Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Coronary Angiography
2.3. OCT Acquisition Technique and Image Analysis
3. Results
3.1. Clinical Data
3.2. Coronary Angiography Data
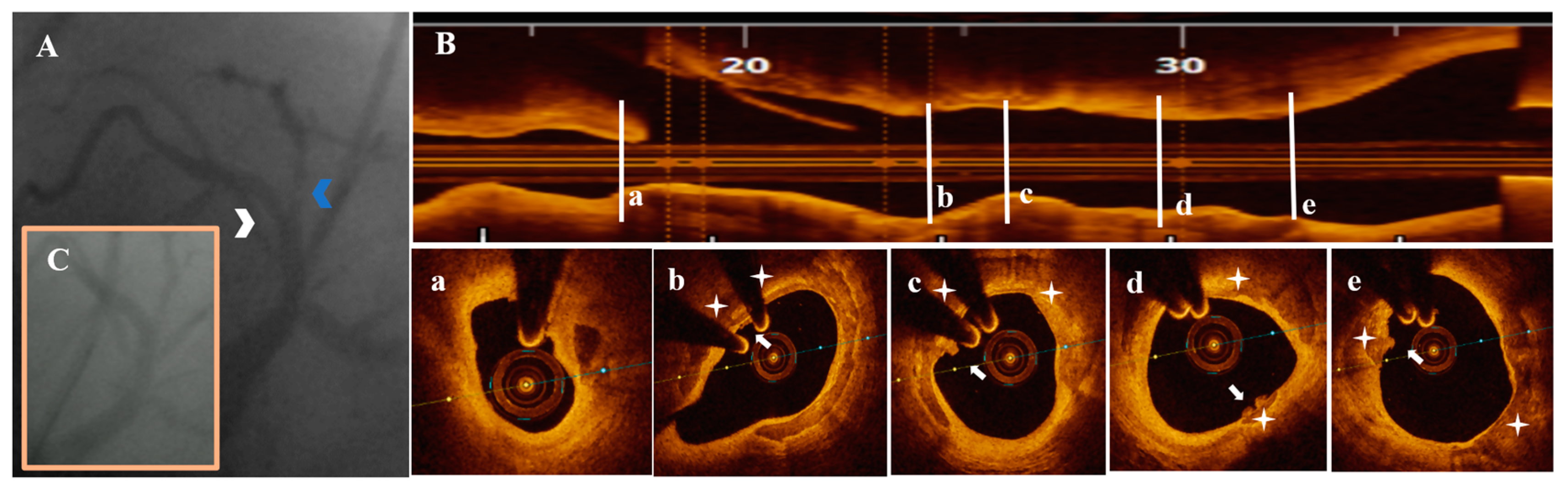
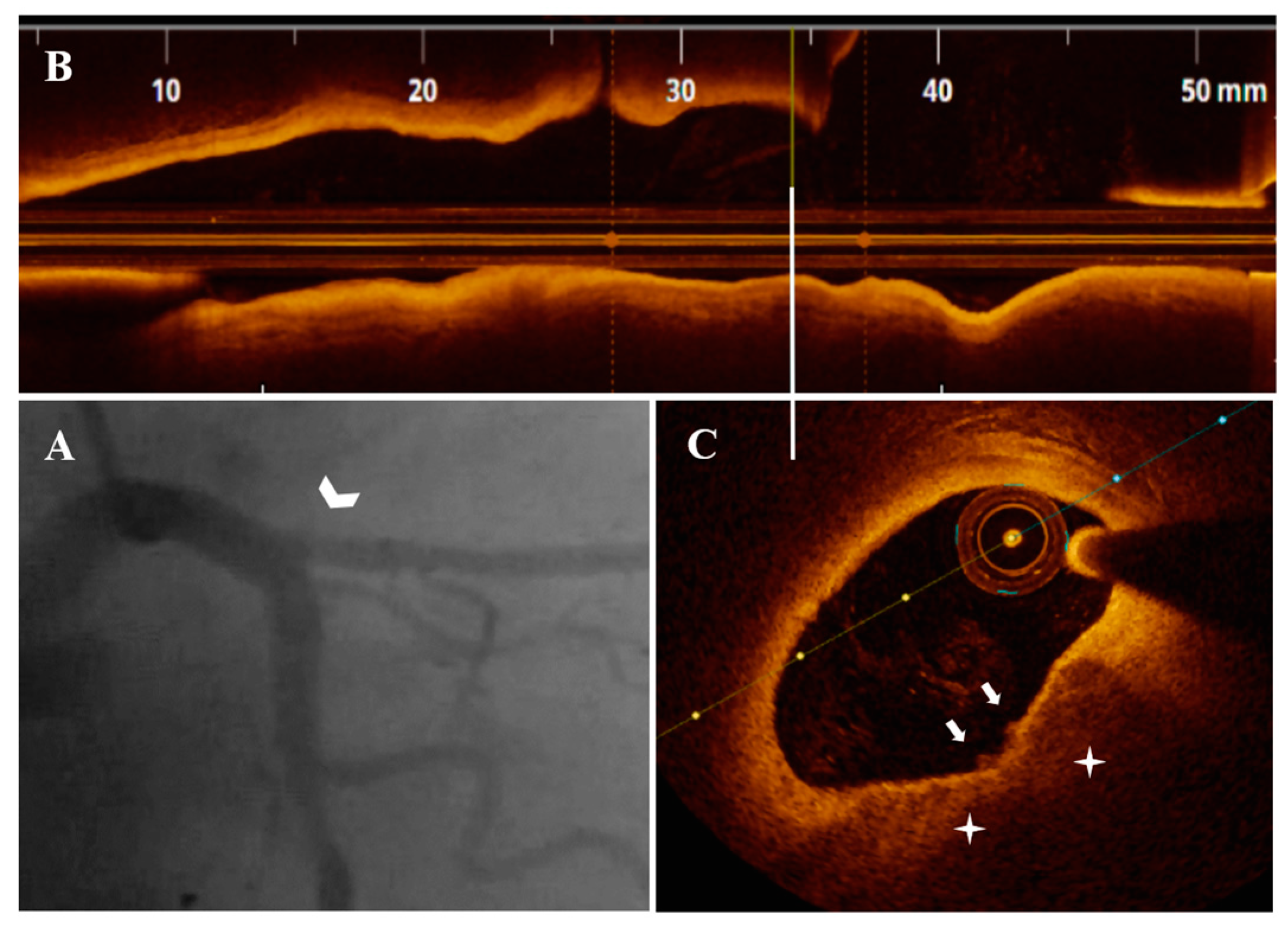
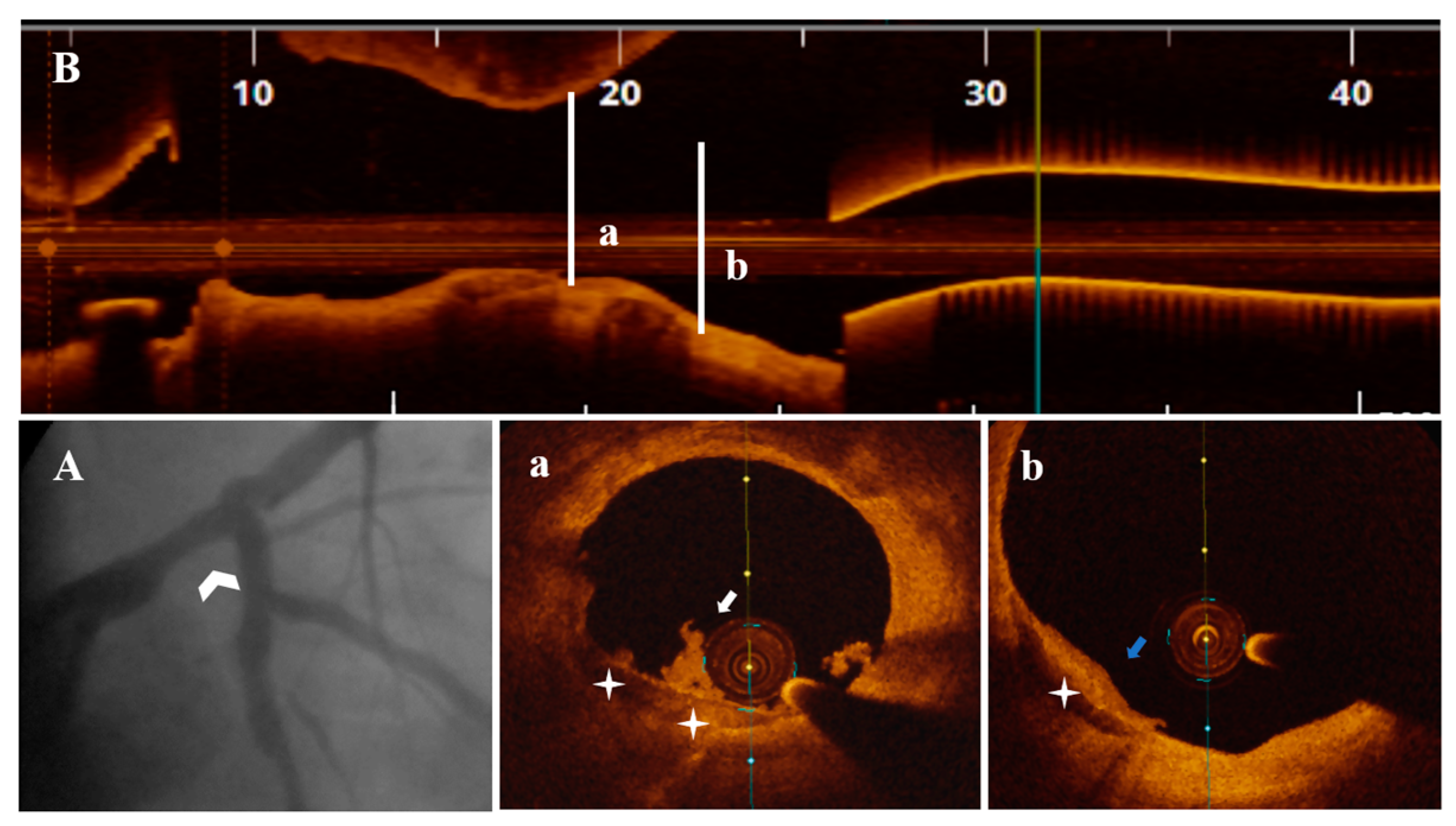
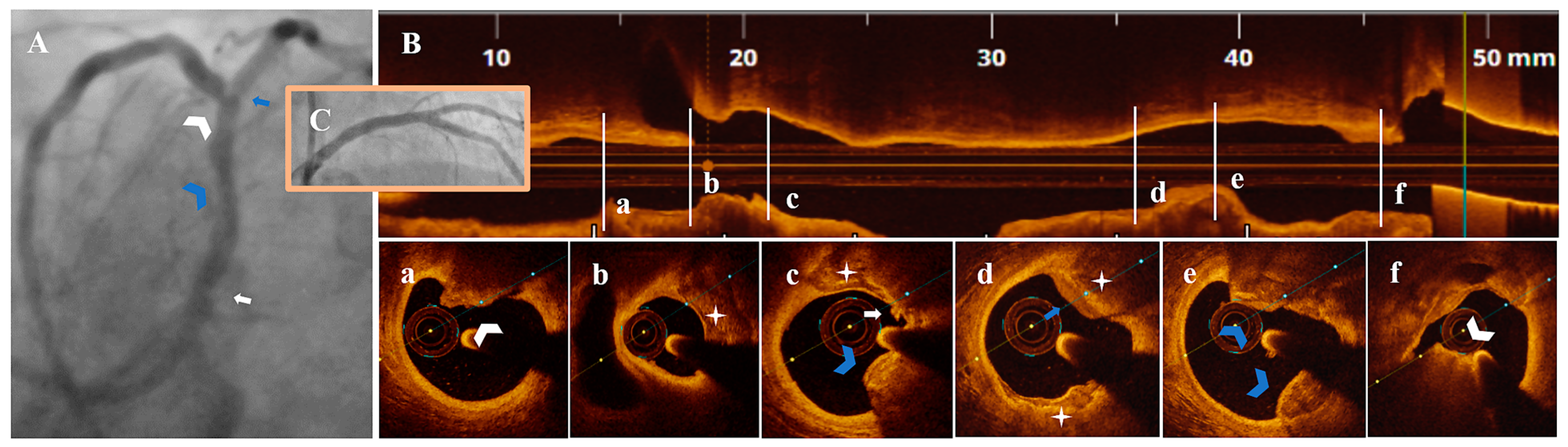
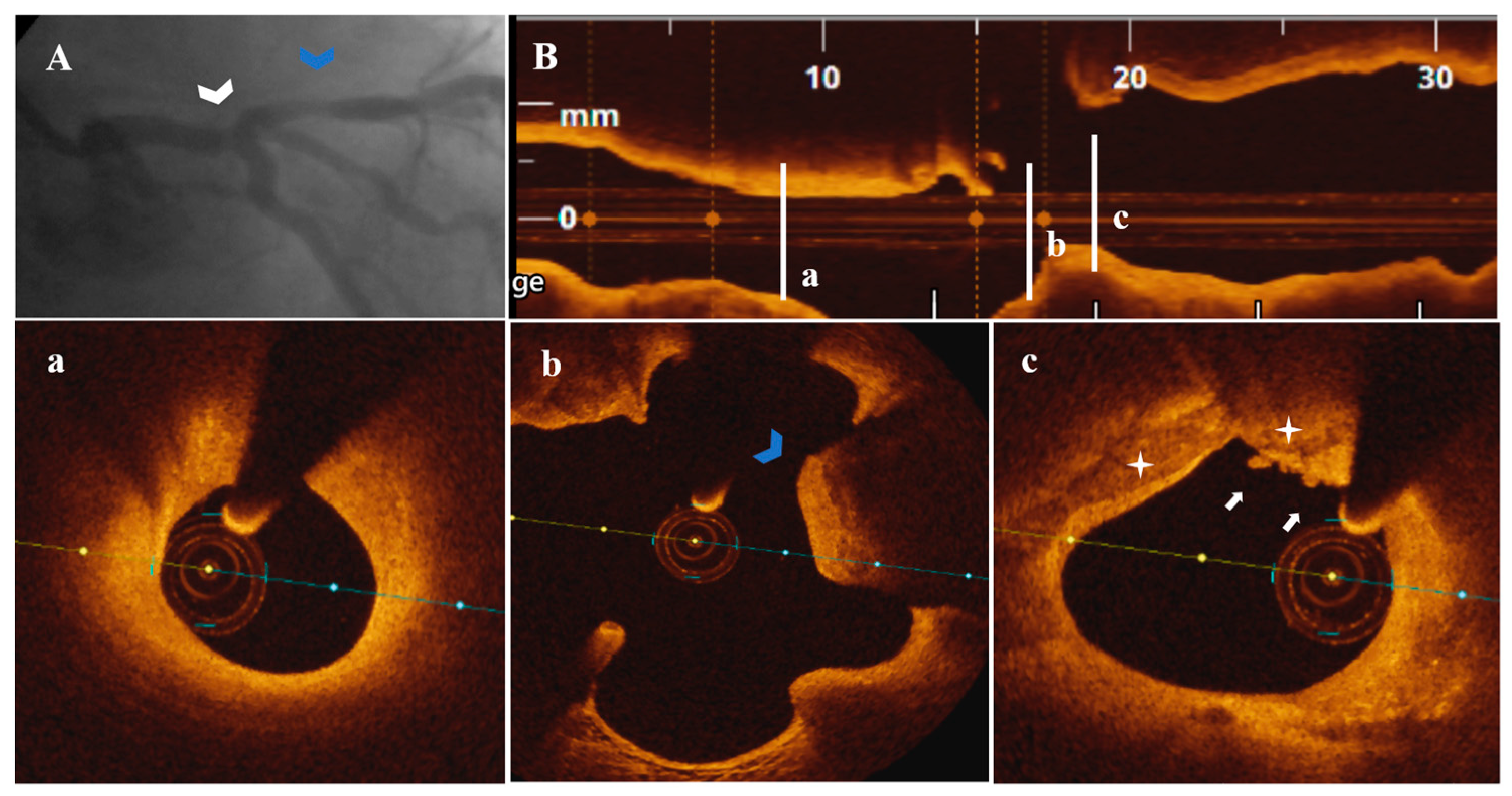
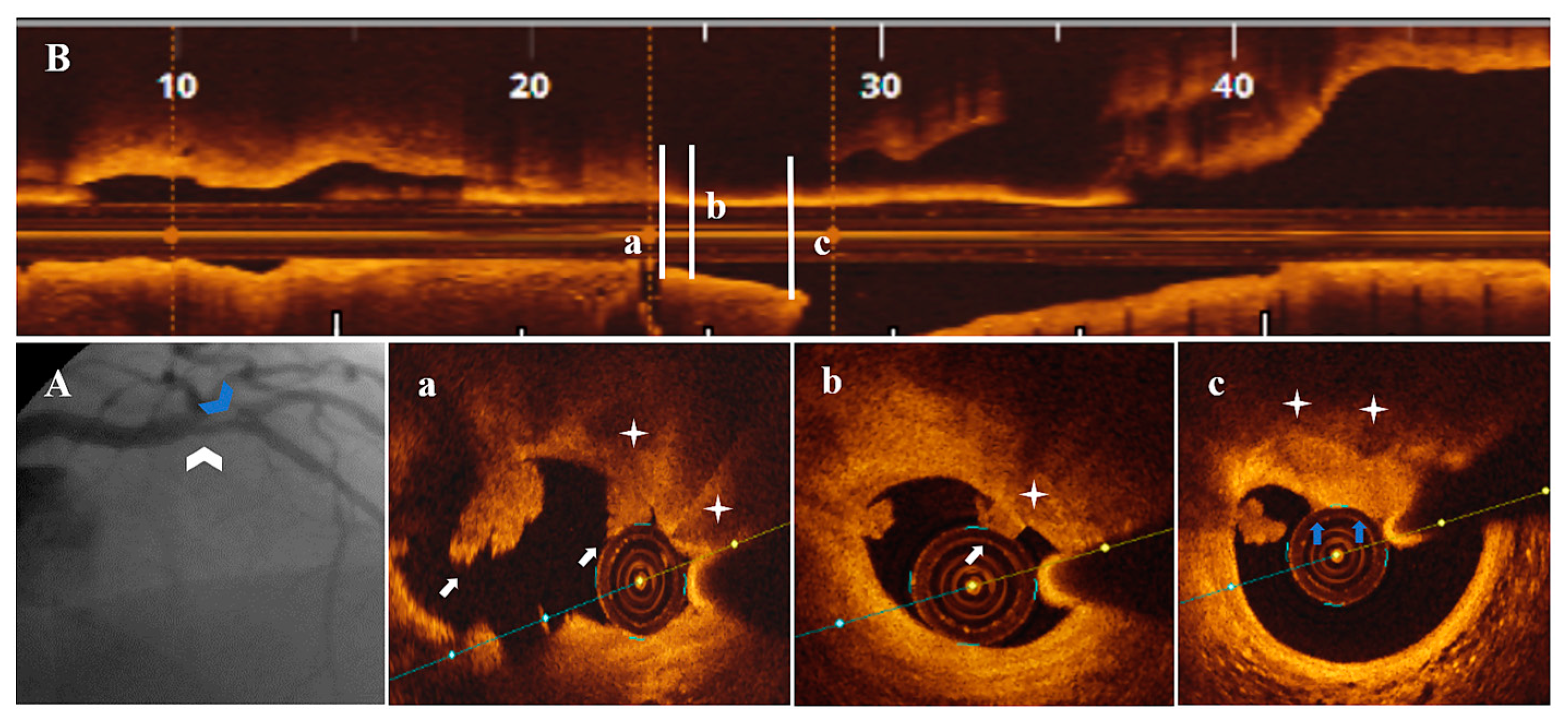
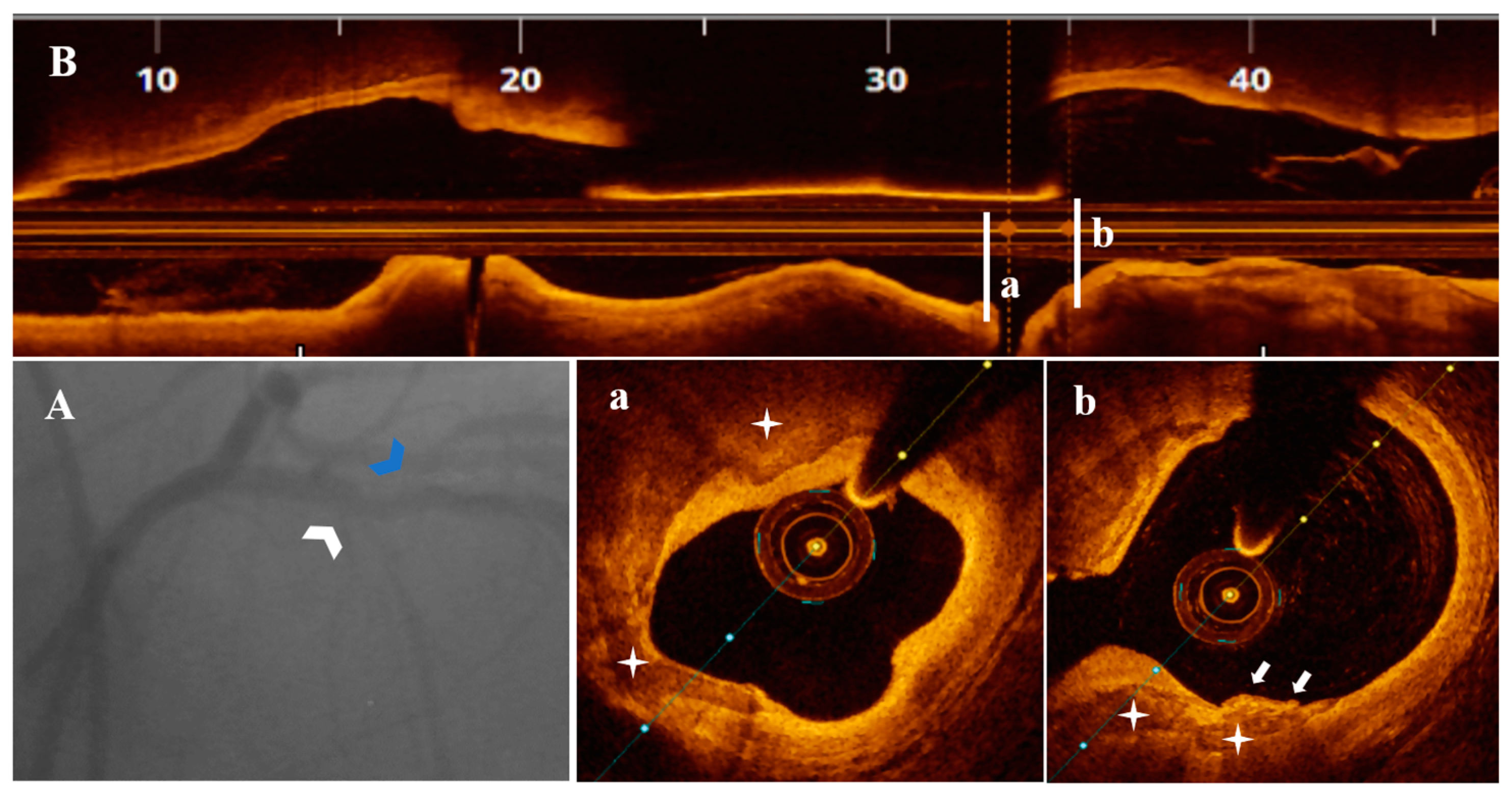
3.3. OCT Data
3.4. Treatment Strategy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crea, F.; Libby, P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation 2017, 136, 1155–1166. [Google Scholar]
- Onea, H.-L.; Spinu, M.; Homorodean, C.; Olinic, M.; Lazar, F.-L.; Ober, M.C.; Stoian, D.; Itu, L.M.; Olinic, D.M. Distinctive Morphological Patterns of Complicated Coronary Plaques in Acute Coronary Syndromes: Insights from an Optical Coherence Tomography Study. Diagnostics 2022, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Spînu, M.; Onea, L.H.; Homorodean, C.; Olinic, M.; Ober, M.C.; Olinic, D.M. Optical Coherence Tomography-OCT for Characterization of Non-Atherosclerotic Coronary Lesions in Acute Coronary Syndromes. J. Clin. Med. 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Onea, H.L.; Lazar, F.L.; Olinic, D.M.; Homorodean, C.; Cortese, B. The role of optical coherence tomography in guiding percutaneous coronary interventions- is left main the final challenge? Minerva Cardiol. Angiol. 2022. [CrossRef] [PubMed]
