Abstract
Atherosclerosis is a significant health concern with a growing incidence worldwide. It is directly linked to an increased cardiovascular risk and to major adverse cardiovascular events, such as acute coronary syndromes. In this review, we try to assess the potential diagnostic role of biomarkers in the early identification of patients susceptible to the development of atherosclerosis and other adverse cardiovascular events. We have collected publications concerning already established parameters, such as low-density lipoprotein cholesterol (LDL-C), as well as newer markers, e.g., apolipoprotein B (apoB) and the ratio between apoB and apoA. Additionally, given the inflammatory nature of the development of atherosclerosis, high-sensitivity c-reactive protein (hs-CRP) or interleukin-6 (IL-6) are also discussed. Additionally, newer publications on other emerging components linked to atherosclerosis were considered in the context of patient evaluation. Apart from the already in-use markers (e.g., LDL-C), emerging research highlights the potential of newer molecules in optimizing the diagnosis of atherosclerotic disease in earlier stages. After further studies, they might be fully implemented in the screening protocols.
1. Introduction
Cardiovascular diseases (CVDs) continue to remain the foremost cause of mortality on a global scale, with coronary artery disease (CAD) emerging as the most prevalent among them [1]. In Europe, CVDs account for approximately 45% of the overall deaths. Furthermore, approximately 4 million individuals die annually due to cardiovascular (CV) events, resulting in a staggering loss of over 60 million years of life due to CVDs in Europe alone [2,3]. While historically, CVDs predominantly affected the elderly population, a shift in paradigm has occurred due to the increasing incidence of diabetes, obesity, and smoking, leading to a growing number of patients under the age of 65 being diagnosed with these pathologies. In light of this, enhancing the existing screening methods and discovering novel ones should be accorded the utmost priority by medical systems worldwide [4].
In general practice, we rely on standardized CV risk scores (SCORE diagram, U-prevent algorithm, ASCVD score) to identify classic CV risk factors such as age, gender, blood pressure, cholesterol levels, smoking status, and diabetes. Even though multiple studies proved their efficiency, it is noteworthy that 50% of the patients who develop CAD are previously classified in the low- or intermediate-risk group [5]. The latest guideline on Chronic Coronary Syndromes from the European Society of Cardiology (ESC) identifies several complementary risk-assessment tools, namely intima-media thickness, coronary artery calcium scores, brachial flow-mediated dilation, ankle-brachial index, high sensitivity C-reactive protein (hs-CRP) and family history of CAD, which may be used in selected patients. However, they recommend using these tools sparingly, due to a lack of scientific proof of their efficiency [6]. Considering this, it is natural to say that there is an urgent need to find alternative instruments able to improve the existing algorithms’ accuracy and give a higher discrimination power to the existing scores.
The United States National Institutes of Health defines biomarkers as “a characteristic that is objectively measured and evaluated as an indication of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”. Even if, after 2010, an increased rate of their use has been registered, they are far from being fully implemented instruments in medical practice, this term dates from as far back as 1970. From infectious diseases and cancers to cardiovascular pathologies such as heart failure (HF), many molecules can serve as indicators of the systemic impact of a particular disease, providing valuable guidance to medical professionals [7]. However, based on our knowledge, the scientific evidence strongly supporting a specific biomarker is lacking when it comes to atherosclerosis and CV risk stratification. Therefore, our article aims to focus on presenting the latest evidence that supports the significance of two particular molecules, interleukin 6 (IL-6) and apolipoprotein B (apoB), as potential biomarkers for atherosclerosis. The article will primarily emphasize their molecular structure and elucidate their involvement in the pathogenesis of atherosclerosis.
Firstly, we will present the most recent studies concerning the pathogenesis of atherosclerosis in the human body, including its underlying processes and influential factors. Secondly, we will expand upon the subjects of low-density lipoproteins (LDL), apoB, hs-CRP, and IL-6. Finally, we will present the potential role of apoB and IL-6 as future screening biomarkers, together with other emerging components.
2. Pathophysiology of Atherosclerosis
Atherosclerosis serves as the underlying cause for various CV events and its manifestations, including CAD and myocardial infarction; peripheral artery disease (PAD) and lower limb amputation; and aortic aneurysm (AA), renal artery stenosis (RAS), and carotid artery stenosis (CAS) associated with strokes [8]. Due to its characteristic risk factors—elevated serum cholesterol, diabetes, smoking, male gender, age, obesity, and chronic systemic inflammation—atherosclerosis tends to be more prevalent in higher-income countries, with a continuous and steady rise in incidence [1].
From the pathophysiological point of view, endothelial dysfunction, hypercholesterolemia, and inflammation represent the central triad of atherosclerosis [8]. The endothelium, the internal layer of the vascular wall, is the interface between the bloodstream and the main site of atherosclerosis. Thus, its integrity is mandatory to prevent lipid accumulation within the arteries. However, phenotypic changes in the endothelium’s cellular structure may alter its protective capacity, opening the path to atherosclerosis, sheer stress playing a central role in this process [9]. Laminar shear stress, physiologically exerted by the bloodstream, has an atheroprotective effect. On the other hand, disturbed blood flow, commonly observed near branch sites, generates low shear stress (frictional drag), activating the endothelial cells, diminishing their vasodilative and anti-thrombotic properties, associating increased oxidative stress, and promoting lipid uptake beneath the endothelium [10]. Once activated, endothelial cells also facilitate atherosclerosis and lipid accumulation by promoting inflammation and intimal hyperplasia via adhesion molecules, cytokines, and growth factors [11]. Adhesion molecules, such as vascular cell adhesion protein 1, intercellular adhesion molecule 1, P-selectin, and E-selectin, bind the circulating inflammatory cells and keep them trapped within the arterial wall. Subsequently, the secreted cytokines and chemokines attract new inflammatory cells to the atherosclerotic site. Lastly, growth factors, including platelet-derived growth factor, determine extracellular matrix deposition and smooth muscle proliferation [8,12].
The effects exerted by the activated endothelium lead to the over-activation of inflammatory cells, primarily targeting the cholesterol deposits beneath the endothelium [13]. This excessive inflammatory state manifests not only at the local site but also systemically, as evidenced by literature documenting a direct correlation between atherosclerosis and specific inflammatory biomarkers [14]. Additionally, the latest guidelines from the ESC on Chronic Coronary Syndromes recommend the use of hs-CRP as a complementary biomarker for assessing atherosclerosis [6]. Thus, comprehending the role of inflammation in the pathophysiology of atherosclerosis is crucial for preventing and treating this condition. The main leukocytes recruited into the vascular wall are monocytes, which become macrophages under the influence of the locally secreted cytokines [15]. Despite their initial protective role in removing cytotoxic oxidized LDL (ox-LDL), which they bind to via scavenger receptors (SR-A1, CD36, and LOX1/SR-E1), macrophages become a central component of the atherosclerotic plaque. Due to their inability to effectively digest LDL, it tends to accumulate within the inflammatory cells, leading to the formation of foam cells [13,16]. Subsequent accumulation of these pathological macrophages leads to fatty streak formation, which is, from the morphopathological point of view, the foundation of the future atherosclerotic plaque. In a desperate attempt to eliminate the abnormal lipids, proinflammatory (IL-1, IL-6, IL-12, IL-15, IL-18) and anti-inflammatory cytokines (IL-10 and TGF-β) are secreted, and the inflammatory response is augmented [17]. Those macrophages that fail to eliminate their lipid content and undergo cell death by apoptosis via endoplasmic reticulum activation are eliminated by other macrophages via efferocytosis [18]. Subsequently, when this process becomes overwhelmed, the foam cells recruit new inflammatory pathways. Dendritic cells play an important role in initiating the adaptative immune response. They are responsible for atherosclerosis-related antigen presentation to T cells. The predominant type of T cells in the atherosclerotic plaque are the T-helper type 1 lymphocytes (Th1), also implicated in the inflammatory process associated with myocardial infarction and HF [19,20]. They are central regulators of the adaptative immune response, able to differentiate into other T-helper subtypes, having either pro- or anti-inflammatory effects [13]. Type B lymphocytes are also present in the atherosclerotic process, being represented by B1, IgM antibody-producing cells and B2, IgG antibody-producing cells [17]. Contrary to the former, which proved to have an anti-atherosclerotic effect mediated by IL-5, IgG antibodies are associated with an increase in the atherosclerotic process [21]. The supplementary activation of the inflammatory response generates new foam cells, which determine the secretion of more cytokines and reactive oxygen species, transforming atherosclerosis into a vicious circle [8,22].
Lipids represent the final, but certainly not the least significant element of the atherosclerotic triad. A proportionality relationship exists between atherosclerosis and serum lipid levels [8,23]. If, theoretically, every human being developed atherosclerosis at some point in their life, the extent of the process and the moment of its development would be tightly correlated to their cholesterolemia [24]. Within the bloodstream, cholesterol particles exist in different forms. However, at least for the general population, LDL represents the most atherogenic fraction. Serum levels of LDL-cholesterol (LDL-C) are not the only determinant of the degree of atherosclerosis. In order to become atherogenic, the lipid particles need to suffer different types of structural changes, such as desialylation, glycation, and oxidation, with native LDL not causing lipid accumulation in the arterial wall [23]. After entering the arterial wall, LDL is internalized by the macrophages and contributes to the vicious circle already presented [8,25].
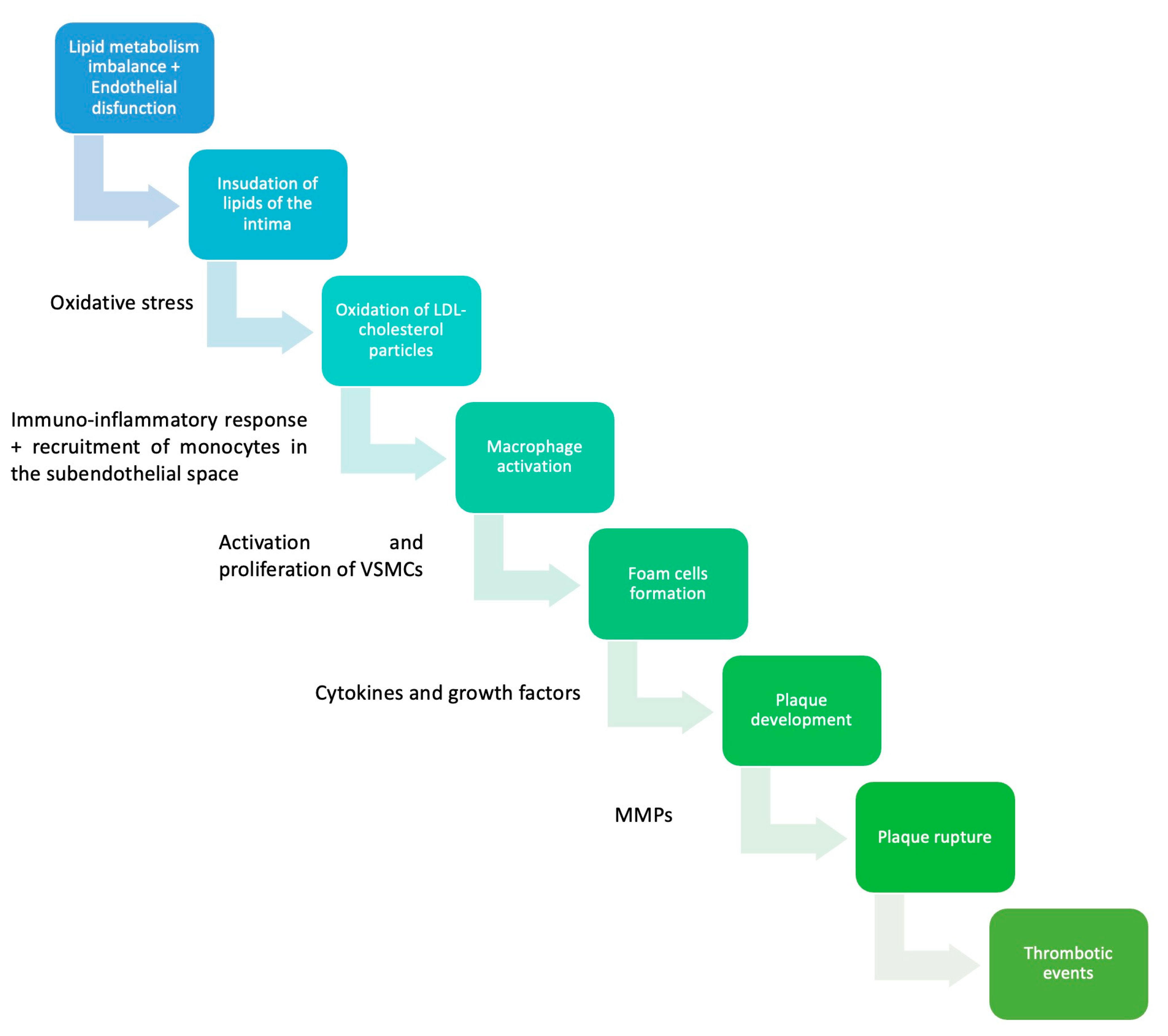
As previously mentioned, the initial manifestation of atherosclerosis is the fatty streak, which develops very early in life, typically during adolescence or even childhood. For the atherosclerotic plaque to progress into a more advanced lesion, there must be an accumulation of extracellular lipids, forming extensive pools beneath the endothelium. These lipid pools serve as the core of the atheroma [26]. In response to inflammatory cytokines such as TNF-alpha, smooth muscle cells and fibroblasts from the outer layers of the arterial wall are recruited to the subendothelial site. Fibroblasts secrete large quantities of collagen, which forms the fibrous layer of the atheroma, giving rise to a new type of atherosclerotic lesion known as fibroatheroma [26,27]. Over time, the fibrous cap becomes weakened by locally secreted proteolytic enzymes, rendering it susceptible to rupture. This exposes the thrombogenic core to the bloodstream. From a clinical perspective, these events underlie some of the most concerning manifestations of atherosclerosis, including unstable angina, myocardial infarction, sudden cardiac death, and stroke [8,22,26]. While it was previously believed that cells producing the fibrous cap in the atherosclerotic plaque originated exclusively from smooth muscle cells, recent evidence has revealed that a significant portion of these cells are derived from endothelial cells and macrophages. This discovery has paved the way for identifying novel therapeutic approaches to enhance plaque stability (see Figure 1; see Table 1) [28].
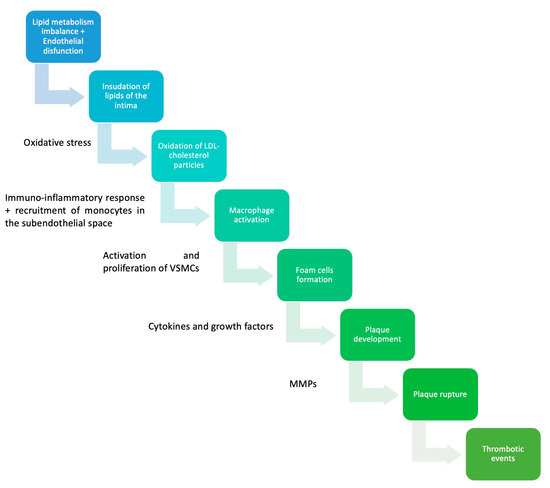
Figure 1.
Pathophysiology of atherosclerosis. VSMCs—vascular smooth muscles cells; MMPs—matrix metalloproteinases [29,30,31].

Table 1.
Factors that contribute to atherosclerosis formation.
3. LDL
LDL serves as the primary carrier of cholesterol and it is recognized as the most abundant atherogenic particle. Many existing therapies are targeted towards this molecule [32,33]. It possesses an average diameter of 22 nm and exhibits a dynamic molecular structure comprised of three layers: the core, which contains approximately 170 triglycerides and 1600 cholesteryl esters; the outer surface layer, primarily composed of phospholipids; and the interfacial layer, formed by the interpenetration of core and surface lipids [32]. Unesterified cholesterol molecules serve as a shared structural component in all three layers, with approximately one-third comprising the core and the remainder constituting the surface layer [34]. LDL has a complex proteome within its structure, apolipoprotein B 100 (apoB-100) being the primary protein in this lipoprotein [35]. It mediates the interactions between LDL and other molecules and receptors, being essential to all lipoprotein-related physiological processes. ApoB-100 accounts for approximately one-fifth of the weight of LDL and consists of three alpha-helical and two beta-structured domains. It has a molecular mass of 550kDa, being the largest monomeric glycoprotein known [36]. As shown by electron microscopy studies, apoB-100 wraps the surface of LDL molecules, forming a dense protein halo protecting the lipidic core and remaining attached to them throughout the entire lipid metabolism, given its hydrophobic character [32,37].
In order to determine LDL-C, multiple options are available. The first method, initially described in 1972, is the Friedewald formula. It estimates LDL-C using total cholesterol, high-density lipoprotein (HDL) cholesterol (HDL-C), and triglycerides (TGs). This formula presents a series of limitations, mainly imposed by TG levels, as in patients with TG > 250mg/dl it becomes inaccurate [38]. A response to the Friedewald’s formula’s limitations has appeared: the Martin equation. Contrary to what we have previously presented, in Martin’s equation, TGs are divided by an adjustable factor according to plasma TGs and nonHDL-C levels. However, neither the Martin equation nor the Friedewald formula are validated for TG > 400mg/dl. A new equation using more advanced and accurate mathematical instruments was introduced recently. We refer to the Sampson equation, which may be used for TG levels up to 800 mg/dl; however, not all studies support its superiority over the Martin equation [39]. LDL-C may also be determined using direct assays, which measure and do not estimate LDL-C. These methods are also called homogenous assays, as, in order to count LDL-C, they do not separate LDL from other lipoproteins but rather consume or mask them. They also impose some limitations, as alterations in the composition and size of lipoproteins decrease these methods’ accuracy [38]. A more recently introduced LDL quantification method uses nuclear magnetic resonance spectrometry to quantify the number and also the size of LDL particles [40].
3.1. Structure-Based Alterations of LDL
While the serum level of LDL-C serves as an important marker of atherogenicity, recent studies have revealed that native LDL does not readily accumulate within the arterial wall. For lipid molecules to become atherogenic, they need to suffer different phenotypical changes that facilitate their entry through the endothelial layer and promote the atherosclerotic process [23]. The existing evidence in the literature indicates that desialylation might be the earliest modification suffered by the native LDL, which promotes its atherogenicity. Desialylated LDL accounts for as much as 60% of the total circulating LDL in patients suffering from CAD [41]. In the molecular structure of LDL, sialic acid is an essential component of apoB, as it represents the terminal carbohydrate of the biantennary sugar chains. Removing sialic acid leads to multiple structural and functional alterations in the LDL particle, including size reduction, increased density, negative electric charge, loss of lipids, and oxidation. These changes collectively contribute to the heightened atherogenicity of desialylated LDL [42,43]. Desialylated LDL may be detected in human serum using a lectin-sorbent assay. However, there are few data supporting the use of this molecule as a biomarker for atherosclerosis [44]. On the other hand, we found a study cited in the literature suggesting the potential utility of desialylated apoB-100, detected using an ELISA assay, in predicting the presence of carotid atherosclerosis [45].
In addition to desialylation, glycation is another extensively studied structural modification of LDL that contributes to its increased atherogenicity [23]. Glycation is a non-enzymatic reaction between glucose and the exposed lysine residues of proteins. While it primarily occurs in proteins, it can also affect lipoproteins, LDL being particularly rich in glycated apoB-100 [46]. Similar to desialylation, glycated LDL is smaller, facilitating its accumulation within the arterial wall. It also has a slower catabolic rate, and due to apoB’s structural alteration, it is not adequately cleared by the LDL receptors [23,47]. Besides the structural changes, recent studies showed that glycated LDL may increase atherosclerotic risk by increasing platelet aggregation. The precise mechanism underlying this effect is not fully understood, but it may be linked to inhibiting the thrombocytes’ membrane Na+/K+ channels and the concomitant stimulation of Ca2+ ATP-ase, which raises intracellular calcium levels, increasing the response of platelets to ADP [48]. Glycated LDL can be measured using ELISA assay. However, this is not a routine technique in daily practice, and the literature lacks data supporting its use as a biomarker of atherosclerosis [49].
Lastly, oxidation is another essential reaction for the atherosclerotic process [50]. Ox-LDL is a strong promoter of atherosclerosis, as it is a major component of foam cells [51]. Compared to native LDL, ox-LDL has a higher affinity for scavenger receptors, making it more readily internalized by macrophages and promoting foam cell formation [52]. Moreover, ox-LDL possesses several biological properties that further contribute to atherosclerosis, including chemotactic activity for monocytes, promotion of growth factor expression, cytotoxic effects, endothelial dysfunction, and stimulation of platelet adhesion. Oxidation of LDL occurs primarily within the arterial wall by resident vascular cells and macrophages [52]. In vitro studies showed that LDL suffers some degree of oxidation during glycation, even in the absence of oxygen free radicals and oxygen-radical-generating processes [47]. Although ox-LDL levels in plasma are generally low due to plasma’s antioxidant properties, advances in ELISA techniques have enabled the detection of these particles with high sensitivity [53,54]. These advances led to a growing interest in researching ox-LDL as a biomarker of CVD. In a meta-analysis, which included 12 studies, increased circulating levels of ox-LDL proved to be associated with an increased risk of CV events [55]. In a prospective study, the ox-LDL/LDL ratio was positively associated with the severity of CAD, assessed using the Gensini score [56]. Additionally, increased levels of ox-LDL were also observed in patients with carotid artery disease. The hypothesis stating that ox-LDL may be a better indicator of the total CV risk is plausible, considering the pathophysiological implications of this molecule [57].
3.2. Lipid-Lowering Therapies and CV Risk
Statins represent the most prescribed drugs used to control the CV risk. They represent the first-line agents used to lower LDL-C. Their efficiency in reducing the rate of cardiovascular events, both as primary and secondary prevention, made them the cornerstone of CV risk management in guidelines [58]. Relatively recent medical research has led to the development of more potent lipid-lowering therapies such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. By regulating the number of LDL receptors responsible for LDL degradation, PCSK9 indirectly influences LDL-C levels. Lower levels of PCSK9 are associated with an increase in LDL receptor density and, consequently, a reduction in LDL-C [59]. Even if the efficiency of these molecules in reducing LDL-C has been proven by numerous randomized controlled studies, whether their effects go beyond cholesterol lowering remains unclear [60,61,62].
HUYGENS is a randomized controlled trial which tested the effects of Evolocumab on atherosclerotic plaques. The study included 161 patients who underwent coronary angiogram for NSTEMI and presented at least one angiographic lesion in addition to the culprit plaque, which implied a stenosis <50%. Other inclusion criteria which concerned the atherosclerotic plaques were having at least one image of fibrous cap thickness ≤120µm and lipidic ac >90°. Patients were randomly assigned to the placebo group or to the Evolocumab group, receiving 420mg/month for 48 weeks. For the atherosclerotic plaque study, optical coherence tomography (OCT) and intravascular ultrasound (IVUS) examination was performed at the baseline and after 50 weeks. At the end of the follow-up period, patients receiving Evolocumab presented a significantly better plaque regression, reflected by fibrous cap thickening (+39.0 µm vs. +22.0 µm, p = 0.015) and lipid arc decrease (−57.5° vs. −31.4°, p = 0.04). Additionally, an interesting finding was that in the Evolocumab group, the length of the vessel containing macrophages was reduced significantly (−3.17 mm vs. −1.45 mm, p = 0.04) [63].
PACMAN-AMI is a similar trial that tried to evaluate the effects on atherosclerotic plaques of the early administration of PCSK9 inhibitors in patients suffering from acute myocardial infarction. This study was centered on Alirocumab and included 300 randomly allocated participants to receive 150mg of Alirocumab or placebo for 52 weeks. To evaluate the effects of Alirocumab at the end of the follow-up period, they used: the percentage of atheroma volume (PAV), determined using IVUS; fibrous cap thickness, measured using OCT; and lipid core burden evaluated using near-infrared spectroscopy. Patients who received the PCSK9 inhibitor showed a greater reduction in PAV compared to placebo (−2.13% vs. −0.92%, p < 0.001). Similar changes were also evident for lipid burden index reduction (−79.42 vs. −37.6, p = 0.006) and fibrous cap thickness (+62.67 µm vs. 33.19 µm, p = 0.001) [64].
PCSK9 inhibitors represent an important step forward when it comes to dyslipidemia treatment and CV risk management, mainly due to their efficiency in LDL-C reduction. Based on their findings regarding the effects of Alirocumab and Evolocumab on plaque stability, HUYGENS and PACMAN-AMI bring us closer to understanding what lies behind the efficiency of these molecules [63,64].
4. Apolipoprotein B
4.1. Structure and Secretion
ApoB is a protein possessing amphipathic properties, serving as a structural framework for all lipoproteins implicated in atherogenesis. These lipoproteins include very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), LDL, and chylomicrons [65]. ApoB exists in two primary forms: apoB-100, a full-length apolipoprotein comprising of 4536 amino acids, predominantly found in VLDL and its metabolic derivatives, and apoB-48, which is synthesized by the intestine and consists of only 2152 amino acids, playing a key role in chylomicrons [66].
ApoB-100 is synthesized by the liver, depending on lipid availability [67]. Contrary to other secretory proteins, apoB-100 becomes exposed to the cytosol of the endoplasmic reticulum very early in the post-translational period. This allows its rapid proteasomal degradation via chaperon protein binding immunoglobulin [68], the liver secretion of apoB-100 being easily regulated proportionally to the need for lipoprotein synthesis. Eventually, apoB-100 is maturated in the Golgi apparatus, where VLDL is formed [69,70].
Structurally, apoB-100 comprises five α-helical structures and β sheet domains, closed by one N-terminal region and another C-terminal one. Each of these structures has specific functions [71]. The βα1 N-terminal domain is a binding site for microsomal triglyceride transfer protein (MTP). MTP is a specific apoB protein needed for VLDL assembly and for scavenger receptors [72]. The β1 domain is essential for lipid binding and lipoprotein formation, being the anchor that facilitates the firm adhesion of apoB-100 to the surface of different lipoproteins [73]. Another important binding site is the β2 domain, located at the C-terminal portion, assuring the interaction between apoB-100 and LDL receptors [74]. Lastly, α2 and C-terminal α3 domains are responsible for core lipid recruitment, forming the flexible region of the apolipoprotein [75].
4.2. Role as a Biomarker
Most lipid-lowering and cardiovascular risk-management protocols currently in use focus primarily on targeting LDL [76]. This approach proves effective for a significant proportion of patients, as achieving the recommended LDL-C levels helps reduce the risk of fatal CV events. However, in certain situations, despite reaching the therapeutic goals, these events may not be prevented, suggesting the existence of a residual atherogenic risk. This residual risk may be related to triglyceride concentrations and cholesterol content within particles rich in triglycerides [67,77]. Thus, the need for an alternative biomarker efficient in these particular situations is evident. As measurement methods for apoB have become more and more accessible, research interest in this molecule has considerably increased, and so has its potential as an atherosclerosis biomarker. A 2011 meta-analysis that incorporated data from 12 epidemiological studies aimed to compare the predictive capabilities of LDL-C, non-HDL cholesterol, and apoB in relation to fatal and non-fatal CV events. The analysis utilized the relative risk ratio (RRR) as the measure of predictive power. The study’s findings indicated that among the three investigated risk factors, LDL-C exhibited the weakest association, with a mean RRR of 1.25, while apoB emerged as the strongest indicator of CV risk, with a mean RRR of 1.34. Furthermore, in direct comparison, the RRR for apoB was found to be 5.7% higher than that of non-HDL cholesterol (95% CI, 2.4% to 9.1%; p < 0.001) and 12% higher than LDL-C (95% CI, 8.5% to 15.4%; p < 0.0001) [78]. Another representative research that evaluated the association of apoB with the total CV risk is AMORIS. This prospective study included 175,553 patients, who were followed up for a mean period of 66.8 months. The study aimed to assess whether apoB adds any predictive power regarding CV events to the classic cholesterol profile. Similarly to the meta-analysis previously presented, apoB not only increased the predictive power of LDL-C when added to statistical models, but it was also a better predictor of the CV risk in individuals with low LDL-C [79]. As a response to the growing data supporting the use of apoB as a CV risk-assessment tool, the latest ESC guidelines for the management of dyslipidemias recommend routine measurement of apoB for patients suffering from pathologies such as hypertriglyceridemia, diabetes, obesity, and metabolic syndrome [76].
Furthermore, the atherogenicity of lipids is influenced by their mass, with smaller particles having a greater propensity to penetrate the arterial wall. Consequently, LDL-C, which measures the total mass of cholesterol contained in LDL particles, may not accurately reflect the CV risk [65]. On the other hand, apoB reflects a number of a wider spectrum of atherogenic particles but does not provide information about their mass. By combining these two biomarkers using the LDL-C/apoB ratio, valuable insights can be gained regarding the average mass of circulating atherosclerotic particles. This information may prove useful in assessing an individual’s CV risk [80]. A retrospective case-cohort study, which included 1058 diabetic patients, aimed to evaluate the association between a low LDL-C/apoB ratio and the presence of coronary heart disease (CHD). Participants were included in the CHD group if they had a positive history of percutaneous coronary intervention, coronary bypass graft, acute coronary syndrome, or myocardial infarction. The study concluded that patients with CHD had a significantly lower LDL-C/apoB ratio (p = 0.001). Moreover, an LDL-C/apoB ratio
1.2 was able to predict the presence of CHD, independently of the ASCVD score (OR = 1.841, p = 0.002) [81]. More importantly, Jung et al. found in their study that the LDL-C/apoB ratio was an important predictor of significant coronary artery stenosis (>50%) and the need for revascularization. Their research included non-diabetic patients, extending the use of this biomarker outside the borders specified in the ESC guidelines. However, in their study, apoB alone did not offer any additional predictive power for coronary stenosis over LDL-C [82].
Contrary to apoB, apolipoprotein A-I (apoA-I) plays a protective role in the atherosclerotic process. It is the main component of HDL, and increased serum levels of apoA-I have proven to be associated with a lower risk of CV events [83,84]. Provided that each of these two biomarkers give separate information regarding the atherogenicity of one’s plasma, several studies attempted to combine their predictive power under the apoB/apoA-I ratio. Liting et al. conducted a prospective study which included over 820 patients with coronary angiography indications. ApoB, apoA-I, and the apoB/apoA-I ratio were measured for each study participant. Statistical analysis revealed that patients with angiographically significant coronary lesions (>50%) had higher levels of apoB and apoB/apoA-I ratio and lower apoA-I levels. Furthermore, when CAD patients were divided into groups according to the severity of their lesions, they observed that the apoB/apoA-I ratio was proportional to the extension of the coronary disease. At the end of the 3-year follow-up period, they also concluded that the apoB/apoA-I ratio better predicted long-term CV events than LDL-C [85].
Lastly, apoB proved to be a more powerful predictor for myocardial infarction than LDL-C and triglycerides. In a prospective study, which included over 430,000 patients, split into two groups (the primary and the secondary prevention group), apoB was the only independent predictor of the risk of myocardial infarction. Additionally, they tested whether the type of lipoprotein (triglyceride-rich lipoproteins or LDL) had any prognostic importance. They evaluated the triglyceride/LDL-C ratio while adjusting for apoB and clinical risk factors and concluded that the type of apoB-containing lipoprotein brought no additional risk-assessment value [86].
5. Interleukin 6
High hs-CRP has proved to be a promising predictor of future CV events in clinical trials conducted worldwide. However, the data supporting it as a routine biomarker for atherosclerosis are insufficient. IL-6 is a central mediator of acute phase response and an essential stimulant for hepatic CRP synthesis [87]. Going “upstream” in the pathophysiological chain of atherosclerosis-related inflammation, IL-6 plays a central role, along with IL-1, in this process. This relationship is supported by genetic research as well. Compared to hs-CRP, IL-6 has a shorter half-life and within-person variability, which limited the research regarding this molecule [88]. However, an increase in both molecules may be a better indicator of a chronic inflammatory process, as atherosclerosis is [89]. Having this in mind, recent studies support its utility for CV risk assessment [90,91]. Structurally, IL-6 is a phosphorylated glycoprotein consisting of four-helix bundles, which form a single chain. The four helices run in different directions; thus, helices A and B have opposite distributions than helices C and D. The linking between these components is made via four loops, connecting the bundles two by two [92].
Regarding its physiological effects, IL-6 is a ubiquitarian molecule secreted by various tissues and cells. In response to infection, trauma, or stress, IL-6 is expressed by monocytes and macrophages. The adipose tissue is another important source of IL-6, the latter being associated with obesity and diabetes. For different neoplastic tissues (myeloma, plasmacytoma, renal cell carcinoma, Kaposi sarcoma), IL-6 serves as a growth factor [93,94,95,96]. Studies in the literature also link IL-6 to the endothelial dysfunction associated with angiotensin-II-induced hypertension [97,98,99].
