Assessment of the Health of Soils Contaminated with Ag, Bi, Tl, and Te by the Intensity of Microbiological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Heavy Metals
2.3. Simulation Experiment
2.4. Determination of the Basal Respiration of Soils and Microbial Biomass
2.5. Statistical Processing
3. Results
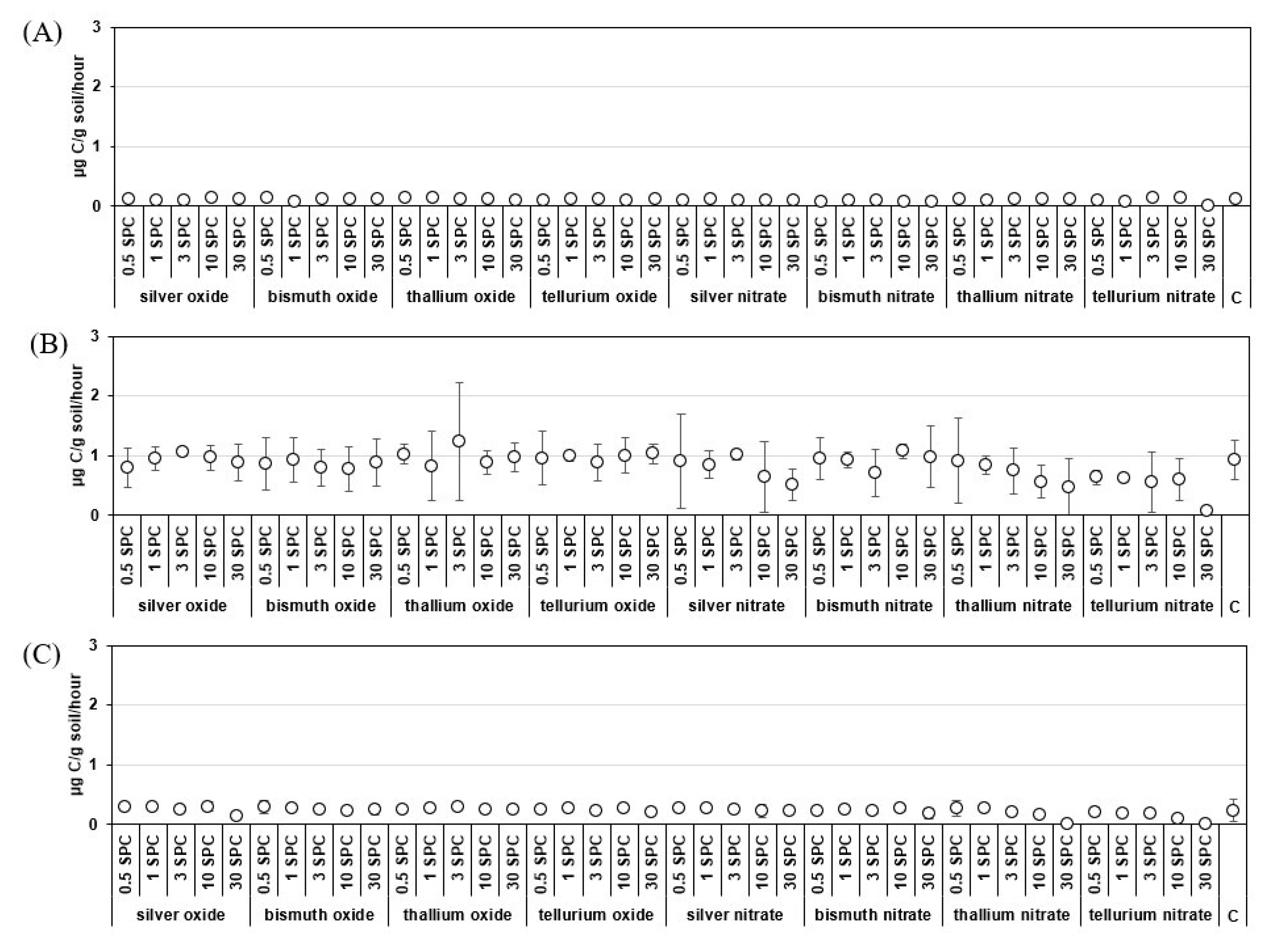
3.1. Basal Respiration of Soils
3.2. Metabolic Coefficient (qCO2)
3.3. Ranking the Toxicity of Elements
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguilera, A.; Bautista, F.; Goguitchaichvili, A.; Garcia-Oliva, F. Health risk of heavy metals in street dust. Front. Biosci.-Landmark 2021, 26, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Battsengel, E.; Murayama, T.; Fukushi, K.; Nishikizawa, S.; Chonokhuu, S.; Ochir, A.; Tsetsgee, S.; Davaasuren, D. Ecological and Human Health Risk Assessment of Heavy Metal Pollution in the Soil of the Ger District in Ulaanbaatar, Mongolia. Int. J. Environ. Res. Public Health 2020, 17, 4668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Danek, T.; Drozdova, J.; Huang, Q.; Qi, W.; Zou, L.; Yang, S.; Zhao, X.; Xiang, Y. Soil heavy metal pollution and risk assessment associated with the Zn-Pb mining region in Yunnan, Southwest China. Env. Monit. Assess. 2018, 190, 194. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; Nemr, A.E.; Madkour, F.F. Environmental Assessment of Heavy Metal Pollution and Human Health Risk. Water Sci. Eng. 2016, 2, 14. [Google Scholar]
