Effects of Dietary Supplemental Chlorogenic Acid and Baicalin on the Growth Performance and Immunity of Broilers Challenged with Lipopolysaccharide
Abstract
1. Introduction
2. Materials and Methods
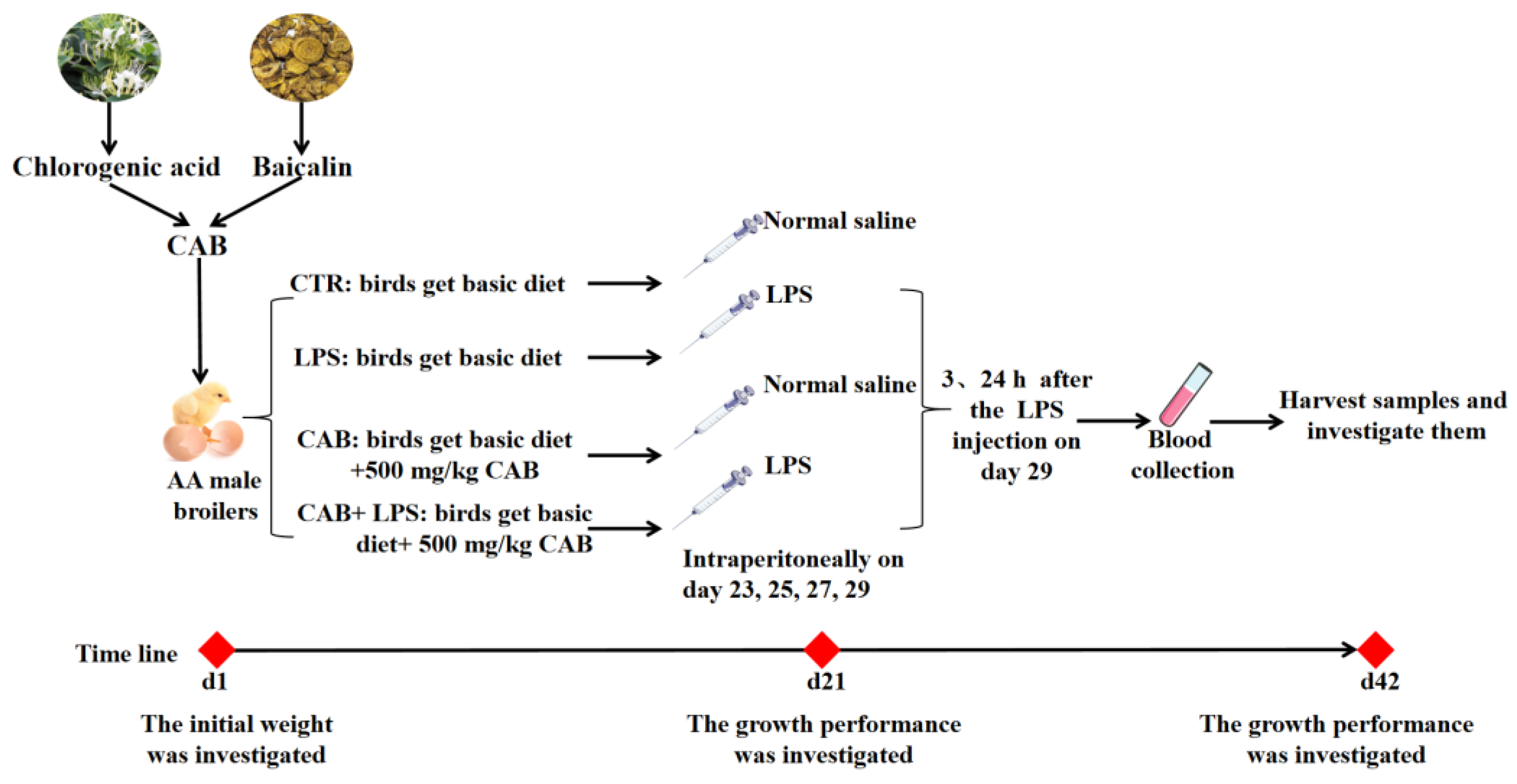
2.1. Experimental Design and Animal Management
2.2. Growth Performance and Organ Index
2.3. Jejunum Morphology
2.4. Serum Levels of Diamine Oxidase, Immunoglobulins, and Antioxidant-Related Enzymes
2.5. The Levels of Cytokines in the Spleen
2.6. Data Analysis
3. Results
3.1. Growth Performance
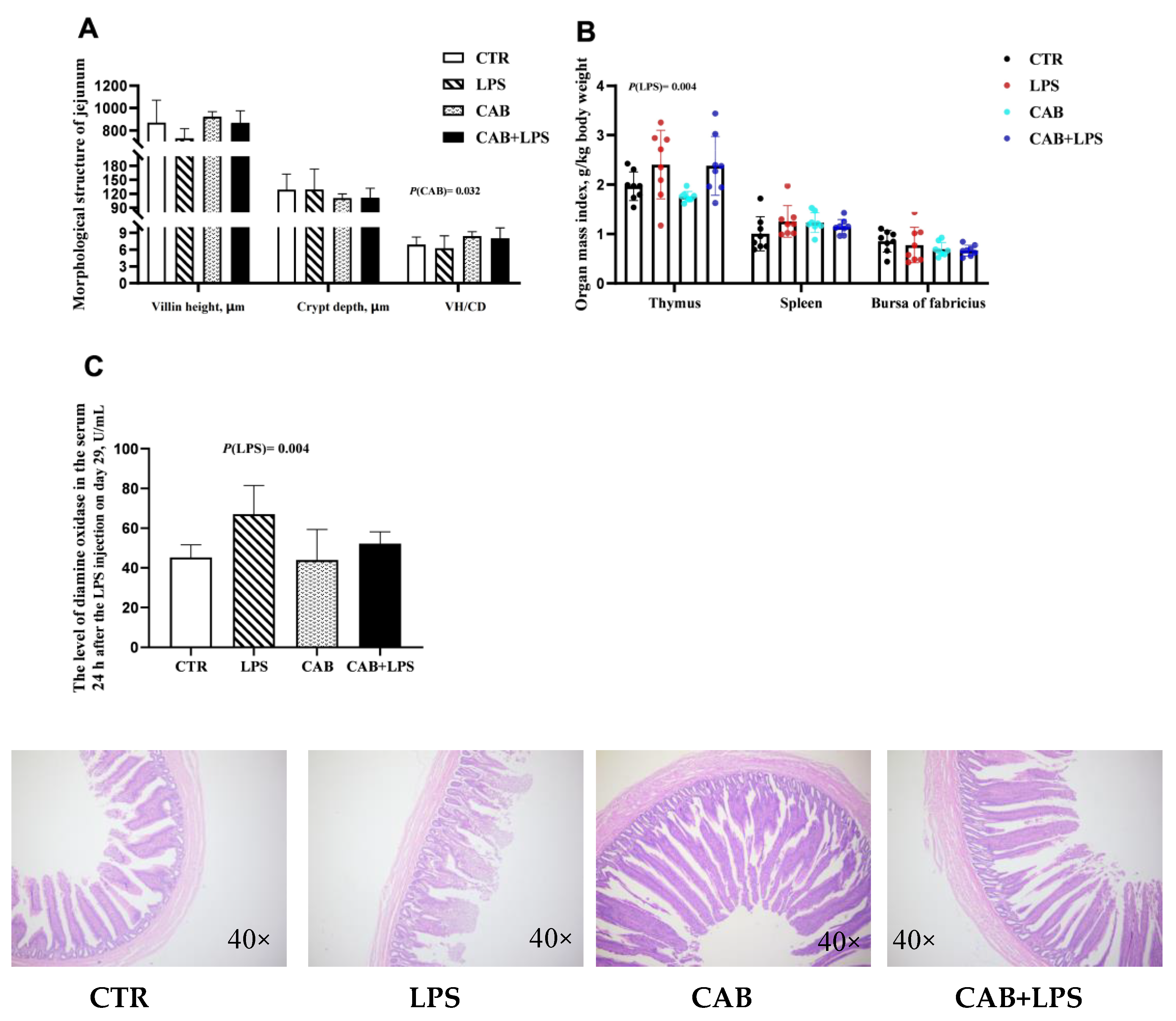
3.2. Intestinal Morphology and Organ Index
3.3. Antioxidant and Immunity Function
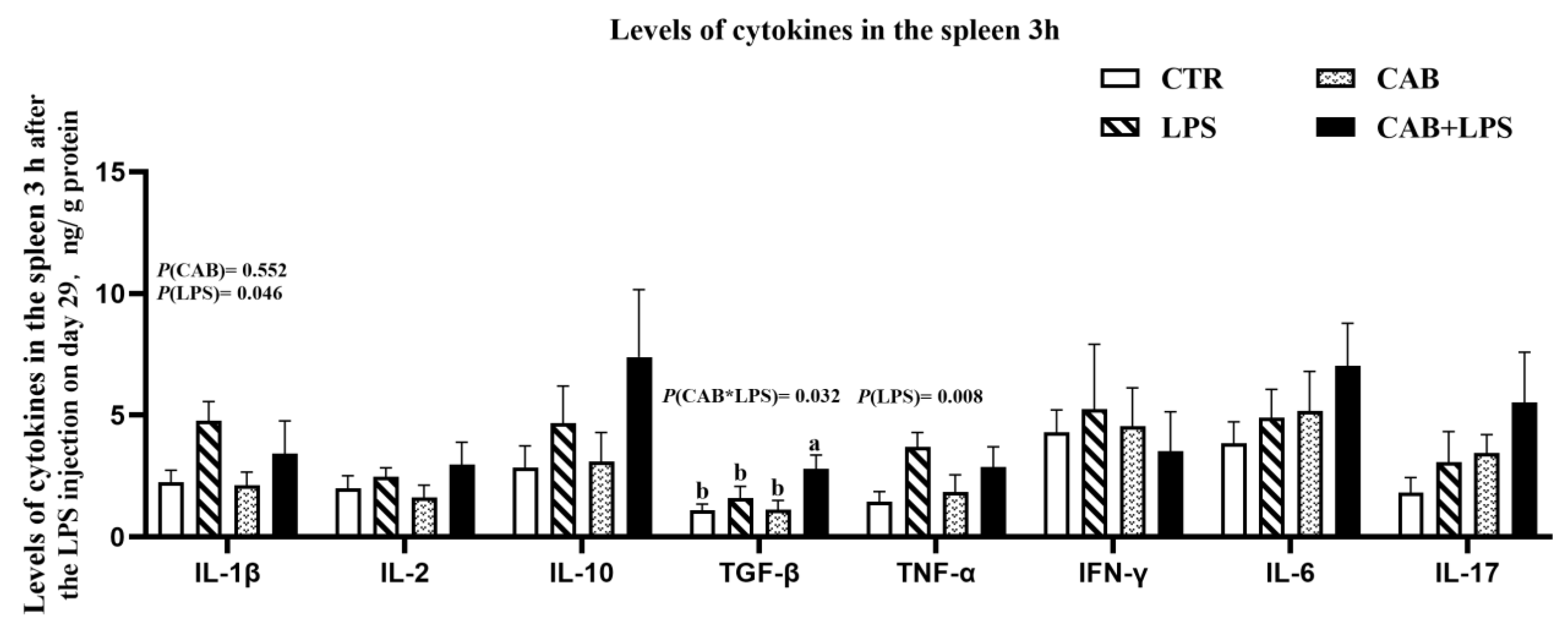
3.4. Spleen Cytokines mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, Z.; Dai, H.; Wei, Q.; Jin, S.; Wang, J.; Wei, X.; Yuan, Y.; Yu, D.; Shi, F. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile. Poult. Sci. 2020, 99, 3411–3427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Z.X.; He, M.L.; Pastor, J.J.; Tedo, G.; Liu, J.X.; Wang, H.F. Olive oil cake extract stabilizes the physiological condition of lipopolysaccharide-challenged piglets by reducing oxidative stress and inflammatory responses and modulating the ileal microbiome. Food Funct. 2021, 12, 10171–10183. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Homedes, J.; Klasing, K.C. Prevention of immunologic stress contributes to the growth-permitting ability of dietary antibiotics in chicks. J. Nutr. 1992, 122, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Nobs, S.P.; Zmora, N.; Elinav, E. Nutrition Regulates Innate Immunity in Health and Disease. Annu. Rev. Nutr. 2020, 40, 189–219. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Shi, A.; Li, T.; Zheng, Y.; Song, Y.; Wang, H.; Wang, N.; Dong, L.; Shi, H. Chlorogenic Acid Improves NAFLD by Regulating gut Microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef]