On the other hand, a potential protective effect of this interleukin was observed in studies conducted on IL-6 depleted mice. Schieffer et al. observed its atheroprotective role, as the deficit of IL-6 not only did not stop plaque formation but was associated with a more pronounced atherosclerotic process in their study [100]. Furthermore, Al-Khalili et al. observed that mice with IL-6 deficiency who developed obesity had a positive glucose and lipid metabolism response after short-term administration of IL-6. This might be explained by the fact that at the level of skeletal muscle, IL-6 ensures short-term energy supply and glucose mobilization [101,102]. However, to our knowledge, these results have yet to be reproduced in human subjects. Additionally, patients suffering from autoimmune diseases characterized by increased IL-6 levels, such as rheumatoid arthritis, tend to have a higher risk of developing CVD, supporting the role of IL-6 as a risk factor for atherosclerosis [103].
IL-6 plays its roles via a cell-surface receptor complex formed by a ligand-binding glycoprotein (IL-6R) and a signal-transducing component (gp130). IL-6R is an alpha-chain containing three domains. There are two types of IL-6R, the membrane-bound one (mIL-6R), mainly expressed in hepatocytes, neutrophils, and monocytes, and a soluble one (sIL-6R), which acts as a transporter for IL-6 [92].
5.1. IL-6 and the Risk of CV Events
The relationship between IL-6 and atherosclerosis is bidirectional. On the one hand, an intense atherosclerotic process is associated with increased IL-6 production due to inflammation. On the other hand, increased IL-6 levels, determined by extrinsic factors such as obesity, social stress, smoking, and pollution, may increase the atherosclerotic risk, as it is associated with increased insulin resistance, hypertension, dyslipidemia, and endothelial dysfunction [104]. Papadopoulos et al. observed in their meta-analysis, which included 11 population-based prospective cohort studies, a linear association between IL-6 levels and the risk of ischemic stroke. For each standard deviation increase in log-IL-6 levels, the risk of ischemic stroke increased by 19%. Their observation was independent of conventional CV risk factors and comparable to other ischemic stroke risk factors (non-HDL cholesterol and blood pressure) [105].
In another meta-analysis, which included eleven epidemiological studies and over 288,738 patients, IL-6 emerged as a predictive factor for CVD. The mean follow-up period was seven years. IL-6 baseline levels were associated with an increased risk of developing coronary heart disease at the end of the follow-up period. Furthermore, IL-6 levels correlated with the incidence of hypertension and hypercholesterolemia [106]. A prospective study conducted over 12 years, which included 1592 patients suffering from peripheral artery disease, showed a linear correlation between CRP, IL-6, and ICAM-1 and the disease progression, reflected by the ankle-brachial index (ABI) evolution. In the same study, IL-6 was the only biomarker able to predict the ABI decline at both 5 and 12 years, showing its superiority over the other molecules [107].
5.2. IL-6 and CAD
The existing literature provides supporting evidence for utilizing IL-6 as an indicator for subclinical CAD. In their prospective study, which included over 300 diabetic subjects, who underwent electron-beam cardiac computed tomography, Saremi et al. observed a significant association (p < 0.01) between IL-6 levels and the severity of CAD, reflected in the calcium score (CAC score). This association remained significant even after the adjustment for other risk factors. This type of relationship was not evident for CRP or Lp-PLA2 and CAC score [108].
Gerin et al. conducted a prospective study over 118 subjects who underwent coronary angiography. The analysis excluded those patients suffering from pathologies that could be associated with increased IL-6 levels. CAD disease was defined as the presence of at least one coronary stenosis >50%. CAD severity was quantified using SYNTAX and GENSINI scores. IL-6 levels correlated with the two severity scores, and they were higher in the CAD group. Furthermore, after the regression analysis, IL-6 proved to be an independent predictor for the presence of CAD, with serum levels >7.91 pg/mL being able to predict significative atherosclerotic plaques with a sensitivity of 78% and a specificity of over 70% [109]. Similar results were found in another recent prospective study, which included 1796 patients who underwent computed tomography angiography. Besides the association between IL-6 levels and the severity of CAD, serum levels >1.8ng/L were associated with an increased risk of death, myocardial infarction, or unstable angina. Additionally, in patients with non-obstructive coronary plaques (<70%), high-sensitivity troponin and IL-6 levels above the median were predictive for major adverse cardiovascular events [110].
These findings have been validated by other prospective and observational studies as well. However, in order to establish a consensus regarding the appropriate cut-off values for IL-6, further research is required [111,112,113].
5.3. Anti-inflammatory Therapy and CV Risk
The existing therapies designed to reduce the CV burden induced by atherosclerotic disease almost exclusively concentrate on cholesterol targets. Even though the inflammation hypothesis of atherothrombosis has been validated by numerous animal-based, epidemiological, and genetic studies, the causality effect remains to be established by future interventional trials. This is why there is a growing interest in exploring the efficiency of anti-inflammatory therapies in reducing CV risk [114]. A recent randomized, double-blind trial (CANTOS) assessed whether Canakinumab, a human monoclonal antibody targeting IL-1β, could reduce the total CV risk without affecting lipid levels. The study separated their cohort of over 10,000 patients into two groups: the placebo group or the Canakinumab group. The primary endpoints were nonfatal myocardial infarction, stroke, or cardiovascular death. The mean follow-up period was 3.7 years. At the end of this period, CRP and IL-6 were significantly reduced in the Canakinumab group. Additionally, compared to placebo, Canakinumab reduced the risk of the primary endpoints by 15% without influencing the lipid levels of the patients (3.86 vs. 4.5 events per 100 person-years) [115].
The fact that IL-1 is a major inducer of IL-6 production, as well as the effects of Canakinumab on IL-6 levels identified in the prior study, prompted the additional investigation. In an IL-6-centered analysis, Ridker et al. observed that the baseline levels of this cytokine were correlated with the risk of future CV events. More importantly, an on-treatment IL-6 level of <1.65 ng/L was associated with a reduction in the risk of major CV events of 32% (p < 0.0001) and a 52% reduction in CV mortality (p < 0.0001). Based on these findings, they concluded that there is a strong correlation between the IL-6 signaling pathway and the risk of CV events. Additionally, this study gives solid clinical arguments for the inflammatory hypothesis of atherothrombosis, supporting, at the same time, the use of IL-6 as a potential biomarker for the CV risk [116].
RESCUE is another recent randomized trial which tested the effects of Ziltivekimab, an antibody-mediated IL-6 inhibitor explicitly developed for atherosclerosis. This study included 264 participants with high CV risk, moderate to severe chronic kidney disease (CKD), and hs-CRP > 2 mg/L. They were randomly assigned to the placebo group or to Ziltivekimab at ascending doses (7.5 mg, 15 mg, 30 mg). At the end of the 24-week follow-up period, hs-CRP was reduced proportionally to the Ziltivekimab dose (77%, 88%, 92%), compared to the 4% reduction in the placebo group. Compared to Canakinumab from the CANTOS trial, Ziltivekimab was almost twice as efficient in reducing hs-CRP levels [117].
In response to the findings from the RESCUE and CANTOS trials, the concept of a new randomized, long-term endpoint trial called ZEUS emerged. The first results from this trial are expected in 2025, and it is meant to compare Ziltivekimab to a placebo among 6200 patients with moderate to severe CKD and elevated hs-CRP. The central question of this trial is whether reducing IL-6 levels can reduce CV event rates, testing one more time the causal effect of inflammation in atherothrombosis [118].
6. Role of Emerging Biomarkers of Atherosclerosis
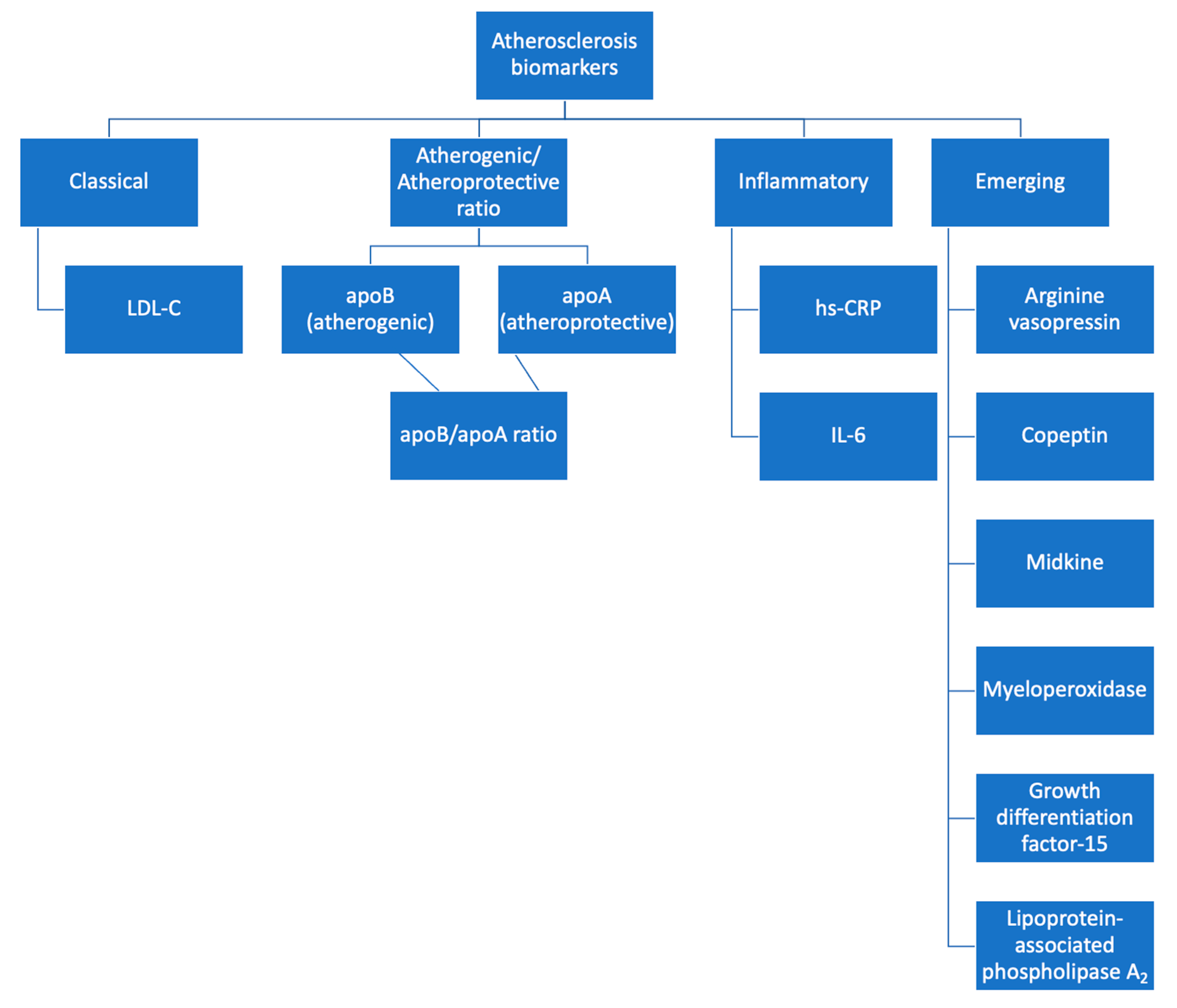
Searching the existing literature, we found numerous other molecules currently under research which may act as biomarkers of atherosclerosis and CVD [51,119] (see Table 2).

Table 2.
Emerging biomarkers of atherosclerosis.
