, is a prevalent disease with global economic implications for broiler chickens [1,2]. The global poultry sector faced significant financial burdens due to this disease, with losses estimated at GBP 10.4 billion in 2016 [3]. These losses arise from factors such as reduced weight gain, decreased feed conversion efficiency, increased medication costs, and increased mortality [3,4,5]. In cases of coccidiosis, damage to the intestinal mucosa—accompanied by oocyst proliferation—results in symptoms like diarrhea, malabsorption, and inflammation. It may also predispose chickens to dysbacteriosis, including the development of Clostridium perfringens-mediated necrotic enteritis [5,6,7]. To mitigate the impact of coccidiosis, anticoccidial drugs and anticoccidial live vaccines (ACVs) have been employed as the primary control strategies [4,8,9].
Recently, there has been growing interest in the use of ACVs for broilers, driven by the emergence of Eimeria strains resistant to anticoccidials [10]. The ACVs contain either attenuated or live wild-type strains of Eimeria and induce a protective immune response in vaccinated birds [9]. These vaccines are typically administered orally—through spraying, gel, or drinking water—making it feasible for mass administration to large flocks of broilers [6]. They stimulate both cellular and humoral immune responses, effectively mimicking the infection process without causing severe disease. While considered an effective approach for controlling coccidiosis in layers and breeding flocks, ACVs present some concerns when used in broilers. The primary issue is the potential for vaccine-induced pathogenicity, as ACVs may occasionally trigger mild to moderate symptoms of coccidiosis or serve as a predisposing factor for bacterial enteritis in vaccinated birds. Such outcomes can affect bird performance, which may not be fully compensated for given the relatively short lifespan of birds. Additionally, the immunity developed is species-specific; hence, birds may remain susceptible to other Eimeria species not included in the vaccine [4,9,11,12,13,14,15].
In light of these challenges, there is a growing interest in exploring natural methods that can support coccidiosis immunoprophylaxis and improve the overall health and productivity of vaccinated broiler chickens. One promising strategy is the use of plant extracts in poultry nutrition as they serve as a rich source of diverse bioactive substances capable of exerting their beneficial effects through various mechanisms of action [16,17,18,19,20].
Dandelion (Taraxacum officinale), a herbaceous plant in the Asteraceae family, is recognized for its various medicinal properties, including anti-inflammatory, antibacterial, antioxidant, hepatoprotective, anticancer, and immunomodulatory effects [21]. The primary bioactive ingredients of dandelion are phenolic compounds, terpenes, carbohydrates, proteins, fatty acids, vitamins, and minerals. Among its antioxidant and anti-inflammatory phenolic compounds, derivatives of hydroxycinnamic acid—specifically chicoric acid, chlorogenic acid, and caffeic acid—have been found in the highest concentrations [22,23]. Various studies have reported dandelions’ ability to improve growth performance in poultry [21,24,25,26]. A recent study demonstrated that dandelion enhances feed efficiency in broilers by enhancing intestinal barrier function, decreasing proinflammatory cytokines, and optimizing the composition of the intestinal microbiota [25].
Thus, it was hypothesized that dandelion extract (DE) could effectively mitigate the potential negative effects of ACV during the development of ACV-induced immunity to coccidiosis, especially concerning the deterioration of growth parameters. Furthermore, when birds are exposed to Eimeria infections, DE has the potential to either enhance the vaccine’s efficacy or alleviate the adverse effects of the infection in unvaccinated birds.
2. Materials and Methods
2.1. Experimental Design, Birds, and Diets
Animal testing procedures received approval from the second Local Ethics Committee for Animal Testing in Krakow, Poland. The experiment followed a 2 × 2 factorial arrangement resulting in four treatment groups. Each treatment group had five replicates (pens), with each pen housing eight male Ross 308 chicks. Experimental factors included either the absence or presence of a single dose of ACV Paracox®-5 (MSD Animal Health, Milton Keynes, UK) administered at 1 d of age. This was combined with either the presence or absence of dietary supplementation with T. officinale extract, also known as dandelion (Herberry Ltd., Stawiguda, Poland).
In total, 160 1-day-old male Ross 308 broiler chickens were purchased from a commercial hatchery (H and P Hatching and Poultry Breeding, Orzesze-Gardawice, Poland) and randomly allotted to different treatment groups at 1 d of age. The experiment lasted for 42 days. Chickens were maintained under conventional environmental conditions, with initial temperatures set at 32 °C on day 1, gradually decreasing to 21 °C by day 21. They were housed in floor pens, each offering 0.76 m2 of space and equipped with two nipple–cup drinking systems and one trough feeder. Wood shavings were employed as bedding to facilitate the recirculation of vaccine-originated oocysts. To minimize the transfer of oocysts and reduce the risk of contamination among unvaccinated groups, PVC sheet barriers were installed between the pens.
The birds had ad libitum access to water and feed. The basal diets were formulated to meet or exceed the nutritional standards for broilers during various feeding phases: starter (1–10 days), grower 1 (11–19 days), grower 2 (20–35 days), and finisher (36–42 days) (Table 1) [27]. The experiment was conducted at the broiler facility within the Experimental Station in Aleksandrowice, operated by the National Research Institute of Animal Production in Poland. This facility maintains a stringent sanitation regimen to mitigate challenges that broilers might encounter in large-scale intensive production systems. To make the diets slightly challenging, wheat and rye (as sources of nonstarch polysaccharides) were incorporated, along with fishmeal. Diets for vaccinated birds were free of in-feed coccidiostats, which is crucial for allowing the development of acquired immunity and the recirculation of vaccine strains. In contrast, feed mixtures for unvaccinated birds provided up to the 20th day of age were supplemented with salinomycin (70 ppm; Sacox 120; Huvepharma, Antwerp, Belgium). The administration of coccidiostats in the diet of unvaccinated groups, along with the high level of sanitation, aimed to prevent the potential transmission of vaccine oocysts and their subsequent proliferation in the avian gastrointestinal tract. This setup allowed the evaluation of the effects of dandelion extract under conditions of either first-time or repeated exposure of the chickens to Eimeria spp.

Table 1.
Composition and content of nutrients in basic feed mixtures.
At 20 d of age, all birds were challenged with Eimeria spp. through individual oral administration of a 25-fold dose of ACV Paracox®-5. The vaccine’s 25-fold dose (0.1 mL) was suspended in 0.24 mL of distilled water and given orally. The composition of this vaccine is detailed in Section 2.2. The attenuated vaccine dose used for the infection was not expected to induce clinical coccidiosis.
2.2. Experimental Factors
At 1 day of age, half of the chickens received an oral administration of a single recommended dose of the live attenuated ACV, Paracox®-5 (MSD Animal Health, Milton Keynes, UK) before being placed in their designated pens. Each vaccine dose contained sporulated oocysts of various Eimeria species in the following amounts: E. acervulina 500–600, E. maxima CP 200–230, E. maxima MFP 100–130, E. mitis HP 1000–1300, and E. tenella HP 500–650. The vaccine dose (0.004 mL) was suspended in 0.24 mL of distilled water and given orally. An equivalent volume of distilled water was administered to the unvaccinated groups of birds.