- Liu, J.; Poojary, M.M.; Zhu, L.; Williams, A.R.; Lund, M.N. Phenolic Acid-Amino Acid Adducts Exert Distinct Immunomodulatory Effects in Macrophages Compared to Parent Phenolic Acids. J. Agric. Food Chem. 2023, 71, 2344–2355. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Wang, J.; Wang, X.; Hu, N.; Wang, S. Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct. 2021, 12, 5637–5649. [Google Scholar] [CrossRef]
- Fu, Y.J.; Xu, B.; Huang, S.W.; Luo, X.; Deng, X.L.; Luo, S.; Liu, C.; Wang, Q.; Chen, J.Y.; Zhou, L. Baicalin prevents LPS-induced activation of TLR4/NF-kappaB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol. Sin. 2021, 42, 88–96. [Google Scholar] [CrossRef]
- Zhao, J.S.; Deng, W.; Liu, H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019, 98, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Shi, S.; Zhou, Y.; Zhang, K.; Wang, Y.; Zhao, J. Chlorogenic acid improves growth performance and intestinal health through autophagy-mediated nuclear factor erythroid 2-related factor 2 pathway in oxidatively stressed broilers induced by dexamethasone. Poult. Sci. 2022, 101, 102036. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, M.; Fu, J.; Yan, S.; Liu, Y.; Mahmood, T.; Lv, Z.; Guo, Y. Dietary soya saponin improves the lipid metabolism and intestinal health of laying hens. Poult. Sci. 2022, 101, 101663. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, L.; Xu, Z.J.; De Marco, M.; Briens, M.; Yan, X.H.; Sun, L.H. Hydroxy-Selenomethionine Improves the Selenium Status and Helps to Maintain Broiler Performances under a High Stocking Density and Heat Stress Conditions through a Better Redox and Immune Response. Antioxidants 2021, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Verwoolde, M.B.; van Baal, J.; Jansen, C.A.; Graat, E.A.M.; Lamot, D.M.; Lammers, A.; van Eck, L. Transgenerational effects of innate immune activation in broiler breeders on growth performance and immune responsiveness. Poult. Sci. 2021, 100, 101413. [Google Scholar] [CrossRef]