Arginine vasopressin (AVP) is a pituitary gland peptide which was proven to be related to CV risk factors such as abdominal obesity and diabetes [120]. Copeptin is the C-terminal fragment of the precursor pre-provasopressin, which is released in the same amount as AVP, but has greater plasma stability [121]. There is a growing interest in researching this molecule and its relationship to the CV risk; however, most of the existing data are based on diabetic patients. Copeptin levels were associated with an increased risk of CAD, HF, and death in this population [122]. It was also proven to be a good indicator of subclinical atherosclerosis as it was also correlated with arterial stiffness [123]. Recently, similar results were obtained in non-diabetic patients. Tasevska et al. concluded that higher copeptin levels were predictive for CAD development, even though the association was stronger for non-diabetics. As for the risk of CV mortality, the relationship with copeptin was equally strong in both groups [124].
Midkine (MK) is a low molecular weight protein, representing the family of growth factors and cytokines. It is associated with both inflammatory and reparative processes [125]. Numerous studies have shown its relationship with CVD [126,127]. In atherosclerosis, MK is responsible for various pathophysiological processes, such as inflammation, neointimal formation, angiogenesis, and foam cell formation [125,128]. In a study conducted on patients referred for peripheral artery revascularization, higher levels of MK were observed in the diseased group compared to controls. Furthermore, as part of a diagnosis panel, MK proved to be efficient in predicting the presence of obstructive peripheral artery disease [129]. Similar results were obtained with a different panel tested for CAD [130].
Myeloperoxidase (MPO) is an inflammation-related enzyme, mainly secreted by phagocytes (monocytes, macrophages, and neutrophils). Its implication in the atherosclerotic process is related to LDL oxidation, a process that MPO supports mainly via oxidant-hypochlorous acid secretion [131]. Additionally, it was found that MPO is also a strong promoter of endothelial dysfunction, being responsible for nitric oxide bioavailability impairment [132]. A recent prospective study found a significant inverse correlation between MPO and brachial flow-mediated dilation, a measure of endothelial function. In the same study, higher levels of MPO were associated with a higher prevalence of subclinical carotid atherosclerosis [133]. In another prospective study, MPO proved to be a strong predictor for developing CAD (p = 0.001). The analysis included over 1100 patients, who were followed up for a mean period of 8 years [134].
Growth differentiation factor-15 (GDF-15) is a cytokine from the transforming growth factor-β superfamily [135]. It is expressed by various cells, including myocardial cells, adipocytes, macrophages, endothelial cells, and vascular smooth muscle cells. However, its serum levels are generally low in healthy individuals [136]. As a biomarker of CVD, GDF-15 proved its efficiency, especially in HF patients, for whom it appeared to be a reliable predictor of mortality [137] and disease severity [138]. As for atherosclerosis, its biological functions are not completely understood. However, GDF-15 seems to support this process by promoting inflammation and angiogenesis [139]. In patients suffering from acute coronary syndromes (ACS), GDF-15 has been demonstrated to be a powerful predictor of death [140,141] and risk of hospitalization for HF [142]. In stable CAD patients, GDF-15 is associated with CV and non-CV death [143] as well as with the risk of developing myocardial infarction [144]. In a retrospective study based on the Framingham Offspring Study cohort, GDF-15 levels were predictive for subclinical carotid atherosclerosis [145]. Further studies assessed the association between GDF-15 and CAD. A small prospective study concluded that increased GDF-15 levels were predictive for the presence of CAD, evaluated angiographically. In the same study, GDF-15 correlated with the Gensini score [146]. A positive correlation was also observed between GDF-15 and the extent of coronary collateral formation, described using the Rentrop grading system, which further linked it with the severity of CAD [147].
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme secreted especially by monocytes and macrophages [148]. It has a high specificity for vascular inflammation, and it circulates in the plasma primarily associated with LDL [149,150]. Lp-PLA2 promotes atherosclerosis by hydrolyzing the oxidized phospholipids in the LDL, which generates bioactive products, such as lysophosphatidylcholine, oxidized fatty acids, and arachidonic acid, capable of recruiting monocytes, activating endothelial cells, and stimulating smooth muscle cell proliferation [151,152]. The quantification of Lp-PLA2 can be performed using two methods: measuring its mass concentration using an ELISA immunoassay or assessing its enzymatic activity using a spectrophotometric assay. However, the latter proved to be more accurate [153]. In stable CAD patients, Lp-PLA2 activity proved to be a predictor of CV events, such as myocardial infarction, CV death, and stroke [154]. In another prospective study, Lp-PLA2 was positively correlated with the presence and the severity of CAD, independently of other CV risk factors (p < 0.05) [155]. Additionally, adding Lp-PLA2 to a biomarker panel, along with hs-CRP, increased the predictive power for atherosclerotic carotid disease, assessed using ultrasonography [156].
Using ultracentrifugation, LDL particles can be separated into four subclasses based on their density: subclass I—large LDL, subclass II—intermediate LDL, and subclasses III and IV, generally assigned as small dense LDL (sdLDL). Small dense LDL is the smallest and also the densest subclass, measuring less than 25.5nm and with a density that ranges from 1.034 g/mL to 1.060 g/mL [157,158]. Experimental studies suggested that compared to other LDL subfractions, sdLDL presents higher atherogenic properties due to several metabolic and structural features, including smaller size, which promotes endothelial penetration [158], a higher affinity for proteoglycans in the arterial wall [159], a lower affinity for LDL receptors [160], and higher susceptibility for oxidation, due to vitamin E deficiency [161]. Based on these results, the idea of using sdLDL as a biomarker for atherosclerosis and CV risk emerged. In a prospective cohort study, which included over 11,000 participants, sdLDL-C was associated with incident CHD (hazard ratio of 1.51, 95% CI, 1.21–1.88). Additionally, sdLDL-C was higher in patients with diabetes [162]. In another prospective trial conducted on patients who suffered an acute ischemic stroke, sdLDL was able to predict short-term mortality (p < 0.05) and also ischemic stroke onset (OR = 4.31, p < 0.001) [163]. This subfraction of LDL was also associated with coronary artery stenosis. In a small case-control study, which included over 190 patients referred for elective coronary angiography, sdLDL-C levels were predictive for the presence of significant coronary stenosis [164]. Additionally, there are studies in the literature, which link sdLDL-C levels to the risk of plaque progression and destabilization, strengthening the use of sdLDL as a marker for CV risk [165,166].
7. The Potential Use of Apolipoprotein B and Interleukin-6 as Future Screening Biomarkers for Cardiovascular Risk
This review aimed to explore the pathophysiological basis that supports the role of these two molecules as potential biomarkers of atherosclerosis. Based on our research, there is a rising number of studies supporting the notion that apoB and IL-6 are able to predict both the risk of future CV events and the presence and severity of CAD. However, further prospective studies are needed in order for these molecules to be implemented in clinical practice (see Figure 2).
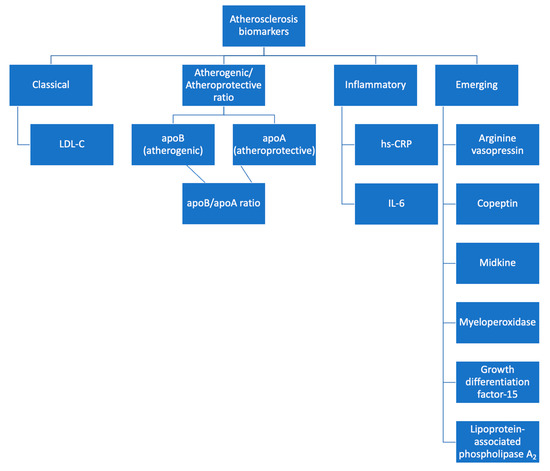
Figure 2.
Biomarkers for atherosclerosis detection.
At the moment, LDL-C represents the only routine biomarker used by the guidelines to estimate the total CV risk, despite its limitations, especially in specific populations. However, based on the current existing data, apoB, the common piece that links all the atherosclerotic lipoproteins, might be a potential substituent for LDL-C, able to cross its boundaries [154].
Furthermore, inflammation plays a key role in the atherosclerotic process, and represents an important risk factor for CV events. Despite this, no inflammatory biomarker is currently used for the CV risk assessment, except for hs-CRP, whose utility is controversial. IL-6 is a strong stimulator of hs-CRP liver secretion and a central inflammation mediator in the atherosclerotic process, making it a good candidate for this position. Moreover, the rising number of prospective studies linking IL-6 serum levels to the severity of CAD supports future research on this topic [108,109,110,111,112,113].
These emerging biomarkers could prove useful in assessing individuals during the early stages of the atherosclerotic process when the cardiovascular risk is still low, thus enabling the faster initiation of the preventive measures before the onset of events such as CAD or stroke. This could also allow us to reclassify the patients based on the cardiovascular risk. However, at the moment, their sensibility and specificity should still be studied in larger groups, in order to fully understand their mechanisms, what influences their levels, and how exactly they correlate to the atherosclerotic process [108,109,110,111,112,113].
8. Conclusions
LDL-C, the classical and most routinely used parameter measured in patients, remains a viable marker of atherosclerosis and cardiovascular risk. More specific would be the measuring of apoB, which includes a wider spectrum of atherogenic particles. On the other hand, apoA is associated with an atheroprotective effect. Therefore, the ratio between the two would be an even more accurate assessment tool. Additionally, given the inflammatory nature of atherosclerosis, hs-CRP and IL-6 levels are indicative. This review offers a comprehensive perspective on the diagnosis of atherosclerosis through the analysis of both established and emerging biomarkers, with a potential to be fully implemented in future screening protocols.
Author Contributions
Conceptualization, R.-A.J., A.-D.C. and R.-S.G.; methodology, R.-A.J., A.-D.C. and A.M.; writing—original draft preparation, R.-A.J., M.Ș.C.H., E.G.B. and A.-D.C.; writing—review and editing, R.-A.J., M.R. and R.M.H.; visualization, R.-A.J., O.M., A.-F.O., R.-S.M., B.-A.T. and S.I.C.; supervision, M.M.L., I.-I.C. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Robinson, J.G.; Williams, K.J.; Gidding, S.; Borén, J.; Tabas, I.; Fisher, E.A.; Packard, C.; Pencina, M.; Fayad, Z.A.; Mani, V.; et al. Eradicating the Burden of Atherosclerotic Cardiovascular Disease by Lowering Apolipoprotein B Lipoproteins Earlier in Life. J. Am. Heart Assoc. 2018, 7, e009778. [Google Scholar] [CrossRef]
- Hoefer, I.E.; Steffens, S.; Ala-Korpela, M.; Bäck, M.; Badimon, L.; Bochaton-Piallat, M.L.; Boulanger, C.M.; Caligiuri, G.; Dimmeler, S.; Egido, J.; et al. Novel methodologies for biomarker discovery in atherosclerosis. Eur. Heart J. 2015, 36, 2635–2642. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers-A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9.23.1–9.23.17. [Google Scholar] [CrossRef]
- Seidman, M.A.; Mitchell, R.N.; Stone, J.R. Pathophysiology of Atherosclerosis. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M.S., Homeister, J.W., Stone, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 221–237. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Avati Nanjundappa, R.P.; Branch, J.R.; Taylor, W.R.; Quyyumi, A.A.; Jo, H.; McDaniel, M.C.; Suo, J.; Giddens, D.; Samady, H. Shear stress and plaque development. Expert. Rev. Cardiovasc. Ther. 2010, 8, 545–556. [Google Scholar] [CrossRef]
- Souilhol, C.; Serbanovic-Canic, J.; Fragiadaki, M.; Chico, T.J.; Ridger, V.; Roddie, H.; Evans, P.C. Endothelial responses to shear stress in atherosclerosis: A novel role for developmental genes. Nat. Rev. Cardiol. 2020, 17, 52–63. [Google Scholar] [CrossRef]
- Déglise, S.; Bechelli, C.; Allagnat, F. Vascular smooth muscle cells in intimal hyperplasia, an update. Front. Physiol. 2022, 13, 1081881. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; Guillermo, G.-C. Vascular Endothelium, Hemodynamics, and the Pathobiology of Atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, J.; Chen, W.; Li, W.; Chen, Z. Vascular Macrophages in Atherosclerosis. J. Immunol. Res. 2019, 2019, 4354786. [Google Scholar] [CrossRef]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidol. 2016, 27, 209–215. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef]
- Kumar, V.; Prabhu, S.D.; Bansal, S.S. CD4+ T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 992653. [Google Scholar] [CrossRef]
- Kumar, V.; Rosenzweig, R.; Asalla, S.; Nehra, S.; Prabhu, S.D.; Bansal, S.S. TNFR1 Contributes to Activation-Induced Cell Death of Pathological CD4+ T Lymphocytes During Ischemic Heart Failure. JACC Basic Transl. Sci. 2022, 7, 1038–1049. [Google Scholar] [CrossRef]
- Binder, C.J.; Hartvigsen, K.; Chang, M.K.; Miller, M.; Broide, D.; Palinski, W.; Curtiss, L.K.; Corr, M.; Witztum, J.L. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Investig. 2004, 114, 427–437. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.F.; Sobenin, I.A.; Orekhov, A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef]
- Eikendal, A.L.; Groenewegen, K.A.; Bots, M.L.; Peters, S.A.; Uiterwaal, C.S.; den Ruijter, H.M. Relation Between Adolescent Cardiovascular Risk Factors and Carotid Intima-Media Echogenicity in Healthy Young Adults: The Atherosclerosis Risk in Young Adults (ARYA) Study. J. Am. Heart Assoc. 2016, 5, e002941. [Google Scholar] [CrossRef]
- Venugopal, S.K.; Jialal, I. Biochemistry, Low Density Lipoprotein. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Insull, W. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Newman, A.A.C.; Serbulea, V.; Baylis, R.A.; Shankman, L.S.; Bradley, X.; Alencar, G.F.; Owsiany, K.; Deaton, R.A.; Karnewar, S.; Shamsuzzaman, S.; et al. Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRβ and bioenergetic mechanisms. Nat. Metab. 2021, 3, 166–181. [Google Scholar] [CrossRef]
- Gopalan, C.; Kirk, E. Atherosclerosis. In Biology of Cardiovascular and Metabolic Diseases; Gopalan, C., Kirk, E., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 85–101. [Google Scholar]
- Wang, T.; Butany, J. Pathogenesis of atherosclerosis. Diagn. Histopathol. 2017, 23, 473–478. [Google Scholar]
- Aprotosoaie, A.C.; Costache, A.D.; Costache, I.I. Therapeutic Strategies and Chemoprevention of Atherosclerosis: What Do We Know and Where Do We Go. Pharmaceutics 2022, 14, 722. [Google Scholar] [CrossRef]
- Hevonoja, T.; Pentikäinen, M.O.; Hyvönen, M.T.; Kovanen, P.T.; Ala-Korpela, M. Structure of low density lipoprotein (LDL) particles: Basis for understanding molecular changes in modified LDL. Biochim. Biophys. Acta 2000, 1488, 189–210. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Bukhari, S.M.A.; Herrera, E.C.; Awuah, W.A.; Lawrence, J.; de Andrade, H.; Patel, N.; Shah, R.; Shaikh, R.; Capriles, C.A.A.; et al. Lipid Lowering Therapy: An Era Beyond Statins. Curr. Probl. Cardiol. 2022, 47, 101342. [Google Scholar] [CrossRef]
- Lund-Katz, S.; Phillips, M.C. Packing of cholesterol molecules in human low-density lipoprotein. Biochemistry 1986, 25, 1562–1568. [Google Scholar] [CrossRef]
- Bancells, C.; Canals, F.; Benítez, S.; Colomé, N.; Julve, J.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. Proteomic analysis of electronegative low-density lipoprotein. J. Lipid Res. 2010, 51, 3508–3515. [Google Scholar] [CrossRef]
- Devaraj, S.; Semaan, J.R.; Jialal, I. Biochemistry, Apolipoprotein B. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Prassl, R.; Laggner, P. Molecular structure of low density lipoprotein: Current status and future challenges. Eur. Biophys. J. 2009, 38, 145–158. [Google Scholar] [CrossRef]
- Wolska, A.; Remaley, A.T. Measuring LDL-cholesterol: What is the best way to do it. Curr. Opin. Cardiol. 2020, 35, 405–411. [Google Scholar] [CrossRef]
- Song, Y.; Lee, H.S.; Baik, S.J.; Jeon, S.; Han, D.; Choi, S.Y.; Chun, E.J.; Han, H.W.; Park, S.H.; Sung, J.; et al. Comparison of the effectiveness of Martin’s equation, Friedewald’s equation, and a Novel equation in low-density lipoprotein cholesterol estimation. Sci. Rep. 2021, 11, 13545. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Ruelland, A.; Gallou, G.; Legras, B.; Paillard, F.; Cloarec, L. LDL sialic acid content in patients with coronary artery disease. Clin. Chim. Acta 1993, 221, 127–133. [Google Scholar] [CrossRef]
- Tertov, V.V.; Sobenin, I.A.; Orekhov, A.N. Characterization of desialylated low-density lipoproteins which cause intracellular lipid accumulation. Int. J. Tissue React. 1992, 14, 155–162. [Google Scholar]
- Tertov, V.V.; Sobenin, I.A.; Gabbasov, Z.A.; Popov, E.G.; Jaakkola, O.; Solakivi, T.; Nikkari, T.; Smirnov, V.N.; Orekhov, A.N. Multiple-modified desialylated low density lipoproteins that cause intracellular lipid accumulation. Isolation, fractionation and characterization. Lab. Investig. 1992, 67, 665–675. [Google Scholar]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxid. Med. Cell Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Ivanova, E.A.; Melnichenko, A.A.; Sobenin, I.A. Circulating desialylated low density lipoprotein. Cor et Vasa 2016, 59, e149–e156. [Google Scholar]
- Younis, N.; Charlton-Menys, V.; Sharma, R.; Soran, H.; Durrington, P.N. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 2009, 202, 162–168. [Google Scholar] [CrossRef]
- Soran, H.; Durrington, P.N. Susceptibility of LDL and its subfractions to glycation. Curr. Opin. Lipidol. 2011, 22, 254–261. [Google Scholar] [CrossRef]
- Ferretti, G.; Rabini, R.A.; Bacchetti, T.; Vignini, A.; Salvolini, E.; Ravaglia, F.; Curatola, G.; Mazzanti, L. Glycated low density lipoproteins modify platelet properties: A compositional and functional study. J. Clin. Endocrinol. Metab. 2002, 87, 2180–2184. [Google Scholar] [CrossRef]
- Cohen, M.P.; Lautenslager, G.; Shea, E. Glycated LDL concentrations in non-diabetic and diabetic subjects measured with monoclonal antibodies reactive with glycated apolipoprotein B epitopes. Eur. J. Clin. Chem. Clin. Biochem. 1993, 31, 707–713. [Google Scholar] [CrossRef][Green Version]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Heart Lung Circ. 2019, 28, 667–677. [Google Scholar] [CrossRef]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef]
- Ling, W.; Lougheed, M.; Suzuki, H.; Buchan, A.; Kodama, T.; Steinbrecher, U.P. Oxidized or acetylated low density lipoproteins are rapidly cleared by the liver in mice with disruption of the scavenger receptor class A type I/II gene. J. Clin. Investig. 1997, 100, 244–252. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Oka, Y.; Katagiri, H. Circulating oxidized LDL: A biomarker and a pathogenic factor. Curr. Opin. Lipidol. 2009, 20, 363–369. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association Between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.; Tang, Z.; Feng, B. Association between circulating oxidized OxLDL/LDL-C ratio and the severity of coronary atherosclerosis, along with other emerging biomarkers of cardiovascular disease in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 191, 110040. [Google Scholar] [CrossRef]
- Li, X.; Guo, D.; Hu, Y.H.; Zhou, H.; Chen, Y. Potential Biomarkers and Therapeutic Targets: Inflammation and Oxidative Stress in Left Carotid Artery Stenosis with Coronary Artery Disease. Curr. Pharm. Des. 2023, 29, 966–979. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Roth, E.M.; Davidson, M.H. PCSK9 Inhibitors: Mechanism of Action, Efficacy, and Safety. Rev. Cardiovasc. Med. 2018, 19, S31–S46. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Navarese, E.P.; Kolodziejczak, M.; Kereiakes, D.J.; Tantry, U.S.; O’Connor, C.; Gurbel, P.A. Proprotein Convertase Subtilisin/Kexin Type 9 Monoclonal Antibodies for Acute Coronary Syndrome: A Narrative Review. Ann. Intern. Med. 2016, 164, 600–607. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef]
- Glavinovic, T.; Thanassoulis, G.; de Graaf, J.; Couture, P.; Hegele, R.A.; Sniderman, A.D. Physiological Bases for the Superiority of Apolipoprotein B Over Low-Density Lipoprotein Cholesterol and Non-High-Density Lipoprotein Cholesterol as a Marker of Cardiovascular Risk. J. Am. Heart Assoc. 2022, 11, e025858. [Google Scholar] [CrossRef]