- Khan, K.; Mohsin, A.; Sharif, H.M.A.; Maryam, A.; Ali, J.; Li, X.; Ibrahim, S.M.; Ayaz, M.; Zhou, Y.; Younas, M. Heavy metal pollution in the soil of a riverine basin: Distribution, source, and potential hazards. Environ. Monit. Assess. 2022, 194, 618. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.; Minnikova, T.; Kazeev, K.; Akimenko, Y.; Evstegneeva, N. Assessment of the Ecotoxicity of Pollution by Potentially Toxic Elements by Biological Indicators of Haplic Chernozem of Southern Russia (Rostov region). Water Air Soil Pollut. 2022, 233, 18. [Google Scholar] [CrossRef]
- Plekhanova, I.O.; Zolotareva, O.A. Ecological regulation of the state of soils contaminated with heavy metals. Agrochemistry 2020, 10, 79–88. [Google Scholar] [CrossRef]
- Spahić, M.P.; Manojlović, D.; Tančić, P.; Cvetković, Ž.; Nikić, Z.; Kovačević, R.; Sakan, S. Environmental impact of industrial and agricultural activities to the trace element content in soil of Srem (Serbia). Environ. Monit. Assess. 2019, 191, 133. [Google Scholar] [CrossRef]
- Teng, Y.; Ke, Y.; Zhou, Q.; Tao, R.; Wang, Y. Derived regional soil-environmental quality criteria of metals based on Anhui soil-crop systems at the regulated level. Sci. Total Environ. 2022, 825, 154060. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Cheng, J.; Li, Y.; Li, F.; Li, Y.; Shi, Z. Pollution Assessment and Source Apportionment of Soil Heavy Metals in a Coastal Industrial City, Zhejiang, Southeastern China. Int. J. Environ. Res. Public Health 2022, 11, 3335. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, W.; Lin, X.; Yan, H.; Ma, M.; He, Z. Assessment of heavy metals pollution of soybean grains in North Anhui of China. Sci. Total Environ. 2019, 646, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, R.; Wu, S.Y.; Jia, Z.B.; Wang, N. Heavy Metal Pollution and Health Risk Assessment of Mine Soil in Yangtze River Economic Belt. Huan Jing Ke Xue 2022, 43, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, K.; