Eosinophil Cationic Protein Variation in Patients with Asthma and CRSwNP Treated with Dupilumab
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Study Design and Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Asthma Control Test |
| ECP | Eosinophil cationic protein |
| EOS | Eosinophil count |
| FeNO | Fractional exhaled nitric oxide |
| FEV1 | Forced expiratory volume in the first second. |
| FVC | Forced vital capacity. |
| GINA | Global Initiative for Asthma |
| IgE | Immunoglobulin E |
| IL-4 | Interleukin-4 |
| IL-4Rα | Interleukin-4 receptor α |
| IL-5 | Interleukin-5 |
| IL-13 | Interleukin-13 |
| ILC2 | Type 2 Innate Lymphoid Cells |
| mAbs | Monoclonal antibodies |
| NO | Nitric Oxide |
| OCS | oral corticosteroids |
| TH2 | T helper 2 |
| SAEs | Severe Adverse Events |
| SNOT22 | Sino-Nasal Outcome Test 22 |
References
- Holgate, S.T.; Thomas, M.; Holgate, S.T.; Thomas, M. Chapter 7—Asthma. In Middleton’s Allergy Essentials; O’Hehir, R.E., Holgate, S.T., Sheikh, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–204. ISBN 9780323375795. [Google Scholar] [CrossRef]
- Pelaia, G.; Vatrella, A.; Busceti, M.T.; Gallelli, L.; Calabrese, C.; Terracciano, R.; Maselli, R. Cellular Mechanisms Underlying Eosinophilic and Neutrophilic Airway Inflammation in Asthma. Mediat. Inflamm. 2015, 2015, 879783. [Google Scholar] [CrossRef]
- Bakakos, A.; Loukides, S. Severe Eosinophilic Asthma. J. Clin. Med. 2019, 8, 1375. [Google Scholar] [CrossRef]
- Gomes, S.C.; Delemarre, T.; Holtappels, G.; Van Zele, T.; Derycke, L.; Bonne, E.; Eeckels, A.-S.; Zhang, N.; Voegels, R.L.; Bachert, C. Olfaction in nasal polyp patients after Reboot surgery: An endotype-based prospective study. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 2821–2830. [Google Scholar] [CrossRef]
- Jonstam, K.; Swanson, B.N.; Mannent, L.P.; Cardell, L.; Tian, N.; Wang, Y.; Zhang, D.; Fan, C.; Holtappels, G.; Hamilton, J.D.; et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy 2019, 74, 743–752. [Google Scholar] [CrossRef]
- Tokunaga, T.; Sakashita, M.; Haruna, T.; Asaka, D.; Takeno, S.; Ikeda, H.; Nakayama, T.; Seki, N.; Ito, S.; Murata, J.; et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: The JESREC Study. Allergy 2015, 70, 995–1003. [Google Scholar] [CrossRef]
- Kambara, R.; Minami, T.; Akazawa, H.; Tsuji, F.; Sasaki, T.; Inohara, H.; Horii, A. Lower Airway Inflammation in Eosinophilic Chronic Rhinosinusitis as Determined by Exhaled Nitric Oxide. Int. Arch. Allergy Immunol. 2017, 173, 225–232. [Google Scholar] [CrossRef]
- Uraguchi, K.; Kariya, S.; Makihara, S.; Okano, M.; Haruna, T.; Oka, A.; Fujiwara, R.; Noda, Y.; Nishizaki, K. Pulmonary function in patients with eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 2018, 45, 476–481. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Mullol, J.; Woessner, K.M.; Amin, N.; Mannent, L.P. Chronic Rhinosinusitis with Nasal Polyps and Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1133–1141. [Google Scholar] [CrossRef]
- Pelaia, C.; Pelaia, G.; Maglio, A.; Tinello, C.; Gallelli, L.; Lombardo, N.; Terracciano, R.; Vatrella, A. Pathobiology of Type 2 Inflammation in Asthma and Nasal Polyposis. J. Clin. Med. 2023, 12, 3371. [Google Scholar] [CrossRef]
- Papi, A.; Corren, J.; Castro, M.; Domingo, C.; Rogers, L.; Chapman, K.R.; Jackson, D.J.; Daizadeh, N.; Pandit-Abid, N.; Gall, R.; et al. Dupilumab reduced impact of severe exacerbations on lung function in patients with moderate-to-severe type 2 asthma. Allergy 2023, 78, 233–243. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Armentaro, G.; Calabrese, C.; Sciacqua, A.; Gallelli, L.; Vatrella, A. Biological Therapy of Severe Asthma with Dupilumab, a Dual Receptor Antagonist of Interleukins 4 and 13. Vaccines 2022, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.R.; Ackerman, S.J. Eosinophil Granule Proteins: Form and Function. J. Biol. Chem. 2014, 289, 17406–17415. [Google Scholar] [CrossRef] [PubMed]
- Laorden, D.; Hernández, I.; Domínguez-Ortega, J.; Romero, D.; Álvarez-Sala, R.; Quirce, S. A real life cohort of Mepolizumab treatment in severe eosinophilic asthma. Eur. Ann. Allergy Clin. Immunol. 2023. [Google Scholar] [CrossRef]
- Lu, P.-C.; Lee, T.-J.; Huang, C.-C.; Chang, P.-H.; Chen, Y.-W.; Fu, C.-H. Serum eosinophil cationic protein: A prognostic factor for early postoperative recurrence of nasal polyps. Int. Forum Allergy Rhinol. 2021, 11, 766–772. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nagase, H.; Sugimoto, N.; Yamamoto, S.; Tanaka, A.; Fukunaga, K.; Atsuta, R.; Tagaya, E.; Hojo, M.; Gon, Y.; et al. Mepolizumab decreased the levels of serum galectin-10 and eosinophil cationic protein in asthma. Asia Pac. Allergy 2021, 11, e31. [Google Scholar] [CrossRef]
- Nielsen, L.P.; Peterson, C.G.B.; Dahl, R. Serum eosinophil granule proteins predict asthma risk in allergic rhinitis. Allergy 2009, 64, 733–737. [Google Scholar] [CrossRef]
- Nair, P.; Ochkur, S.I.; Protheroe, C.; Radford, K.; Efthimiadis, A.; Lee, N.A.; Lee, J.J. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy 2013, 68, 1177–1184. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Mozzanica, F.; Preti, A.; Gera, R.; Gallo, S.; Bulgheroni, C.; Bandi, F.; Ottaviani, F.; Castelnuovo, P. Cross-cultural adaptation and validation of the SNOT-22 into Italian. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. Affil. Ger. Soc. Oto-Rhino-Laryngol. Head Neck Surg. 2017, 274, 887–895. [Google Scholar] [CrossRef]
- Pini, L.; Caruso, C.; Colantuono, S.; Bagnasco, D.; Maxwell, A.; Price, R.G.; Howarth, P.; Canonica, G.W. Prospective Italian real-world study of mepolizumab in severe eosinophilic asthma validates retrospective outcome reports. Clin. Transl. Allergy 2021, 11, e12067. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Am. J. Respir. Crit. Care Med. 2022, 205, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Louis, R.; Satia, I.; Ojanguren, I.; Schleich, F.; Bonini, M.; Tonia, T.; Rigau, D.; Brinke, A.T.; Buhl, R.; Loukides, S.; et al. European Respiratory Society guidelines for the diagnosis of asthma in adults. Eur. Respir. J. 2022, 60, 2101585. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Drick, N.; Fuge, J.; Welte, T.; Suhling, H. Eosinophilic cationic protein as marker for response to antibody therapy in severe asthma. ERJ Open Res. 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.W.; Ocampo, C.J.; Berdnikovs, S.; Sakashita, M.; Mahdavinia, M.; Suh, L.; Takabayashi, T.; Norton, J.E.; Hulse, K.E.; Conley, D.B.; et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rioja, A.; Chacón, P.; Fernández-Delgado, L.; Doukkali, B.; Rodríguez, A.d.V.; Perkins, J.R.; Ranea, J.A.G.; Dominguez-Cereijo, L.; Pérez-Machuca, B.M.; Palacios, R.; et al. Regulation and directed inhibition of ECP production by human neutrophils. Front. Immunol. 2022, 13, 1015529. [Google Scholar] [CrossRef]
- Shah, S.N.; Grunwell, J.R.; Mohammad, A.F.; Stephenson, S.T.; Lee, G.B.; Vickery, B.P.; Fitzpatrick, A.M. Performance of Eosinophil Cationic Protein as a Biomarker in Asthmatic Children. J. Allergy Clin. Immunol. Pract. 2021, 9, 2761–2769.e2. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Scott, G.; Asrat, S.; Allinne, J.; Lim, W.K.; Nagashima, K.; Birchard, D.; Srivatsan, S.; Ajithdoss, D.K.; Oyejide, A.; Ben, L.-H.; et al. IL-4 and IL-13, not eosinophils, drive type 2 airway inflammation, remodeling and lung function decline. Cytokine 2023, 162, 156091. [Google Scholar] [CrossRef]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef]
- Sánchez, J.; Biol, A.S.; Biol, M.M.; García, E.; López, J.-F. Immunoglobulin E and G autoantibodies against eosinophil proteins in children and adults with asthma and healthy subjects. World Allergy Organ. J. 2023, 16, 100742. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Nuss, S.J.; Rothe, T.; Siebenhüner, A.; Akdis, C.A.; Menz, G. Differential serum protein markers and the clinical severity of asthma. J. Asthma Allergy 2014, 7, 67–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujieda, S.; Matsune, S.; Takeno, S.; Ohta, N.; Asako, M.; Bachert, C.; Inoue, T.; Takahashi, Y.; Fujita, H.; Deniz, Y.; et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status. Allergy 2022, 77, 186–196. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Dupilumab (n = 21) | Control Group (n = 21) |
|---|---|---|
| Age (years) Median ± IQR | 55 ± 17.3 | 45 ± 27.3 |
| Female, n | 11 | 10 |
| Never smoker (%) | 71 | 66 |
| CRSwNP (%) | 100 | 100 |
| Asthma (%) | 85 | 100 |
| Atopic | 9 | 11 |
| ECP | Minimum | Median | Maximum | IQR | p Value |
|---|---|---|---|---|---|
| Control group | 4.5 | 69.5 | 147 | 65 | 0.35 |