In the treatment groups intended for obtaining T. officinale extract (Herberry Ltd., Stawiguda, Poland), a feed additive was incorporated into the basic feed mixtures at a dose of 2 g/kg of feed. The DE was prepared from the radix and herba of T. officinale using the following method: an aqueous extract was obtained at a plant-to-water ratio of 1:10 (DER 1:10). This extract was then concentrated 10-fold using a rotary evaporator under reduced pressure. The concentrated extract was subsequently blended with maltodextrin in a ratio of 2726 g of extract to 700 g of maltodextrin. This mixture was then spray-dried, yielding 1002 g of powder. The concentration of chicoric acid, the major phenolic compound in T. officinale, was determined in the final product. Quantitative analysis of chicoric acid was performed using a PerkinElmer Altus™ HPLC system equipped with a Diode Array Detector (DAD) (PerkinElmer, Shelton, CT, USA) utilizing detection wavelength of 330 nm. Chromatographic separation was achieved on a LiChrospher 100, C18, 5 µm column (125 × 4 mm; Merck KGaA, Darmstadt, Germany) maintained at 35 °C. The mobile phase consisted of 0.1% formic acid in ultrapure water and 0.1% formic acid in acetonitrile, with a flow rate of 0.75 mL/min. The injection volume was set at 10 μL and the detection wavelength was 330 nm. The chicoric acid content in the final preparation was determined to be 0.65 g/kg.
2.3. Sample Collection and Analytical Procedure
Weekly feed intake (FI) measurements were taken and the broiler weight was recorded on days 1, 10, 20, 35, and 42. Growth performance parameters, including body weight gain (BWG), FI, and the feed conversion ratio corrected for mortality (FCR) were calculated for each feeding phase. These parameters were analyzed on a pen basis (n = 5).
To determine oocyst excretion, the concentration of oocysts per gram of feces (OPG) was measured using the McMaster concentration method in a McMaster counting chamber [28]. OPG assessments were carried out on pooled fecal samples collected from each experimental pen (n = 5). In the vaccinated groups, these assessments took place on days 7, 14, and 20 postvaccination (PV). In all experimental groups, they were conducted on days 5, 6, 7, and 15 post infection (PI). To achieve a normal distribution, OPG values underwent a logarithmic transformation [log10 (OPG + 1)].
At 25 days of age (5 d PI), five birds per treatment (one bird per replicate pen; n = 5) were slaughtered by decapitation after 8 h of fasting and electrical stunning using STZ6 apparatus (Koma Ltd., Wilkanowo, Poland). Blood samples were collected in heparin tubes and the intestines were immediately excised for lesion scoring (LS) due to the Eimeria challenge and for histological analysis. Lesions in the duodenum, jejunum/ileum, and ceca were evaluated using the Johnson and Reid method, with a scale ranging from 0 (no visible lesion) to 4 (most severe lesion) [29]. For histological analysis, two 20-mm sections were collected from the duodenum (2 cm behind the gizzard), jejunum (from the midpoint of this intestinal segment), and ileum (5 cm before the ceca). These were preserved in 10% formalin, following standard histological procedures as previously described [30]. After a 24-h fixation period, tissue samples were washed with tap water, dehydrated, embedded in paraffin, and cut into 4 μm sections (Microm HM 360, Microm, Walldorf, Germany). They were then stained with Goldner’s trichrome. Microscopic observations were conducted using optical microscopes (BX63 and CX43, Olympus, Tokyo, Japan). Various histomorphometric parameters, such as villus height (VH) and thickness, crypt depth (CD) and width, as well as the thickness of the mucosa, submucosa, and lamina muscularis (both longitudinal and circular), were measured [31]. The ratio of villus height to crypt depth (VH/CD) for individual pairs of villus and crypt was determined and the mucosal surface absorptive area was calculated as detailed in the previous literature [32]. For each parameter, eight histomorphometry measurements per bird were taken using graphic analysis software (ImageJ 1.51; National Institutes of Health, Bethesda, MD, USA).
The jejunum tissue slices were utilized for immunohistochemical (IHC) staining, following a protocol previously described [30]. To label tight junctions (TJs), rabbit polyclonal antiZO-1 (zonula occludens-1) antibody (orb11587, Biorbyt, St. Louis, MO, USA) and rabbit polyclonal anticlaudin 3 antibody (AB15102; Abcam, Cambridge, UK) were used as the primary antibodies. These sections were subsequently treated with the secondary antibody: peroxidase-conjugated goat antirabbit (#611-1302, Rockland Immunochemicals, Inc., Limerick, PA, USA). The sections were then exposed to 3,30-diaminobenzidine tetrahydrochloride (DAB D5905; Sigma-Aldrich, St. Louis, MO, USA) for chromogenic development and counterstained with Mayer’s hematoxylin (MHS32-1L; Sigma-Aldrich, St. Louis, MO, USA). IHC images were captured using CX43 and BX63 light microscopes (Olympus, Tokyo, Japan) and converted into 8-bit grayscale format. This allowed for the quantitative measurement of the intensity of the immunoreaction (IR) for ZO-1 in villi and claudin-3 in crypts by comparing the optical density (OD) values of the images. OD calculations followed the ImageJ protocol and used a Kodak 3-step calibration tablet in conjunction with ImageJ’s integrated Rodbard function. This translated the 8-bit pixel values into calibrated OD readings [33]. Representative images of the IHC reactions for zonula occludens-1 and claudin-3 on formaldehyde-fixed sections from the jejunum are presented as supplementary material (Figure S1).
The blood samples were centrifuged at 3000× g for 10 min and the resulting plasma was analyzed for concentrations of total immunoglobulin Y, M, and A (IgY, IgM, and IgA), tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), ceruloplasmin (Cp), and fibrinogen (Fb). Commercial quantitative ELISA kits (FineTest, Wuhan Fine Biotech Co., Ltd., Wuhan, China) were used for these analyses.
Additionally, blood plasma samples were further evaluated for specific biochemical parameters using commercial kits from Cormay Co. (PZ Cormay Inc., Lomianki, Poland). These parameters included the total protein (TP), triacylglycerols (TG), total cholesterol (TC), and glucose (GLU) levels as well as enzyme activities for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH).
At 42 d of age, two chickens per pen (n = 10) were slaughtered following electrical stunning and their cecal digesta were collected and immediately frozen for the determination of volatile fatty acids (VFAs). Upon thawing, a 5 g aliquot of the digesta sample was diluted in 50 mL of water. Subsequently, 5 mL of the filtrate was mixed with 1 mL of 24% sulfuric acid and centrifuged. The supernatant was then used for chromatographic analysis to determine the concentration of cecal VFAs. The analysis was performed using a GC-2010 Plus gas chromatograph (Shimadzu Corp., Kyoto, Japan) equipped with a capillary column (CP-WAX 58 FFAP 25 m × 0.53 mm; Agilent Technologies, Palo Alto, CA, USA).