- Cortese, B.; Piraino, D.; Gentile, D.; Onea, H.; Lazar, L. Intravascular imaging for left main stem assessment: An update on the most recent clinical data. Catheter. Cardiovasc. Interv. 2022, 100, 1220–1228. [Google Scholar] [CrossRef]
- Moț, D.C.; Șerban, A.M.; Achim, A.; Dădârlat-Pop, A.; Tomoaia, R.; Pop, D. Clinical Characteristics and Outcomes following Percutaneous Coronary Intervention in Unprotected Left Main Disease: A Single-Center Study. Diagnostics 2023, 13, 1333. [Google Scholar] [CrossRef]
- Olinic, D.M.; Spinu, M.; Homorodean, C.; Ober, M.C.; Olinic, M. Real-Life Benefit of OCT Imaging for Optimizing PCI Indications, Strategy, and Results. J. Clin. Med. 2019, 8, 437. [Google Scholar] [CrossRef]
- Achim, A.; Kákonyi, K.; Nagy, F.; Jambrik, Z.; Varga, A.; Nemes, A.; Chan, J.S.K.; Toth, G.G.; Ruzsa, Z. Radial Artery Calcification in Predicting Coronary Calcification and Atherosclerosis Burden. Cardiol. Res. Pract. 2022, 2022, 5108389. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Dai, J.; Fang, C.; Zhang, S.; Hou, J.; Xing, L.; Li, L.; Wang, Y.; Wang, J.; Wang, Y.; Tu, Y.; et al. Not All Plaque Erosions Are Equal: Novel Insights From 1,660 Patients with STEMI: A Clinical, Angiographic, and Intravascular OCT Study. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 516–518. [Google Scholar] [CrossRef]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar]
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; et al. Calcified Plaques in Patients With Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [PubMed]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.-M.; Chowdhary, S.; et al. International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [PubMed]
- de la Torre Hernandez, J.M.; Hernandez, F.H.; Alfonso, F.; Rumoroso, J.R.; Lopez-Palop, R.; Sadaba, M.; Carrillo, P.; Rondan, J.; Lozano, I.; Nodar, J.M.R.; et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J. Am. Coll. Cardiol. 2011, 58, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Joner, M.; Prati, F.; Virmani, R.; Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 2014, 11, 379–389. [Google Scholar] [CrossRef]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wu, T.; Zhao, J.; Du, Z.; Wang, Z.; Li, L.; Wei, G.; Tian, J.; Jia, H.; Mintz, G.S.; et al. Focal Geometry and Characteristics of Erosion-Prone Coronary Plaques in vivo Angiography and Optical Coherence Tomography Study. Front. Cardiovasc. Med. 2021, 8, 709480. [Google Scholar] [CrossRef]
- Alfonso, F.; Gonzalo, N.; Nuñez-Gil, I.; Bañuelos, C. Coronary thrombosis from large, nonprotruding, superficial calcified coronary plaques. J. Am. Coll. Cardiol. 2013, 62, 2254. [Google Scholar] [CrossRef]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736, Erratum in: Arterioscler. Thromb. Vasc. Biol. 2014, 34, e17. [Google Scholar] [CrossRef]
- Saita, T.; Fujii, K.; Hao, H.; Imanaka, T.; Shibuya, M.; Fukunaga, M.; Miki, K.; Tamaru, H.; Horimatsu, T.; Nishimura, M.; et al. Histopathological validation of optical frequency domain imaging to quantify various types of coronary calcifications. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 342–349. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Sato, Y.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawakami, R.; Gadhoke, N.V.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis 2020, 306, 85–95. [Google Scholar] [CrossRef]
- Nakajima, A.; Araki, M.; Kurihara, O.; Minami, Y.; Soeda, T.; Yonetsu, T.; Crea, F.; Takano, M.; Higuma, T.; Kakuta, T.; et al. Comparison of post-stent optical coherence tomography findings among three subtypes of calcified culprit plaques in patients with acute coronary syndrome. Catheter. Cardiovasc. Interv. 2021, 97, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Yin, Y.; Liu, X.; Fang, C.; Jiang, S.; Xu, X.; Sun, S.; Pei, X.; Jia, R.; Tang, C.; et al. Clinical Outcomes of Different Calcified Culprit Plaques in Patients with Acute Coronary Syndrome. J. Clin. Med. 2022, 11, 4018. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Thondapu, V.; Poon, E.; Sugiyama, T.; Fracassi, F.; Dijkstra, J.; Lee, H.; Ooi, A.; Barlis, P.; Jang, I.-K. Endothelial Shear Stress and Plaque Erosion: A Computational Fluid Dynamics and Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2019, 12, 374–375. [Google Scholar] [CrossRef]
- Richardson, P.D.; Davies, M.J.; Born, G.V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 1989, 2, 941–944. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Y.; Yang, J.; Zhai, G.; Zhou, Y.; Guo, Q. Prevalence of Healed Plaque and Factors Influencing Its Characteristics Under Optical Coherence Tomography in Patients With Coronary Artery Disease: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Cardiovasc. Med. 2021, 8, 761208. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion): A 1-Year Follow-Up Report. Circ. Cardiovasc. Interv. 2017, 10, e005860. [Google Scholar] [CrossRef]
- Ino, Y.; Kubo, T.; Tanaka, A.; Kuroi, A.; Tsujioka, H.; Ikejima, H.; Okouchi, K.; Kashiwagi, M.; Takarada, S.; Kitabata, H.; et al. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome: An optical coherence tomography study. JACC Cardiovasc. Interv. 2011, 4, 76–82. [Google Scholar] [CrossRef]
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age, years | 74 | 69 | 51 | 79 | 79 | 58 | 70 |
| Gender | F | F | M | F | M | F | M |
| Diagnosis | UAP | UAP | NSTEMI | NSTEMI | UAP | NSTEMI | UAP |
| Risk factors | |||||||
| Hypertension | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Diabetes mellitus | No | No | No | Yes | No | Yes | Yes |
| Hyperlipidemia | Yes | Yes | Yes | Yes | No | Yes | No |
| Smoking habit | No | No | Yes | No | No | Yes | No |
| Overweight | No | Yes | No | No | No | No | No |
| Clinical history | |||||||
| Previous MI | No | No | Inferior MI | No | Inferior MI | Inferior MI | No |
| Previous PCI | No | No | BMS/Cx | DES/RCA | BMS/RCA | DES/LM; BMS/RCA | No |
| Previous stroke | No | No | No | Yes | Yes | Yes | No |
| PAD | No | No | Yes | No | Yes | No | Yes |
| CKD | No | No | No | Yes | Yes | No | No |
| Atrial fibrillation | Yes | No | No | Yes | No | No | No |
| Clinical presentation at admission | Daily resting angina the past 2 weeks | De-novo resting angina and dyspnea | Ongoing prolonged angina and ventricular tachycardia | 24 h after diagnosis of high-risk NSTEMI | Recurrent angina episodes at rest the past 3 days | Prolonged resting angina; exercise angina for 1 year | Worsening angina the last 2 weeks |
| Physical examination | Grade II systolic murmur RUSB | Normal | Absent pulses left femoral artery | Normal | Absent pulses left popliteal artery | Normal | Low-grade systolic murmur RUSB; diminished bilateral distal pulses |
| Previous medication | |||||||
| Aspirin | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Statin | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Medication at discharge | |||||||
| Aspirin | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| P2Y12 inhibitor | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Statin | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Beta blocker | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ACEI/ARB | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Electrocardiography | Right bundle branch block + left anterior hemiblock | Voltage criteria left ventricular hypertrophy | Normal | ST segment depression V4-V6, DI, aVL | Right bundle branch block | Normal | Negative T waves V4-V6, DI, aVL |
| Echocardiogram | Moderate aortic stenosis, EF 55% | Normal, EF 60% | Left ventricular dilation, inferior akinesia, anterolateral hypokinesia, EF 30–35% | Basal septum and inferolateral hypokinesia, EF 40% | Inferior wall hypokinesia, EF 45% | Septum and anterolateral kypokinesia, EF 45% | Inferior wall akinesia, mild aortic stenosis, EF 45% |
| Laboratory data | |||||||
| TC, mg/dL | 232 | 215 | 149 | 154 | 152 | 182 | 94 |
| LDL-C, mg/dL | 157 | 142 | 75 | 102 | 80 | 109 | 18 |
| HDL-C, mg/dL | 48 | 58 | 33 | 39 | 41 | 45 | 20 |
| Triglycerides, mg/dL | 136 | 409 | 206 | 216 | 157 | 140 | 279 |
| Creatinine, mg/dL | 1.03 | 0.9 | 0.77 | 1.97 | 1.22 | 0.7 | 0.88 |
| Peak CK-MB, U/L | 11 | 8 | 77 | 127 | 19 | 101 | 24 |
| Leucocytes, 109/L | 8.7 | 12.6 | 6.89 | 10.4 | 6.25 | 7.09 | 8.46 |
| Hemoglobin, g/dL | 14 | 14.3 | 14.2 | 12.3 | 12 | 14.4 | 13.3 |
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Angiographic data | |||||||
| Culprit vessel | LAD | LAD | LM | LM-LAD | LM | LAD | LAD |
| Multivessel disease * | Yes; LAD and D2 | No | No | Yes; LM, LAD, D2, CTO Cx (patent RCA stent) | Yes; D1, OM1 (patent RCA stent) | Yes; LAD, D1 (patent LM and RCA stents) | Yes; D1 and CTO RCA |
| Lesion length, mm | 32 | 23 | 25 | 45 | 27 | 28 | 40 |
| Lesion severity, % | 50 | 30 | 40 | 55 (LM)/80 (mid-LAD) | 40 | 50 | 50 |
| TIMI flow | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| OCT data | |||||||
| Reference lumen diameter, mm | 2.53 | 3.08 | 4.66 | 4.1 (LM)/2.99 (LAD) | 3.91 | 2.38 | 3.55 |
| MLD, mm | 0.66 | 1.72 | 2.85 | 1.22 (LM)/0.58 (mid-LAD) | 2.13 | 0.70 | 1.86 |
| Diameter stenosis, % | 74 | 44.1 | 38.8 | 70.2 (LM)/80.6 (mid-LAD) | 45.5 | 70.6 | 47.6 |
| Reference lumen area, mm2 | 5.02 | 7.44 | 17.14 | 13.2 (LM)/7.01 (LAD) | 12.8 | 4.45 | 9.9 |
| MLA, mm2 | 1.31 | 4.61 | 9.77 | 2.06 (LM)/1.22 (mid-LAD) | 7.05 | 1.13 | 4.3 |
| Area stenosis, % | 73.9 | 38 | 42.9 | 84.4 (LM)/82.6 (mid-LAD) | 44.9 | 74.6 | 56.5 |
| Calcification length, mm | 28 | 10 | 25 | 39 | 12 | 14 | 32 |
| Calcification quadrant, n | 4 | 2 | 3 | 4 | 2 | 2 | 4 |