- Douglass, J.D.; Dorfman, M.D.; Fasnacht, R.; Shaffer, L.D.; Thaler, J.P. Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 2017, 6, 366–373. [Google Scholar] [CrossRef]
- Cheng, X.; Cao, Z.; Luo, J.; Hu, R.; Cao, H.; Guo, X.; Xing, C.; Yang, F.; Zhuang, Y.; Hu, G. Baicalin ameliorates APEC-induced intestinal injury in chicks by inhibiting the PI3K/AKT-mediated NF-kappaB signaling pathway. Poult. Sci. 2022, 101, 101572. [Google Scholar] [CrossRef]
- Wickramasuriya, S.S.; Park, I.; Lee, K.; Lee, Y.; Kim, W.H.; Nam, H.; Lillehoj, H.S. Role of Physiology, Immunity, Microbiota, and Infectious Diseases in the Gut Health of Poultry. Vaccines 2022, 10, 172. [Google Scholar] [CrossRef]
- Krauze, M.; Cendrowska-Pinkosz, M.; Matuseviĉius, P.; Stępniowska, A.; Jurczak, P.; Ognik, K. The Effect of Administration of a Phytobiotic Containing Cinnamon Oil and Citric Acid on the Metabolism, Immunity, and Growth Performance of Broiler Chickens. Animals 2021, 11, 399. [Google Scholar] [CrossRef]
- Dieryck, I.; De Backere, J.; Paeshuyse, J. Effect of hatching system and prophylactic antibiotic use on serum levels of intestinal health biomarker diamine oxidase in broilers at an early age. Animal 2022, 16, 100493. [Google Scholar] [CrossRef]
- Duangnumsawang, Y.; Zentek, J.; Goodarzi, B.F. Development and Functional Properties of Intestinal Mucus Layer in Poultry. Front. Immunol. 2021, 12, 745849. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. Influence of Plant Bioactive Compounds on Intestinal Epithelial Barrier in Poultry. Mini Rev. Med. Chem. 2020, 20, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Zhang, L.; Gao, F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021, 100, 100909. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Meng, X.; Chen, S.; Huang, D.; Xia, Y.; Zhu, S. Antioxidant activities of chlorogenic acid derivatives with different acyl donor chain lengths and their stabilities during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 357, 129904. [Google Scholar] [CrossRef]
- Paudel, K.R.; Kim, D.W. Microparticles-Mediated Vascular Inflammation and its Amelioration by Antioxidant Activity of Baicalin. Antioxidants 2020, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Amasheh, S.; Aschenbach, J.R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3237–3266. [Google Scholar] [CrossRef]
- Ding, D.; Mou, D.; Zhao, L.; Jiang, X.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Zhuo, Y.; Li, J.; et al. Maternal organic selenium supplementation alleviates LPS induced inflammation, autophagy and ER stress in the thymus and spleen of offspring piglets by improving the expression of selenoproteins. Food Funct. 2021, 12, 11214–11228. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef]
- Das, Q.; Shay, J.; Gauthier, M.; Yin, X.; Hasted, T.L.; Ross, K.; Julien, C.; Yacini, H.; Kennes, Y.M.; Warriner, K.; et al. Effects of Vaccination Against Coccidiosis on Gut Microbiota and Immunity in Broiler Fed Bacitracin and Berry Pomace. Front. Immunol. 2021, 12, 621803. [Google Scholar] [CrossRef]
- Song, B.; Tang, D.; Yan, S.; Fan, H.; Li, G.; Shahid, M.S.; Mahmood, T.; Guo, Y. Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 42. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Wang, Y.; Tian, Y.; Chen, X.; Wu, Z.; Lan, T.; Li, Y.; Bai, M.; Liu, J.; et al. Major depression accompanied with inflammation and multiple cytokines alterations: Evidences from clinical patients to macaca fascicularis and LPS-induced depressive mice model. J. Affect. Disord. 2020, 271, 262–271. [Google Scholar] [CrossRef] [PubMed]



| Items | d 1–d 21 | d 22–d 42 |
|---|---|---|
| Corn, 7.8% protein | 56.73 | 59.95 |
| Soybean meal, 46% protein | 31.80 | 27.40 |
| corn gluten meal, 44% protein | 2.50 | 2.50 |
| Cottonseed meal, 43.5% protein | 2.00 | 2.50 |
| Soybean oil | 2.88 | 4.00 |
| CaHPO₄ | 1.81 | 1.51 |
| Stone powder | 1.28 | 1.22 |
| NaCl | 0.35 | 0.35 |
| DL-Methionine, 98% | 0.21 | 0.12 |
| L-Lysine HCl, 78% | 0.12 | 0.13 |
| Vitamin premix ① | 0.02 | 0.02 |
| Mineral premix ② | 0.20 | 0.20 |
| Choline chloride, 50% | 0.10 | 0.10 |
| Total | 100 | 100 |
| Nutrition levels ③ | ||
| ME (MJ/kg) | 12.34 | 12.76 |
| Crude protein, % | 21.50 | 19.50 |
| Calcium, % | 1.00 | 0.90 |
| Total phosphorus, % | 0.71 | 0.64 |
| Available phosphorus, % | 0.45 | 0.40 |
| Lysine, % | 1.10 | 1.00 |
| Methionine, % | 0.50 | 0.40 |
| Methionine+ Cystine, % | 0.92 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Li, P.; Wang, Z.; Gao, M.; Li, G.; Nie, W.; Xiao, L.; Lv, Z.; Guo, Y. Effects of Dietary Supplemental Chlorogenic Acid and Baicalin on the Growth Performance and Immunity of Broilers Challenged with Lipopolysaccharide. Life 2023, 13, 1645. https://doi.org/10.3390/life13081645
Lv H, Li P, Wang Z, Gao M, Li G, Nie W, Xiao L, Lv Z, Guo Y. Effects of Dietary Supplemental Chlorogenic Acid and Baicalin on the Growth Performance and Immunity of Broilers Challenged with Lipopolysaccharide. Life. 2023; 13(8):1645. https://doi.org/10.3390/life13081645
Chicago/Turabian StyleLv, Huiyuan, Peng Li, Zhiming Wang, Mingkun Gao, Guang Li, Wei Nie, Lei Xiao, Zengpeng Lv, and Yuming Guo. 2023. "Effects of Dietary Supplemental Chlorogenic Acid and Baicalin on the Growth Performance and Immunity of Broilers Challenged with Lipopolysaccharide" Life 13, no. 8: 1645. https://doi.org/10.3390/life13081645
APA StyleLv, H., Li, P., Wang, Z., Gao, M., Li, G., Nie, W., Xiao, L., Lv, Z., & Guo, Y. (2023). Effects of Dietary Supplemental Chlorogenic Acid and Baicalin on the Growth Performance and Immunity of Broilers Challenged with Lipopolysaccharide. Life, 13(8), 1645. https://doi.org/10.3390/life13081645