The basic compound feeds formulated for each feeding phase were analyzed using AOAC standard methods [34]. The analyses included measurements for moisture, crude fat, crude protein, ash, amino acids, calcium, and total phosphorus content, following method 930.15, method 920.39, method 984.13, method 942.05, method 982.30, method 968.08, and method 965.17, respectively.
2.4. Statistical Analysis
Data were analyzed using a two-way analysis of variance (ANOVA) through STATISTICA software (version 13.3; StatSoft Inc., Tulsa, OK, USA) to determine the main effects of the treatments. In this analysis, ACV and DE dietary supplementation were considered as the primary influencing factors and potential interactions between them were also evaluated. The effect of DE supplementation on OPG results in vaccinated chickens was assessed using a one-way ANOVA. To identify differences between the treatments, Duncan’s multiple range post hoc test was used. Statistical significance was set at p < 0.05.
3. Results
3.1. Growth Performance
The effects of ACV and DE on basic growth performance results across various feeding phases (1–10 days for the starter phase, 11–20 days for grower 1, 21–35 days for grower 2, 36–42 days for the finisher phase, and 1–42 days overall) are presented in Table 2. No significant interactions between the experimental factors were observed during any of the analyzed periods.

Table 2.
Effects of experimental factors on the growth performance.
Regarding independent effects, during the starter feeding phase, DE significantly reduced FI, which consequently led to a decrease in BWG. The impact of ACV became noticeable starting from the grower 1 feeding phase. In this phase, ACV significantly increased FI, resulting in increased FCR, although it did not affect BWG. During the same period, DE showed a tendency to increase BWG by 2.2% (p = 0.051).
After the experimental challenge with Eimeria at 20 days of age, vaccination with ACV led to a significant improvement in BWG during the grower 2 phase and increased FI during the finisher phase. No impact from DE was observed in the grower 2 phase. However, in the finisher phase, supplementation with the extract significantly increased BWG and showed a tendency to lower the FCR by 6.3% compared to the unsupplemented groups. For the entire experimental period, neither treatment showed an effect on FCR. Nevertheless, a significant impact of ACV was noted, resulting in 3.1% and 4,6% increases in BWG and FI, respectively, compared to unvaccinated groups. Additionally, a tendency towards increased BWG was observed in birds supplemented with DE, showing a 2.41% increase compared to unsupplemented groups.
3.2. Oocyst Shedding and Lesion Score
Figure 1 illustrates the oocyst shedding profile in vaccinated bird groups at 7, 14, and 20 days PV. Statistically significant differences were observed at 14 days PV, during which birds receiving the diet supplemented with DE shed a higher number of oocysts. By 20 days PV, the OPG values in all vaccinated birds were comparable. No oocysts were detected in unvaccinated groups up to 20 d of age.
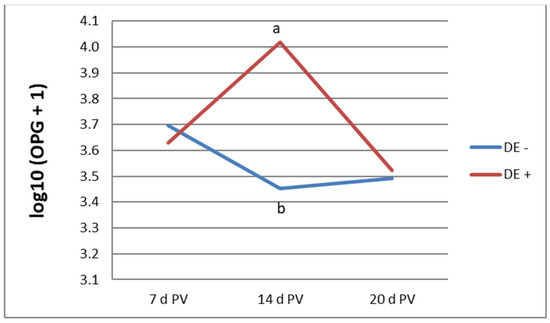
Figure 1.
Oocyst output in the vaccinated groups [log10 (OPG + 1)]; a, b—mean values not sharing a common letter are significantly different (p ≤ 0.05); OPG—oocysts per gram, pv—postvaccination, and DE—dandelion extract.
The profile of PI oocyst shedding and the results of LS conducted 5 days after Eimeria spp. infection are presented in Table 3. A significant interaction between ACV and DE was only recorded in OPG at 5 days PI. During this period, supplementation with DE resulted in a decrease in OPG, with no effect observed in unvaccinated groups. In terms of independent effects, vaccinated birds shed significantly fewer oocysts at 5, 6, and 7 d PI. Supplementation with DE lowered OPG at 7 and 15 d PI, regardless of vaccination status. During LS, no cecal lesions were detected in any of the groups. A significant effect of lowered LS in the duodenum and a tendency (p = 0.059) toward lowered LS in the jejunum and ileum were observed. DE supplementation did not affect LS at 5 d PI.

Table 3.
Eimeria oocyst counts per gram of feces [log10 (OPG + 1)] collected post infection (PI) with Eimeria spp. and intestinal lesion scoring at 5 d PI.
3.3. Intestinal Morphology and Epithelial Barrier Characteristics
The results of histomorphometry measurements for the duodenum, jejunum, and ileum are detailed in Table 4, Table 5 and Table 6, respectively. No significant interactions between ACV and DE were observed in any section of the intestine.

Table 4.
Effects of experimental factors on the histometric measurements of the duodenum sampled at 5 d post infection with Eimeria spp.

Table 5.
Effects of experimental factors on the histometric measurements of the jejunum sampled at 5 d post infection with Eimeria spp.

Table 6.
Effects of experimental factors on the histometric measurements of the ileum sampled at 5 d post infection with Eimeria spp.
Concerning independent effects, neither ACV nor DE had a significant impact on the morphology of the duodenum.
In the jejunum, ACV significantly increased villus thickness and the VH/CD ratio and reduced CD by 17.2%, 17.6%, and 15.6%, respectively. DE supplementation also led to a 19.2% increase in villus thickness.
In the ileum, the effect of ACV diverged; vaccinated birds displayed deeper crypts and a reduced VH/CD ratio by 25.0% and 20.6%, respectively, compared to unvaccinated birds. Additionally, the ileum’s longitudinal muscularis lamina in birds supplemented with DE was 17.8% thinner than that in unsupplemented chickens.
The results of the quantitative analysis examining the intensity of zonula occludens-1 (ZO-1) and claudin-3 IR in the jejunum of broiler chickens at 5 days post Eimeria infection are presented in Table 7. This table presents the OD values for ZO-1 in the villi and claudin-3 in the crypts.

Table 7.
Quantitative analysis of the intensity of zonula occludens-1 and claudin-3 immunoreaction (IR) of the jejunum of broiler chickens at 5 days post infection.
A significant interaction between ACV and DE was observed for both ZO-1 and claudin-3 (p ≤ 0.001 for both) in addition to a significant independent effect of ACV.
In vaccinated birds, DE supplementation led to higher OD values, signifying stronger ZO-1 IR in the villi. Conversely, in unvaccinated birds, DE supplementation was associated with lower OD values for ZO-1 IR.
Regarding claudin-3, dietary DE supplementation was associated with lower OD values, indicating weaker IR in vaccinated birds. However, it did not affect this parameter in unvaccinated birds.
3.4. Immunoglobulin, Cytokines, and Acute Phase Protein Levels
The effects of the experimental factors on plasma immunoglobulin, cytokines, and acute phase protein levels in broiler chickens at 5 days post Eimeria spp. infection are presented in Table 8.