| Thrombus type | White | White | White | White/red | White | White | Red |
| Thrombus intraplaque site | Proximal/medial | Proximal | Proximal | Medial | Proximal | Proximal | Medial |
| Procedural data | |||||||
| Number of stents, n | 1 | N/A | N/A | 1 | N/A | N/A | N/A |
| Stent length, mm | 23 | N/A | N/A | 40 | N/A | N/A | N/A |
| Stent diameter, mm | 2.75 | N/A | N/A | 3 | N/A | N/A | N/A |
| Management | PCI | Medical | Medical | PCI | Medical | coronary artery bypass grafting | Medical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onea, H.-L.; Spinu, M.; Homorodean, C.; Ober, M.C.; Olinic, M.; Lazar, F.-L.; Achim, A.; Tataru, D.A.; Olinic, D.M. Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes. Life 2023, 13, 1732. https://doi.org/10.3390/life13081732
Onea H-L, Spinu M, Homorodean C, Ober MC, Olinic M, Lazar F-L, Achim A, Tataru DA, Olinic DM. Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes. Life. 2023; 13(8):1732. https://doi.org/10.3390/life13081732
Chicago/Turabian StyleOnea, Horea-Laurentiu, Mihail Spinu, Calin Homorodean, Mihai Claudiu Ober, Maria Olinic, Florin-Leontin Lazar, Alexandru Achim, Dan Alexandru Tataru, and Dan Mircea Olinic. 2023. "Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes" Life 13, no. 8: 1732. https://doi.org/10.3390/life13081732
APA StyleOnea, H.-L., Spinu, M., Homorodean, C., Ober, M. C., Olinic, M., Lazar, F.-L., Achim, A., Tataru, D. A., & Olinic, D. M. (2023). Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes. Life, 13(8), 1732. https://doi.org/10.3390/life13081732