- Chen, S.H.; Yang, C.Y.; Chen, P.F.; Setzer, D.; Tanimura, M.; Li, W.H.; Gotto, A.M.; Chan, L. The complete cDNA and amino acid sequence of human apolipoprotein B-100. J. Biol. Chem. 1986, 261, 12918–12921. [Google Scholar]
- Carr, S.S.; Hooper, A.J.; Sullivan, D.R.; Burnett, J.R. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology 2019, 51, 148–154. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Lipoprotein physiology. Endocrinol. Metab. Clin. N. Am. 1998, 27, 503–519. [Google Scholar] [CrossRef]
- Olofsson, S.O.; Borèn, J. Apolipoprotein B: A clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 2005, 258, 395–410. [Google Scholar] [CrossRef]
- Tiwari, S.; Siddiqi, S.A. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1079–1086. [Google Scholar] [CrossRef]
- Segrest, J.P.; Jones, M.K.; Mishra, V.K.; Anantharamaiah, G.M.; Garber, D.W. apoB-100 has a pentapartite structure composed of three amphipathic alpha-helical domains alternating with two amphipathic beta-strand domains. Detection by the computer program LOCATE. Arterioscler. Thromb. 1994, 14, 1674–1685. [Google Scholar] [CrossRef]
- Manchekar, M.; Kapil, R.; Sun, Z.; Segrest, J.P.; Dashti, N. Relationship between Amphipathic β Structures in the β1 Domain of Apolipoprotein B and the Properties of the Secreted Lipoprotein Particles in McA-RH7777 Cells. Biochemistry 2017, 56, 4084–4094. [Google Scholar] [CrossRef]
- Shelness, G.S.; Hou, L.; Ledford, A.S.; Parks, J.S.; Weinberg, R.B. Identification of the lipoprotein initiating domain of apolipoprotein B. J. Biol. Chem. 2003, 278, 44702–44707. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, S.H.; Gianturco, S.H.; Bradley, W.A.; Sparrow, J.T.; Tanimura, M.; Li, W.H.; Sparrow, D.A.; DeLoof, H.; Rosseneu, M. Sequence, structure, receptor-binding domains and internal repeats of human apolipoprotein B-100. Nature 1986, 323, 738–742. [Google Scholar] [CrossRef]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef]
- Zeitouni, M.; Sabouret, P.; Kerneis, M.; Silvain, J.; Collet, J.P.; Bruckert, E.; Montalescot, G. 2019 ESC/EAS Guidelines for management of dyslipidaemia: Strengths and limitations. Eur. Heart. J. Cardiovasc. Pharmacother. 2021, 7, 324–333. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Williams, K.; Contois, J.H.; Monroe, H.M.; McQueen, M.J.; de Graaf, J.; Furberg, C.D. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 337–345. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I.; Holme, I.; Aastveit, A.H.; Kolar, W.; Steiner, E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet 2001, 358, 2026–2033. [Google Scholar] [CrossRef]
- St-Pierre, A.C.; Cantin, B.; Dagenais, G.R.; Mauriège, P.; Bernard, P.M.; Després, J.P.; Lamarche, B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 553–559. [Google Scholar] [CrossRef]
- Rabizadeh, S.; Rajab, A.; Mechanick, J.I.; Moosaie, F.; Rahimi, Y.; Nakhjavani, M.; Esteghamati, A. LDL/apo B ratio predict coronary heart disease in Type 2 diabetes independent of ASCVD risk score: A case-cohort study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1477–1485. [Google Scholar] [CrossRef]
- Jung, H.W.; Ra, M.; Bae, H.J.; Hong, S.P. The LDL-C/Apo B predicts coronary atherosclerotic heart disease in non-diabetic patients without high LDL-C. Medicine 2023, 102, e32596. [Google Scholar] [CrossRef]
- Deng, S.; Xu, Y.; Zheng, L. HDL Structure. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1377, pp. 1–11. [Google Scholar] [CrossRef]
- Khuseyinova, N.; Koenig, W. Apolipoprotein A-I and risk for cardiovascular diseases. Curr. Atheroscler. Rep. 2006, 8, 365–373. [Google Scholar] [CrossRef]
- Liting, P.; Guoping, L.; Zhenyue, C. Apolipoprotein B/apolipoprotein A1 ratio and non-high-density lipoprotein cholesterol. Predictive value for CHD severity and prognostic utility in CHD patients. Herz 2015, 40 (Suppl. S1), 1–7. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Melloni, G.E.M.; Park, J.G.; Morrill, V.; Blazing, M.A.; Ference, B.; Stein, E.; Stroes, E.S.; Braunwald, E.; et al. Association of Apolipoprotein B-Containing Lipoproteins and Risk of Myocardial Infarction in Individuals with and without Atherosclerosis: Distinguishing Between Particle Concentration, Type, and Content. JAMA Cardiol. 2022, 7, 250–256. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Gager, G.M.; Biesinger, B.; Hofer, F.; Winter, M.P.; Hengstenberg, C.; Jilma, B.; Eyileten, C.; Postula, M.; Lang, I.M.; Siller-Matula, J.M. Interleukin-6 level is a powerful predictor of long-term cardiovascular mortality in patients with acute coronary syndrome. Vasc. Pharmacol. 2020, 135, 106806. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef]
- Danesh, J.; Kaptoge, S.; Mann, A.G.; Sarwar, N.; Wood, A.; Angleman, S.B.; Wensley, F.; Higgins, J.P.; Lennon, L.; Eiriksdottir, G.; et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Med. 2008, 5, e78. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Van Damme, J.; Opdenakker, G.; Simpson, R.J.; Rubira, M.R.; Cayphas, S.; Vink, A.; Billiau, A.; Van Snick, J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J. Exp. Med. 1987, 165, 914–919. [Google Scholar] [CrossRef]
- Kawano, M.; Hirano, T.; Matsuda, T.; Taga, T.; Horii, Y.; Iwato, K.; Asaoku, H.; Tang, B.; Tanabe, O.; Tanaka, H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 1988, 332, 83–85. [Google Scholar] [CrossRef]
- Miki, S.; Iwano, M.; Miki, Y.; Yamamoto, M.; Tang, B.; Yokokawa, K.; Sonoda, T.; Hirano, T.; Kishimoto, T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989, 250, 607–610. [Google Scholar] [CrossRef]
- Miles, S.A.; Rezai, A.R.; Salazar-González, J.F.; Vander Meyden, M.; Stevens, R.H.; Logan, D.M.; Mitsuyasu, R.T.; Taga, T.; Hirano, T.; Kishimoto, T. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc. Natl. Acad. Sci. USA 1990, 87, 4068–4072. [Google Scholar] [CrossRef]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef]
- Schrader, L.I.; Kinzenbaw, D.A.; Johnson, A.W.; Faraci, F.M.; Didion, S.P. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2576–2581. [Google Scholar] [CrossRef]
- Coles, B.; Fielding, C.A.; Rose-John, S.; Scheller, J.; Jones, S.A.; O’Donnell, V.B. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am. J. Pathol. 2007, 171, 315–325. [Google Scholar] [CrossRef]
- Schieffer, B.; Selle, T.; Hilfiker, A.; Hilfiker-Kleiner, D.; Grote, K.; Tietge, U.J.; Trautwein, C.; Luchtefeld, M.; Schmittkamp, C.; Heeneman, S.; et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 2004, 110, 3493–3500. [Google Scholar] [CrossRef]
- Schuett, H.; Luchtefeld, M.; Grothusen, C.; Grote, K.; Schieffer, B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb. Haemost. 2009, 102, 215–222. [Google Scholar] [CrossRef]
- Al-Khalili, L.; Bouzakri, K.; Glund, S.; Lönnqvist, F.; Koistinen, H.A.; Krook, A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol. Endocrinol. 2006, 20, 3364–3375. [Google Scholar] [CrossRef]
- Jarlborg, M.; Gabay, C. Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. Cytokine 2022, 149, 155742. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link. Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Palaiopanos, K.; Björkbacka, H.; Peters, A.; de Lemos, J.A.; Seshadri, S.; Dichgans, M.; Georgakis, M.K. Circulating Interleukin-6 Levels and Incident Ischemic Stroke: A Systematic Review and Meta-analysis of Prospective Studies. Neurology 2022, 98, e1002–e1012. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.L.; Zhao, C.R.; Pan, C.L.; Zhang, Z. Interleukin-6 as a Predictor of the Risk of Cardiovascular Disease: A Meta-Analysis of Prospective Epidemiological Studies. Immunol. Investig. 2018, 47, 689–699. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005, 112, 976–983. [Google Scholar] [CrossRef]
- Saremi, A.; Anderson, R.J.; Luo, P.; Moritz, T.E.; Schwenke, D.C.; Allison, M.; Reaven, P.D. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT). Atherosclerosis 2009, 203, 610–614. [Google Scholar] [CrossRef]
- Gerin, F.; Durmuş, E.; Yaman, A.; Sünbül, M.; Mammadov, C.; Bozbay, M.; Sarı, İ.; Kıvrak, T. Relation of Interleukin-6 Level with Coronary Artery Disease Severity in Patients Undergoing Coronary Angiography. Eur. J. Ther. 2020, 23, 117–121. [Google Scholar] [CrossRef]
- Ferencik, M.; Mayrhofer, T.; Lu, M.T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Voora, D.; et al. Coronary Atherosclerosis, Cardiac Troponin, and Interleukin-6 in Patients with Chest Pain: The PROMISE Trial Results. JACC Cardiovasc. Imaging 2022, 15, 1427–1438. [Google Scholar] [CrossRef]
- Mossmann, M.; Wainstein, M.V.; Mariani, S.; Machado, G.P.; de Araújo, G.N.; Andrades, M.; Gonçalves, S.C.; Bertoluci, M.C. Increased serum IL-6 is predictive of long-term cardiovascular events in high-risk patients submitted to coronary angiography: An observational study. Diabetol. Metab. Syndr. 2022, 14, 125. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Yang, Y. Association between interleukin-6 and the risk of cardiac events measured by coronary computed tomography angiography. Int. J. Cardiovasc. Imaging 2017, 33, 1237–1244. [Google Scholar] [CrossRef]
- Tajfard, M.; Latiff, L.A.; Rahimi, H.R.; Moohebati, M.; Hasanzadeh, M.; Emrani, A.S.; Esmaeily, H.; Taghipour, A.; Mirhafez, S.R.; Ferns, G.A.; et al. Serum concentrations of MCP-1 and IL-6 in combination predict the presence of coronary artery disease and mortality in subjects undergoing coronary angiography. Mol. Cell Biochem. 2017, 435, 37–45. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, andomized, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef]
- Ridker, P.M. From RESCUE to ZEUS: Will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction. Cardiovasc. Res. 2021, 117, e138–e140. [Google Scholar] [CrossRef]
- Surma, S.; Czober, T.; Lepich, T.; Sierka, O.; Bajor, G. Selected Biomarkers of Atherosclerosis—Clinical Aspects. Acta Angiol. 2020, 26, 28–39. [Google Scholar]
- Enhörning, S.; Bankir, L.; Bouby, N.; Struck, J.; Hedblad, B.; Persson, M.; Morgenthaler, N.G.; Nilsson, P.M.; Melander, O. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: The prospective Malmö Diet and Cancer Study cardiovascular cohort. Int. J. Obes. 2013, 37, 598–603. [Google Scholar] [CrossRef]
- Katan, M.; Morgenthaler, N.; Widmer, I.; Puder, J.J.; König, C.; Müller, B.; Christ-Crain, M. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the Individual stress level. Neuroendocrinol. Lett. 2008, 29, 341–346. [Google Scholar]
- Enhörning, S.; Hedblad, B.; Nilsson, P.M.; Engström, G.; Melander, O. Copeptin is an independent predictor of diabetic heart disease and death. Am. Heart J. 2015, 169, 549–556. [Google Scholar] [CrossRef]
- Wiromrat, P.; Bjornstad, P.; Vinovskis, C.; Chung, L.T.; Roncal, C.; Pyle, L.; Lanaspa, M.A.; Johnson, R.J.; Cherney, D.Z.; Reznick-Lipina, T.K.; et al. Elevated copeptin, arterial stiffness, and elevated albumin excretion in adolescents with type 1 diabetes. Pediatr. Diabetes 2019, 20, 1110–1117. [Google Scholar] [CrossRef]
- Tasevska, I.; Enhörning, S.; Persson, M.; Nilsson, P.M.; Melander, O. Copeptin predicts coronary artery disease cardiovascular and total mortality. Heart 2016, 102, 127–132. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Wang, G.; Yin, S.H.; Yu, X.H. Midkine: A multifaceted driver of atherosclerosis. Clin. Chim. Acta 2021, 521, 251–257. [Google Scholar] [CrossRef]
- Kinoshita, D.; Shishido, T.; Takahashi, T.; Yokoyama, M.; Sugai, T.; Watanabe, K.; Tamura, H.; Nishiyama, S.; Takahashi, H.; Arimoto, T.; et al. Growth Factor Midkine Aggravates Pulmonary Arterial Hypertension via Surface Nucleolin. Sci. Rep. 2020, 10, 10345. [Google Scholar] [CrossRef]
- Kitahara, T.; Shishido, T.; Suzuki, S.; Katoh, S.; Sasaki, T.; Ishino, M.; Nitobe, J.; Miyamoto, T.; Miyashita, T.; Watanabe, T.; et al. Serum midkine as a predictor of cardiac events in patients with chronic heart failure. J. Card. Fail. 2010, 16, 308–313. [Google Scholar] [CrossRef]
- Takemoto, Y.; Horiba, M.; Harada, M.; Sakamoto, K.; Takeshita, K.; Murohara, T.; Kadomatsu, K.; Kamiya, K. Midkine Promotes Atherosclerotic Plaque Formation through Its Pro-Inflammatory, Angiogenic and Anti-Apoptotic Functions in Apolipoprotein E-Knockout Mice. Circ. J. 2017, 82, 19–27. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Shrestha, S.; Ibrahim, N.; van Kimmenade, R.R.J.; Gaggin, H.K.; Mukai, R.; Magaret, C.; Barnes, G.; Rhyne, R.; Garasic, J.M.; et al. Performance of a clinical/proteomic panel to predict obstructive peripheral artery disease in patients with and without diabetes mellitus. Open Heart 2019, 6, e000955. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L.; Magaret, C.A.; Gaggin, H.K.; Rhyne, R.F.; Gandhi, P.U.; Kelly, N.; Simon, M.L.; Motiwala, S.R.; Belcher, A.M.; et al. A Clinical and Biomarker Scoring System to Predict the Presence of Obstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 1147–1156. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, M.R.; Frei, B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: Reaction pathways and antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1716–1723. [Google Scholar] [CrossRef]
- Maiocchi, S.L.; Ku, J.; Thai, T.; Chan, E.; Rees, M.D.; Thomas, S.R. Myeloperoxidase: A versatile mediator of endothelial dysfunction and therapeutic target during cardiovascular disease. Pharmacol. Ther. 2021, 221, 107711. [Google Scholar] [CrossRef]
- Iana, A.; Sirbu, E. Linking myeloperoxidase with subclinical atherosclerosis in adults with metabolic syndrome. Wien. Klin. Wochenschr. 2020, 132, 150–154. [Google Scholar] [CrossRef]
- Meuwese, M.C.; Stroes, E.S.; Hazen, S.L.; van Miert, J.N.; Kuivenhoven, J.A.; Schaub, R.G.; Wareham, N.J.; Luben, R.; Kastelein, J.J.; Khaw, K.T.; et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: The EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 2007, 50, 159–165. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Duffin, K.L.; Chintharlapalli, S.; Wu, X. GDF15 and Growth Control. Front. Physiol. 2018, 9, 1712. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef]
- Miftode, R.S.; Constantinescu, D.; Cianga, C.M.; Petris, A.O.; Costache, I.I.; Mitu, O.; Miftode, I.L.; Mitu, I.; Timpau, A.S.; Duca, S.T.; et al. A Rising Star of the Multimarker Panel: Growth Differentiation Factor-15 Levels Are an Independent Predictor of Mortality in Acute Heart Failure Patients Admitted to an Emergency Clinical Hospital from Eastern Europe. Life 2022, 12, 1948. [Google Scholar] [CrossRef]
- Rullman, E.; Melin, M.; Mandić, M.; Gonon, A.; Fernandez-Gonzalo, R.; Gustafsson, T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin. Res. Cardiol. 2020, 109, 655–672. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Yang, X.; Zhong, J. Roles of Growth Differentiation Factor 15 in Atherosclerosis and Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e012826. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Peter, T.; Olofsson, S.; James, S.; Johnston, N.; Lindahl, B.; Horn-Wichmann, R.; Brabant, G.; Simoons, M.L.; et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation 2007, 115, 962–971. [Google Scholar] [CrossRef]
- Kempf, T.; Björklund, E.; Olofsson, S.; Lindahl, B.; Allhoff, T.; Peter, T.; Tongers, J.; Wollert, K.C.; Wallentin, L. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur. Heart J. 2007, 28, 2858–2865. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Morrow, D.A.; Braunwald, E.; Cannon, C.P.; Jiang, S.; Breher, S.; Sabatine, M.S.; Kempf, T.; Wallentin, L.; Wollert, K.C. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: Observations from PROVE IT-TIMI 22. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 203–210. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Guo, W.; Bai, Y.; Li, H.; Chen, H.; Han, L.; Lyu, L.; Xu, C.; Liu, H. Multiple Biomarkers in the Context of Conventional Risk Factors in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 2769–2770. [Google Scholar] [CrossRef]
- Schopfer, D.W.; Ku, I.A.; Regan, M.; Whooley, M.A. Growth differentiation factor 15 and cardiovascular events in patients with stable ischemic heart disease (The Heart and Soul Study). Am. Heart J. 2014, 167, 186–192.e1. [Google Scholar] [CrossRef]
- Gopal, D.M.; Larson, M.G.; Januzzi, J.L.; Cheng, S.; Ghorbani, A.; Wollert, K.C.; Kempf, T.; D’Agostino, R.B.; Polak, J.F.; Ramachandran, V.S.; et al. Biomarkers of cardiovascular stress and subclinical atherosclerosis in the community. Clin. Chem. 2014, 60, 1402–1408. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.L.; Zhang, Q. Increased Serum Level of Growth Differentiation Factor 15 (GDF-15) is Associated with Coronary Artery Disease. Cardiovasc. Ther. 2016, 34, 138–143. [Google Scholar] [CrossRef]
- Sun, T.; Huang, Y.; Phillips, M.I.; Luo, X.; Zhu, J.; Shi, H.; Li, J. Growth differentiation factor 15 and coronary collateral formation. Clin. Cardiol. 2010, 33, E1–E5. [Google Scholar] [CrossRef]
- Tellis, C.C.; Tselepis, A.D. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim. Biophys. Acta 2009, 1791, 327–338. [Google Scholar] [CrossRef]
- Li, D.; Zhao, L.; Yu, J.; Zhang, W.; Du, R.; Liu, X.; Liu, Y.; Chen, Y.; Zeng, R.; Cao, Y.; et al. Lipoprotein-associated phospholipase A2 in coronary heart disease: Review and meta-analysis. Clin. Chim. Acta 2017, 465, 22–29. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Y.; Yang, Y.; Li, W.; Lu, J.; Hu, Y. Lipoprotein-associated phospholipase A2 and oxidized low-density lipoprotein in young patients with acute coronary syndrome in China. Sci. Rep. 2017, 7, 16092. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Gonçalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Björkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef]
- Fras, Z.; Tršan, J.; Banach, M. On the present and future role of Lp-PLA2 in atherosclerosis-related cardiovascular risk prediction and management. Arch. Med. Sci. 2021, 17, 954–964. [Google Scholar] [CrossRef]
- Wallentin, L.; Held, C.; Armstrong, P.W.; Cannon, C.P.; Davies, R.Y.; Granger, C.B.; Hagström, E.; Harrington, R.A.; Hochman, J.S.; Koenig, W.; et al. Lipoprotein-Associated Phospholipase A2 Activity Is a Marker of Risk but Not a Useful Target for Treatment in Patients with Stable Coronary Heart Disease. J. Am. Heart Assoc. 2016, 5, e003407. [Google Scholar] [CrossRef]
- Cai, A.; Li, G.; Chen, J.; Li, X.; Li, L.; Zhou, Y. Increased serum level of Lp-PLA2 is independently associated with the severity of coronary artery diseases: A cross-sectional study of Chinese population. BMC Cardiovasc. Disord. 2015, 15, 14. [Google Scholar] [CrossRef]
- Liu, H.; Yao, Y.; Wang, Y.; Ji, L.; Zhu, K.; Hu, H.; Chen, J.; Yang, J.; Cui, Q.; Geng, B.; et al. Association between high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A cross-sectional study. J. Cell Mol. Med. 2018, 22, 5145–5150. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Zou, Y.L.; Guo, S.D. Low-density lipoprotein particles in atherosclerosis. Front. Physiol. 2022, 13, 931931. [Google Scholar] [CrossRef]
- Hirayama, S.; Miida, T. Small dense LDL: An emerging risk factor for cardiovascular disease. Clin. Chim. Acta 2012, 414, 215–224. [Google Scholar] [CrossRef]
- Anber, V.; Griffin, B.A.; McConnell, M.; Packard, C.J.; Shepherd, J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis 1996, 124, 261–271. [Google Scholar] [CrossRef]
- Packard, C.; Caslake, M.; Shepherd, J. The role of small, dense low density lipoprotein (LDL): A new look. Int. J. Cardiol. 2000, 74 (Suppl. S1), S17–S22. [Google Scholar] [CrossRef]
- Tribble, D.L.; Rizzo, M.; Chait, A.; Lewis, D.M.; Blanche, P.J.; Krauss, R.M. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am. J. Med. 2001, 110, 103–110. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef]
- Zeljkovic, A.; Vekic, J.; Spasojevic-Kalimanovska, V.; Jelic-Ivanovic, Z.; Bogavac-Stanojevic, N.; Gulan, B.; Spasic, S. LDL and HDL subclasses in acute ischemic stroke: Prediction of risk and short-term mortality. Atherosclerosis 2010, 210, 548–554. [Google Scholar] [CrossRef]
- Toft-Petersen, A.P.; Tilsted, H.H.; Aarøe, J.; Rasmussen, K.; Christensen, T.; Griffin, B.A.; Aardestrup, I.V.; Andreasen, A.; Schmidt, E.B. Small dense LDL particles—A predictor of coronary artery disease evaluated by invasive and CT-based techniques: A case-control study. Lipids Health Dis. 2011, 10, 21. [Google Scholar] [CrossRef]
- Sekimoto, T.; Koba, S.; Mori, H.; Sakai, R.; Arai, T.; Yokota, Y.; Sato, S.; Tanaka, H.; Masaki, R.; Oishi, Y.; et al. Small Dense Low-Density Lipoprotein Cholesterol: A Residual Risk for Rapid Progression of Non-Culprit Coronary Lesion in Patients with Acute Coronary Syndrome. J. Atheroscler. Thromb. 2021, 28, 1161–1174. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Patel, N.; Li, H.; Hong, C.G.; Sampson, M.; O’Hagan, R.; Florida, E.M.; Teague, H.L.; Playford, M.P.; Chen, M.Y.; et al. Estimated sdLDL-C for predicting high-risk coronary plaque features in psoriasis: A prospective observational study. Lipids Health Dis. 2023, 22, 55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).