Table 8.
Effects of experimental factors on plasma immunoglobulin, cytokines, and acute phase proteins levels of broiler chickens at 5 d post infection with Eimeria spp.
A significant interaction between ACV and DE (ACV × DE) was observed for IgY. However, post hoc analysis showed that the only significant difference occurred between the group of unvaccinated birds supplemented with DE and the group of vaccinated birds also receiving DE in their diet.
Similarly, a significant interaction between ACV and DE was noted for IgM. The highest IgM levels were observed in vaccinated chickens that were also supplemented with DE whereas DE had no effect on IgM levels in unvaccinated birds.
For IL-6, a significant effect of ACV was evident as vaccinated chickens displayed IL-6 levels that were 19.4% lower than those found in unvaccinated birds.
For IL-1β, a trend toward lower levels was observed in broilers supplemented with DE, irrespective of their vaccination status (p = 0.076).
Regarding IgA, TNF-α, Cp, and Fb, neither ACV nor DE demonstrated significant effects, nor were any significant interactions between these factors detected.
3.5. Biochemical Parameters
The effects of the experimental factors on the biochemical indices of chicken blood collected at 5 days PI are summarized in Table 9. While all parameters were within the normal range for poultry, some were significantly affected by the experimental treatments. A significant interaction between ACV and DE was noted for ALT, TP, and TC levels.

Table 9.
Biochemical parameters of chicken blood sampled at 5 d PI.
In the case of ALT, vaccinated birds exhibited relatively low activity levels that were not influenced by DE supplementation. However, for unvaccinated birds showing the highest ALT activity, DE supplementation led to a reduction in ALT activity, bringing it to a level similar to that observed in vaccinated groups.
For TP, an increased level was noted in birds that were both vaccinated and supplemented with DE. In contrast, DE did not affect this parameter in unvaccinated birds.
For TC, a significant ACV × DE interaction was also observed. The highest TC concentration was found in groups that were both vaccinated and DE-supplemented, compared to either of the unvaccinated groups.
No significant independent effects of ACV or DE, nor any significant interactions between these factors, were detected for the activities of AST, LDH, ALP, or TG and GLU concentrations.
3.6. Volatile Fatty Acids in Cecal Digesta
The effects of experimental factors on the concentrations of VFAs in the cecal digesta of broiler chickens at 42 days of age are presented in Table 10. The dominant VFA was acetic acid, followed by butyric and propionic acids.

Table 10.
Effects of experimental factors on the volatile fatty acid concentrations in the cecal digesta of broiler chickens at 42 d of age.
No significant interactions between ACV and DE were noted for any of the VFAs. Similarly, neither ACV nor DE showed significant effects on the concentrations of acetic, propionic, isobutyric, isovaleric, valeric, branched-chain fatty acids (BCFAs), or total VFAs. However, a significant effect of DE was observed for butyric acid. Chickens supplemented with DE exhibited a significantly higher concentration of butyric acid (by 27%) in the cecal digesta compared to unsupplemented chickens.
4. Discussion
Immunoprophylaxis with anticoccidial vaccines offers several advantages over conventional chemoprophylaxis. These include reduced reliance on anticoccidial drugs, which help to mitigate concerns about drug residues in poultry products and the potential development of drug-resistant parasites [9].
The deterioration of growth performance is a major concern for poultry producers contemplating the use of immunoprophylaxis with live anticoccidial vaccines. According to vaccine manufacturers, immunity should be established by 14 days PV through exposure to vaccine-derived oocysts. These oocysts initially administered through a vaccine dose undergo a developmental cycle in the intestinal epithelial cells. Subsequently, the resulting progeny oocysts are excreted into the environment where they sporulate and are later ingested by birds. For effective immunity development, 2–3 such cycles within the intestinal epithelium are necessary [35]. It is crucial to avoid the use of anticoccidials or other feed additives with coccidiostatic properties during this period. Such additives could interfere with the developmental cycle of vaccine strains, thereby impacting immunity development and potentially diminishing the efficacy of vaccination in the event of a coccidiosis outbreak.
Our analysis of oocyst shedding up to 20 days PV revealed that dietary supplementation with DE did not negatively impact the proliferation of vaccine-derived oocysts. In fact, at 14 days PV, we noted even higher oocyst counts in the group of chickens receiving DE compared to those that were unsupplemented. Oocyst proliferation in the intestinal epithelium often disrupts intestinal integrity, impairs nutrient absorption, and induces inflammation. These changes can negatively impact production parameters during this period [5,6,7]. In the current study, in vaccinated birds we observed increases in FI and FCR without affecting BWG at 20 days of age. It is presumed that, after achieving immunity to coccidiosis, the birds initiated a recovery phase and activated compensatory mechanisms. Previous studies also noted a decline in performance during the first 2–3 weeks after ACV [36,37,38]. Similar to our findings, Das et al. [39] reported that bird performance was particularly affected during the growing phase from 10 to 20 days of age.
In subsequent growing periods after the infection, no deterioration in growth performance was observed among the vaccinated birds. Moreover, the current study affirmed the efficacy of the applied anticoccidial vaccine, Paracox®-5. Vaccinated birds exhibited higher BWG PI, which can be linked to significantly lower oocyst counts in the feces and reduced LS in the small intestine. The significant impact of ACV was also evident in increased BWG for the entire experimental period. The effectiveness of ACVs in inducing immunity and protecting against subsequent Eimeria infections, as well as mitigating their adverse effects on performance, is well-documented. This is evidenced by increased BWG, reduced FCR, and fewer intestinal lesions in vaccinated chickens compared to unvaccinated ones [40,41]. Even if temporary performance setbacks were observed in the first two weeks of PV [42], the overall efficacy of ACVs was confirmed. However, reports suggest that the negative impact of ACV on growth performance in infected chickens can persist until the end of the rearing period. This discrepancy may arise from differences in the species composition of the vaccine and the inoculum used during infection [36]. In such cases, ACV fails to offer complete protection against the challenge, as resistance to Eimeria is species-specific.
No interaction between ACV and DE was observed in any of the experimental periods with respect to growth parameters. During the starter feeding phase, DE significantly reduced FI, which correspondingly led to a decrease in BWG in both vaccinated and unvaccinated groups. In the study by Mao et al. [25], administering 500 mg of dried dandelion per kg of feed led to reduced FI but resulted in a lowered FCR of up to 21 d of age. However, when the dandelion dose was increased to 1000 mg/kg, this effect was not observed. Qureshi et al. [26] reported that dietary supplementation with 0.5% dried dandelion leaves did not impact FI but did reduce FCR and improve BWG for birds aged 1–3 weeks. In the current study, the negative effects of DE were not observed from day 11–20 or thereafter, which could suggest that the applied dose was too high for chicks during the early rearing period. It is worth noting that dandelion is considered a plant with very low toxicity [43]. From another perspective, Yang et al. [38] suggested that the administration of essential oils (EOs) may be more beneficial starting from the grower phase rather than the starter phase given that the gut microbiota is not yet fully established and stable at that early stage.