| Dupilumab group | 20.3 | 120 | 318 | 128 |
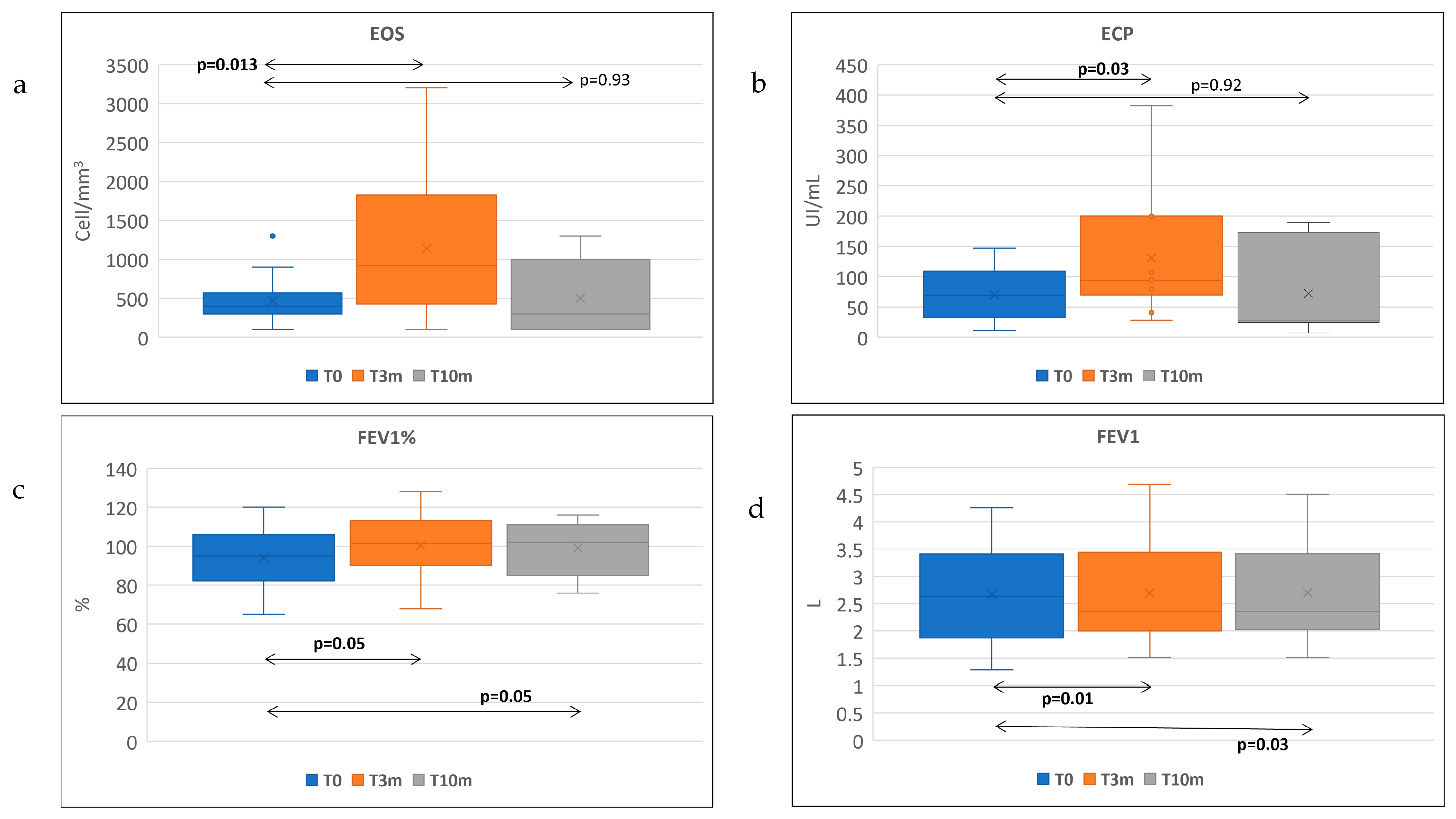
| T0 (Median ± IQR) | T3 (Median ± IQR) | p Value (T0–T3) | T0 (Median ± IQR) | T10 (Median ± IQR) | p Value (T0–T10) | |
|---|---|---|---|---|---|---|
| Eosinophil count (/μL) | 450 ± 233 | 860 ± 1079 | p = 0.1 | 450 ± 275 | 300 ± 833 | p = 0.93 |
| ECP (μg/L) | 69.5 ± 69 | 107 ± 118 | p = 0.03 | 100 ± 65 | 36.5 ± 155 | p = 0.90 |
| Total IgE (IU/mL) | 247 ± 333 | 99 ± 191 | p = 0.002 | 195.5 ± 210 | 53.2 ± 78 | p = 0.01 |
| FEV1 % | 96.5 ± 28 | 104 ± 9 | p = 0.01 | 92 ± 19 | 104 ± 16 | p = 0.03 |
| FEV1 | 2.99 ± 1.4 | 2.34 ± 1.35 | p = 0.52 | 2.23 ± 2.35 | 2.36 | p = 0.05 |
| FeNO (ppb) | 48 ± 44 | 13 ± 18 | p = 0.04 | 49 ± 27.5 | 46 ± 28 | p = 0.01 |
| ACT | 21 ± 6 | 22.5 ± 7 | p = 0.08 | 21 ± 11 | 22 ± 6 | p = 0.09 |
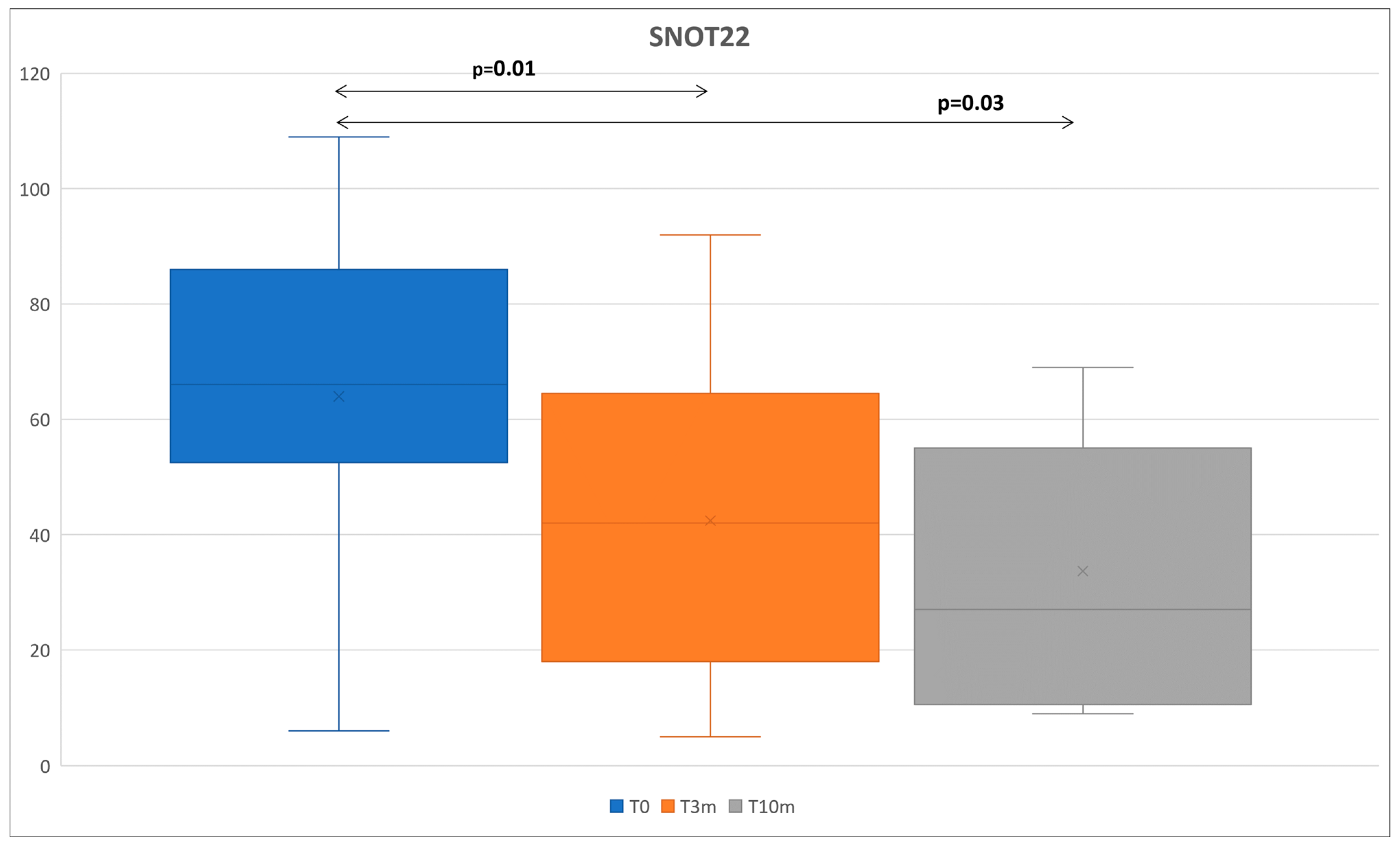
| SNOT-22 | 66.5 ± 34 | 42 ±46 | p = 0.01 | 53 ± 45 | 27 ± 44 | p = 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledda, A.G.; Costanzo, G.; Sambugaro, G.; Caruso, C.; Bullita, M.; Di Martino, M.L.; Serra, P.; Firinu, D.; Del Giacco, S. Eosinophil Cationic Protein Variation in Patients with Asthma and CRSwNP Treated with Dupilumab. Life 2023, 13, 1884. https://doi.org/10.3390/life13091884
Ledda AG, Costanzo G, Sambugaro G, Caruso C, Bullita M, Di Martino ML, Serra P, Firinu D, Del Giacco S. Eosinophil Cationic Protein Variation in Patients with Asthma and CRSwNP Treated with Dupilumab. Life. 2023; 13(9):1884. https://doi.org/10.3390/life13091884
Chicago/Turabian StyleLedda, Andrea Giovanni, Giulia Costanzo, Giada Sambugaro, Cristiano Caruso, Martina Bullita, Maria Luisa Di Martino, Paolo Serra, Davide Firinu, and Stefano Del Giacco. 2023. "Eosinophil Cationic Protein Variation in Patients with Asthma and CRSwNP Treated with Dupilumab" Life 13, no. 9: 1884. https://doi.org/10.3390/life13091884
APA StyleLedda, A. G., Costanzo, G., Sambugaro, G., Caruso, C., Bullita, M., Di Martino, M. L., Serra, P., Firinu, D., & Del Giacco, S. (2023). Eosinophil Cationic Protein Variation in Patients with Asthma and CRSwNP Treated with Dupilumab. Life, 13(9), 1884. https://doi.org/10.3390/life13091884