In the finisher phase, DE supplementation significantly increased BWG and showed a tendency to reduce FCR in our study. This suggests that DE supplementation may have a more pronounced effect on growth performance during later stages and supports the idea of a recovery phase after the onset of infection.
A significant positive effect of DE on decreased oocyst shedding was also found at 7 and 15 days PI. Oocyst output, along with growth performance and LS, is considered one of the crucial criteria for assessing vaccine efficacy [6]. In vaccinated birds, DE supplementation resulted in fewer oocysts being shed at 5 days PI. This outcome may be attributable to the immunomodulatory effects of DE, which enhanced the immune response of the vaccinated birds, thereby increasing their resistance to Eimeria spp. infection. This was reflected in elevated levels of IgY and IgM in vaccinated birds receiving DE in their diet. However, it is important to note that the immunoglobulins measured were not specific to Eimeria but rather represented total antibody levels for the particular class. The cellular response is generally considered to be of greater importance in natural immunity against Eimeria infection and serum antibody levels are thought to be of lesser relevance for immune protection following such an infection [6].
During coccidiosis, the intestinal epithelial tissue suffers damage as oocysts invade and proliferate within intestinal cells, destroying them in the process. Common adverse effects of Eimeria infection on intestinal morphology include a decrease in VH, deeper crypts, and a lower VH/CD ratio. Shorter villi, which are associated with a smaller absorptive area, can lead to malabsorption. Meanwhile, deeper crypts indicate rapid tissue turnover and an increased need for new tissue [44]. In the present study, both vaccination and DE significantly improved morphometric parameters in the jejunum. ACV and DE independently increased villus thickness while vaccinated birds also exhibited shallower crypts and a lower VH/CD ratio. However, in the ileum, ACV led to deeper crypts and a reduced VH/CD ratio without affecting VH. This effect may represent an adaptive mechanism for intestinal epithelial recovery, enhancing cell proliferation and boosting enterocyte turnover following damage by Eimeria development. The use of DE as a dietary supplement was also found to significantly thin the muscularis longitudinal layer, potentially due to a reduction in the accumulation of inflammatory cells. Healthy broilers fed a diet containing 0.5% dandelion showed less cellular infiltration in the mucosa and submucosa compared to the control group, with no effect on villi and crypt architecture [45].
The decrease in the concentration of cecal VFA has been previously reported as a negative effect of Eimeria infection, which could be linked with a shift in microbiota [7,46]. In the current study, neither the concentrations of individual nor total VFAs in cecal digesta were affected by ACV, DE, or their interaction, except for an increase in butyric acid in both groups of birds receiving DE in the diet. However, it is important to note that the cecal digesta were collected after the growth test at 42 d of age. Any potential differences between treatments that might have occurred directly after the infection at 20 d of age may no longer be visible.
The increase in butyrate levels in DE-supplemented groups points to the positive impact of this phytogenic additive on shifting the microbiota towards butyrate-producing species. An elevated level of butyric acid in ileal digesta, along with an affected microbiota profile, has also been previously observed in broilers given dandelion in their diet [25]. This metabolite of microbial fermentation is not only an energy source for enterocytes but also contributes to various other functions. These include gut tissue development, gene expression regulation, and cell differentiation, as well as immune and microbial modulation. Furthermore, it plays a role in reducing oxidative stress and controlling diarrhea [47].
Under healthy conditions, intestinal epithelial TJs effectively prevent the paracellular entry of harmful substances and antigens from the gastrointestinal tract, such as bacteria, toxins, and degraded food components [48,49]. However, in conditions like Eimeria infection, the integrity of the intestinal barrier is compromised, leading to increased permeability to luminal antigens. This “leakiness” allows foreign antigens to penetrate the intestinal tissue [50]. Antigen-presenting cells and T-lymphocytes process these antigens, triggering an inflammatory response. In coccidiosis, there is increased synthesis of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α [51], which further disrupt the TJ barrier and amplify the inflammatory process by allowing more luminal antigens to permeate [48]. There are conflicting reports regarding the response of TJs to Eimeria infection. In a study with graded E. maxima infection in broiler chickens, gene expressions of occludin, ZO-1, claudin-2, and intestinal mucin 2 linearly and quadratically decreased, while the gene expression of claudin-1 was significantly upregulated at 5 days PI. Concurrently, growth performance worsened and gut permeability increased linearly in response to higher levels of E. maxima challenges [52]. Castro et al. [53] observed increased gut permeability at 5 days PI in broilers challenged with Eimeria. There was also an upregulation of serum claudin-1 gene expression by day 6 PI, while the expressions of ZO-1, ZO-2, claudin-2, and occludin remained unaffected compared to unchallenged birds. On the other hand, Alagbe et al. [54] observed upregulated levels of TNF-α, IL-1β, IL-6, and IL-10 with no affected expression of claudin-1 and occludin at 6 d PI. Similarly, Mohsin et al. [55] did not record any difference in the expression of ZO-1 and claudin-1 between the group challenged with E. tenella and the unchallenged group. In our study, at 5 days PI, a significant independent effect of ACV leading to reduced IL-6 levels was observed, along with a trend towards decreased IL-1β in groups supplemented with DE. At the same time, in vaccinated birds, DE supplementation increased the IR intensity for villi ZO-1 but decreased it for claudin-3 IR in the jejunum crypts. In unvaccinated birds, DE reduced ZO-1 IR intensity. ZO-1 is located on the intracellular side of the cell membrane, close to the tight junction strands, and is thought to play a crucial role in tight junction functionality. On the other hand, claudins, a primary component of tight junctions, establish a barrier that regulates paracellular transport within the intestinal epithelium [49]. To our knowledge, as of now, no study on the effect of dandelion on the intestinal barrier in chickens infected with Eimeria has been published. However, in healthy broilers, Mao et al. [25] discovered that a 500 mg/kg dandelion supplementation reduced TNF-α levels and enhanced ileal gene expression for TJ proteins, including claudin, occludin-1, and mucin 1. This was associated with improved feed utilization, without affecting ZO-1 expression. Therefore, the variations in the TJ protein profile observed in the current study in challenged birds might be an adaptive response to infection. Furthermore, the modification in TJ protein composition, combined with the proinflammatory cytokine results from our study in vaccinated birds fed DE-supplemented diets, suggests that these birds may have experienced milder effects from the challenge and maintained a better-protected epithelial barrier.
Increased liver enzymatic activity is often observed during coccidiosis [55]. In the current study, DE significantly lowered ALT activity in unvaccinated birds. This aligns with previous information about the hepatoprotective effect of dandelion, which has been shown in healthy birds to positively influence both enzymatic activity modulation [26] and histological liver examinations by reducing Kupffer cell hyperplasia and perivascular mononuclear cell infiltration [45]. Additionally, the absence of a negative impact of the feed additive on liver enzymatic activity suggests that it does not have a toxic interaction at the dose used in the experiment. An increase in plasma total protein levels was also noted in the group of vaccinated birds in the current study. Previous research has documented a decrease in serum TP levels on days 5–7 following infection with E. acervulina, E. tenella, and E. maxima [56]. Thus, higher TP levels may indicate less damage to the intestinal mucosa from infection, leading to better nutrient absorption or reduced leakage into the intestinal lumen. Previous studies on chickens have demonstrated that dandelion reduces the concentrations of TG, TC, and GLU in the blood of uninfected chickens [24,26]. However, such hypoglycemic and hypocholesterolemic effects at 5 d PI were not observed in the current study.
To sum up the current situation, the practical use of live ACVs in broilers is likely to increase given the current trends to minimize the use of chemotherapeutics due to growing parasite resistance and consumer expectations. As third-generation vaccines (subunit vaccines) are still under development and may take a considerable time to enter the market, poultry producers will continue to rely on live vaccines [9]. Although ACVs are generally effective in protecting against severe effects of coccidiosis, there can be temporary deterioration in growth performance PV, which may not be fully compensated for by the end of the rearing period. Moreover, field infections might arise from species not included in the ACV, in which case the vaccine may not offer full protection as immunity to Eimeria is species-specific. Therefore, nutritional approaches that support immune system development and mitigate the negative effects on growth performance, while also combating infection, are promising. Phytogenic feed additives are, next to probiotics, the most frequently tested alternatives to standard programs against coccidiosis [16,17,18,19,20,57]. T. officinale could be considered a medicinal plant due to its rich content of bioactive substances, which have demonstrated broad pharmacological properties in both in vitro and in vivo studies [21,22,23]. Although this plant is widespread and grows in various soil conditions, being found on every continent except Antarctica [22], its use in poultry nutrition in Poland and the EU is still not very common. To our knowledge, the effectiveness of DE has not yet been tested as part of a coccidiosis control measure in broilers, with the exception of a study in chickens infected with E. tenella where dandelion was part of herbal powder “Shi Ying Zi” consisting of Cnidium monnieri (L.) Cuss, Taraxacum mongolicum Hand.-Mazz., and sodium chloride [58]. The current study validates the effectiveness of DE in mitigating the effects of Eimeria infection in birds. It also confirms the safety of using DE as a feed additive in conjunction with ACV. Importantly, in the current experiment, DE showed no direct oocyst-damaging coccidiocidal effect as indicated in previous studies for oregano, garlic [59], or artemisia, tea tree, thyme, and clove EOs [60], thereby allowing for the continued circulation of vaccine oocysts. The results of this study could support poultry production with reduced or no use of coccidiostats in favor of phytonutrients and/or immunoprophylaxis. However, future studies in large-scale farming conditions are needed to corroborate our results. Also, further in-depth research on the effects of individual selected active substances contained in dandelion would be advisable to determine their exact mode of action in terms of ACV and/or Eimeria challenge.
5. Conclusions
As revealed in our study, ACV effectively mitigated the negative impacts of homologous infection and improved the final BWG in chickens. DE on its own had a positive effect on the growth performance of birds infected with Eimeria in the finisher phase. It reduced PI oocyst shedding, improved intestinal microarchitecture, and positively affected the activity of intestinal microbiota stimulating the cecal production of butyric acid. Furthermore, DE also enhanced the effectiveness of ACV by improving both the humoral immune response and the integrity of the intestinal barrier, as reflected in elevated plasma IgM levels and jejunal TJs proteins profile (ZO-1 and claudin-3), respectively.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13091927/s1, Figure S1: Representative images of the immunohistochemical reactions of zonula occludens-1 and claudin-3 carried out on formaldehyde-fixed sections from the jejunum of broiler chickens sampled at 5 d post challenging with Eimeria spp.; ACV vaccination at 1 d of age with anticoccidial vaccine and DE dietary supplementation with Taraxacum officinale (dandelion) extract. Examples of the reaction are marked with white arrows. All the scale bars represent 100 μm.
Author Contributions
Conceptualization: A.A.-W., S.Ś. and D.J.; methodology: A.A.-W., S.Ś. and D.J.; formal analysis: A.A.-W., E.T., S.M. and P.D.; investigation: A.A.-W., E.T., S.M. and P.D.; resources: A.A.-W., E.T., S.M. and P.D.; data curation: A.A.-W., E.T., S.M. and P.D.; writing—original draft preparation: A.A.-W.; writing—review and editing. A.A.-W., S.Ś., E.T., S.M. and D.J.; visualization: A.A.-W., E.T., S.M. and P.D.; supervision: A.A.-W. and S.Ś.; project administration: A.A.-W., S.Ś. and D.J.; funding acquisition: A.A.-W., S.Ś. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Centre for Research and Development in Poland and conducted within the Biostrateg program: “GUTFEED—innovative nutrition in sustainable poultry production” BIOSTRATEG1/267659/7/NCBR/2015.
Institutional Review Board Statement
The protocols for this study were approved by the second Local Ethical Committee on Animal Testing, Cracow, Poland (Authorization No. 1186/2015). This study was carried out in compliance with Directive 2010/63/EU and by the Act on Experiments on Animals of 21 January 2005 and the Regulation of the Minister of Science and Information Technology of 29 July 2005 on the National Committee for Animal Experimentation.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We thank Herberry Sp. z o.o., especially Andrzej Juszczuk, for providing the extract for our research and assisting with the methodological section of this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mohsin, M.; Li, Y.; Zhang, X.; Wang, Y.; Huang, Z.; Yin, G.; Zhang, Z. Development of CRISPR-CAS9 Based RNA Drugs against Eimeria tenella Infection. Genomics 2021, 113, 4126–4135. [Google Scholar] [CrossRef]
- Mohsin, M.; Li, L.; Huang, X.; Aleem, M.T.; Habib, Y.J.; Ismael, A.; Afzal, M.Z.; Abbas, R.Z.; Abbas, A.; Yin, G. Immunogenicity and Protective Efficacy of Probiotics with EtIMP1C against Eimeria tenella Challenge. Pak. Vet. J. 2021, 41, 274–278. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Cherry, T.E.; Danforth, H.D.; Richards, G.; Shirley, M.W.; Williams, R.B. Sustainable Coccidiosis Control in Poultry Production: The Role of Live Vaccines. Int. J. Parasitol. 2002, 32, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B. Intercurrent Coccidiosis and Necrotic Enteritis of Chickens: Rational, Integrated Disease Management by Maintenance of Gut Integrity. Avian Pathol. 2005, 34, 159–180. [Google Scholar] [CrossRef]
- Soutter, F.; Werling, D.; Tomley, F.M.; Blake, D.P. Poultry Coccidiosis: Design and Interpretation of Vaccine Studies. Front. Vet. Sci. 2020, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yan, Y.; Jian, F.; Ning, C. Coccidia-Microbiota Interactions and Their Effects on the Host. Front. Cell. Infect. Microbiol. 2021, 11, 751481. [Google Scholar] [CrossRef]
- Lin, X. Evaluation of Immunogenicity and Protective Efficacy of Eimeria Maxima Immune Mapped Protein 1 with EDA Adjuvant in Chicken. Pak. Vet. J. 2020, 40, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Tomley, F.M. Securing Poultry Production from the Ever-Present Eimeria Challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Peek, H.W.; Landman, W.J.M. Coccidiosis in Poultry: Anticoccidial Products, Vaccines and Other Prevention Strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef]
- Orso, C.; Stefanello, T.B.; Franceschi, C.H.; Mann, M.B.; Varela, A.P.M.; Castro, I.M.S.; Frazzon, J.; Frazzon, A.P.G.; Andretta, I.; Ribeiro, A.M.L. Changes in the Ceca Microbiota of Broilers Vaccinated for Coccidiosis or Supplemented with Salinomycin. Poult. Sci. 2021, 100, 100969. [Google Scholar] [CrossRef] [PubMed]
- Lehman, R.; Moran, E.T.; Hess, J.B. Response of Coccidiostat- versus Vaccination-Protected Broilers to Gelatin Inclusion in High and Low Crude Protein Diets. Poult. Sci. 2009, 88, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Broussard, C.; Fitz-Coy, S.; Burke, P.; Eckert, N.H.; Stevens, S.M.; Anderson, P.N.; Anderson, S.M.; Caldwell, D.J. Evaluation of Live Oocyst Vaccination or Salinomycin for Control of Field-Strain Eimeria Challenge in Broilers on Two Different Feeding Programs. J. Appl. Poult. Res. 2009, 18, 458–464. [Google Scholar] [CrossRef]
- Allen, P.C.; Fetterer, R.H. Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry. Clin. Microbiol. Rev. 2002, 15, 58–65. [Google Scholar] [CrossRef]
- Lee, Y.; Lu, M.; Lillehoj, H.S. Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines 2022, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Arczewska-Włosek, A.; Świątkiewicz, S. Nutrition as a Modulatory Factor of the Efficacy of Live Anticoccidial Vaccines in Broiler Chickens. Worlds Poult. Sci. J. 2014, 70, 81–92. [Google Scholar] [CrossRef]
- de Andrade, R.M.; Pagnussatt, H.; Talian, L.E.; Santo, A.D.; Ribeiro, A.B.; Leite, F.; Mis, G.; Hoinoski, G.; Aniecevski, E.; Fabiani, L.M.; et al. Interaction between Live Vaccines for Coccidiosis and Phytogenic Compounds in the Diet of Broilers. Parasitol. Int. 2022, 89, 102584. [Google Scholar] [CrossRef]
- Saeed, Z.; Alkheraije, K.A. Botanicals: A Promising Approach for Controlling Cecal Coccidiosis in Poultry. Front. Vet. Sci. 2023, 10, 1157633. [Google Scholar] [CrossRef]
- Park, I.; Nam, H.; Wickramasuriya, S.S.; Lee, Y.; Wall, E.H.; Ravichandran, S.; Lillehoj, H.S. Host-Mediated Beneficial Effects of Phytochemicals for Prevention of Avian Coccidiosis. Front. Immunol. 2023, 14, 1145367. [Google Scholar] [CrossRef]
- Yang, C.; Das, Q.; Rehman, M.A.; Yin, X.; Shay, J.; Gauthier, M.; Lau, C.H.-F.; Ross, K.; Diarra, M.S. Microbiome of Ceca from Broiler Chicken Vaccinated or Not against Coccidiosis and Fed Berry Pomaces. Microorganisms 2023, 11, 1184. [Google Scholar] [CrossRef]
- Qureshi, S.; Adil, S.; El-Hack, M.E.A.; Alagawany, M.; Farag, M.R. Beneficial Uses of Dandelion Herb (Taraxacum officinale) in Poultry Nutrition. Worlds Poult. Sci. J. 2017, 73, 591–602. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; de Falco, B.; Lanzotti, V.; Bonanomi, G. Common Dandelion: A Review of Its Botanical, Phytochemical and Pharmacological Profiles. Phytochem. Rev. 2019, 18, 1115–1132. [Google Scholar] [CrossRef]
- Jędrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of Antioxidant Activity of Phenolic Fractions from the Leaves and Petals of Dandelion in Human Plasma Treated with H2O2 and H2O2/Fe. Chem. Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.S.; Kadhim, A.H.; Ali, M.A. The Effect of Feeding Different Levels of Dandelion Leaf Powder (Taraxacum officinale) in the Diet on the Productive and Physiological Performance of Broiler Chickens, Strain Ross-308. IOP Conf. Ser. Earth Environ. Sci. 2021, 722, 012002. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Wang, W.; Duan, T.; Yin, N.; Guo, T.; Guo, H.; Liu, N.; An, X.; Qi, J. Effects of Taraxacum mongolicum Hand.-Mazz. (Dandelion) on Growth Performance, Expression of Genes Coding for Tight Junction Protein and Mucin, Microbiota Composition and Short Chain Fatty Acids in Ileum of Broiler Chickens. BMC Vet. Res. 2022, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Banday, M.T.; Adil, S.; Shakeel, I.; Munshi, Z.H. Effect of Dandelion Leaves and Fenugreek Seeds with or without Enzyme Addition on Performance and Blood Biochemistry of Broiler Chicken, and Evaluation of Their in Vitro Antibacterial Activity. Indian J. Anim. Sci. 2015, 85, 1248–1254. [Google Scholar] [CrossRef]
- Smulikowska, S.; Rutkowski, A. Recommended Allowances and Nutritive Value of Feedstuffs. Poultry Feeding Standards, 4th ed.; The Kielanowski Institute of Animal Physiology and Nutrition, PAS, Polish Branch of WPSA: Jabłonna, Poland, 2005; ISBN 83-917097-7-9. (In Polish) [Google Scholar]
- FAO. Epidemiology, Diagnosis and Control of Poultry Parasites; Manuel FAO de Santé Animale; FAO: Rome, Italy, 1998; ISBN 92-5-104215-2. [Google Scholar]
- Johnson, J.; Reid, W.M. Anticoccidial Drugs: Lesion Scoring Techniques in Battery and Floor-Pen Experiments with Chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.; Świątkiewicz, S.; Arczewka-Włosek, A.; Muszyński, S.; Szymańczyk, S.; Arciszewski, M.B.; Siembida, A.Z.; Kras, K.; Piedra, J.L.V.; Schwarz, T.; et al. Modern Hybrid Rye, as an Alternative Energy Source for Broiler Chickens, Improves the Absorption Surface of the Small Intestine Depending on the Intestinal Part and Xylanase Supplementation. Animals 2021, 11, 1349. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Dobrowolski, P.; Arciszewski, M.B.; Donaldson, J.; Kras, K.; Abramowicz, B.; Kuc, D.; Muszyński, S. Basal Intestinal Morphology, Immunolocalization of Leptin and Ghrelin and Their Receptors in Newborn Wistar Rats after Prenatal Exposure to Fumonisins. Animals 2023, 13, 1538. [Google Scholar] [CrossRef] [PubMed]
- Kisielinski, K.; Willis, S.; Prescher, A.; Klosterhalfen, B.; Schumpelick, V. A Simple New Method to Calculate Small Intestine Absorptive Surface in the Rat. Clin. Exp. Med. 2002, 2, 131–135. [Google Scholar] [CrossRef]
- Muszyński, S.; Hułas-Stasiak, M.; Dobrowolski, P.; Arciszewski, M.B.; Hiżewska, L.; Donaldson, J.; Mozel, S.; Rycerz, K.; Kapica, M.; Puzio, I.; et al. Maternal Acrylamide Exposure Changes Intestinal Epithelium, Immunolocalization of Leptin and Ghrelin and Their Receptors, and Gut Barrier in Weaned Offspring. Sci. Rep. 2023, 13, 10286. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006; ISBN 978-0-935584-77-6. [Google Scholar]
- Sharman, P.A.; Smith, N.C.; Wallach, M.G.; Katrib, M. Chasing the Golden Egg: Vaccination against Poultry Coccidiosis. Parasite Immunol. 2010, 32, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peebles, E.D.; Kiess, A.S.; Wamsley, K.G.S.; Zhai, W. Effects of Coccidial Vaccination and Dietary Antimicrobial Alternatives on the Growth Performance, Internal Organ Development, and Intestinal Morphology of Eimeria-Challenged Male Broilers. Poult. Sci. 2019, 98, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Lee, K.W.; Bravo, D.; Lillehoj, E.P. Effects of Dietary Supplementation with Phytonutrients on Vaccine-Stimulated Immunity against Infection with Eimeria tenella. Vet. Parasitol. 2011, 181, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of Encapsulated Cinnamaldehyde and Citral on the Performance and Cecal Microbiota of Broilers Vaccinated or Not Vaccinated against Coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef]
- Das, Q.; Shay, J.; Gauthier, M.; Yin, X.; Hasted, T.-L.; Ross, K.; Julien, C.; Yacini, H.; Kennes, Y.M.; Warriner, K.; et al. Effects of Vaccination Against Coccidiosis on Gut Microbiota and Immunity in Broiler Fed Bacitracin and Berry Pomace. Front. Immunol. 2021, 12, 621803. [Google Scholar] [CrossRef]
- Crouch, C.F.; Andrews, S.J.; Ward, R.G.; Francis, M.J. Protective Efficacy of a Live Attenuated Anticoccidial Vaccine Administered to 1-Day-Old Chickens. Avian Pathol. 2003, 32, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.J.; Livingston, M.L.; Nogal, B.; Hoang, V.; Wang, Y.-T.; Crespo, R.; Livingston, K.A. Effect of Coccidial Challenge and Vaccination on the Performance, Veterinary Postmortem Scores, and Blood Biochemistry of Broiler Chickens. Poult. Sci. 2020, 99, 3831–3840. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.F.; Allee, G.L.; Knight, C.D.; Dibner, J.J. Impact of Glutamine and Oasis Hatchling Supplement on Growth Performance, Small Intestinal Morphology, and Immune Response of Broilers Vaccinated and Challenged with Eimeria Maxima. Poult. Sci. 2005, 84, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A Review on Its Phytochemical and Pharmacological Profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Choct, M. Managing Gut Health through Nutrition. Br. Poult. Sci. 2009, 50, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Banday, M.T.; Shakeel, I.; Adil, S.; Mir, M.S.; Beigh, Y.A.; Amin, U. Histomorphological Studies of Broiler Chicken Fed Diets Supplemented with Either Raw or Enzyme Treated Dandelion Leaves and Fenugreek Seeds. Vet. World 2016, 9, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Gordillo Jaramillo, F.X.; Kim, D.-H.; Lee, S.H.; Kwon, S.-K.; Jha, R.; Lee, K.-W. Role of Oregano and Citrus Species-Based Essential Oil Preparation for the Control of Coccidiosis in Broiler Chickens. J. Anim. Sci. Biotechnol. 2021, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Bedford, A.; Gong, J. Implications of Butyrate and Its Derivatives for Gut Health and Animal Production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of Cytokine Modulation of Epithelial Tight Junction Barrier. Front. Biosci. J. Virtual Libr. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef]
- De Meyer, F.; Eeckhaut, V.; Ducatelle, R.; Dhaenens, M.; Daled, S.; Dedeurwaerder, A.; De Gussem, M.; Haesebrouck, F.; Deforce, D.; Van Immerseel, F. Host Intestinal Biomarker Identification in a Gut Leakage Model in Broilers. Vet. Res. 2019, 50, 46. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Min, W.; Dalloul, R.A. Recent Progress on the Cytokine Regulation of Intestinal Immune Responses to Eimeria1. Poult. Sci. 2004, 83, 611–623. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Yadav, S.; Castro, F.L.d.S.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria Challenge Linearly Regulated Growth Performance, Dynamic Change of Gastrointestinal Permeability, Apparent Ileal Digestibility, Intestinal Morphology, and Tight Junctions of Broiler Chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Teng, P.-Y.; Yadav, S.; Gould, R.L.; Craig, S.; Pazdro, R.; Kim, W.K. The Effects of L-Arginine Supplementation on Growth Performance and Intestinal Health of Broiler Chickens Challenged with Eimeria spp. Poult. Sci. 2020, 99, 5844–5857. [Google Scholar] [CrossRef]
- Alagbe, E.O.; Schulze, H.; Adeola, O. Growth Performance, Nutrient Digestibility, Intestinal Morphology, Cecal Mucosal Cytokines and Serum Antioxidant Responses of Broiler Chickens to Dietary Enzymatically Treated Yeast and Coccidia Challenge. J. Anim. Sci. Biotechnol. 2023, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Zhang, Z.; Yin, G. Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella. Vaccines 2022, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Ruff, M.D.; Augustine, P.C. Effects of Coccidiosis on the Electrophoretic Pattern of Serum Proteins in Chickens. J. Parasitol. 1982, 68, 107–111. [Google Scholar] [CrossRef] [PubMed]
- El-Shall, N.A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical Control of Poultry Coccidiosis: A Review. Poult. Sci. 2022, 101, 101542. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Chen, S.; Jia, R.; Huang, Y.; Zou, Y.; Li, L.; Zhao, X.; Yin, Z. Anticoccidial Effect of Herbal Powder “Shi Ying Zi” in Chickens Infected with Eimeria tenella. Animals 2020, 10, 1484. [Google Scholar] [CrossRef]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Baldo, D.; Ratti, C.; Piva, A.; Grilli, E. Investigating the Effects of Essential Oils and Pure Botanical Compounds against Eimeria tenella in Vitro. Poult. Sci. 2023, 102, 102898. [Google Scholar] [CrossRef]
- Remmal, A.; Achahbar, S.; Bouddine, L.; Chami, N.; Chami, F. In Vitro Destruction of Eimeria Oocysts by Essential Oils. Vet. Parasitol. 2011, 182, 121–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).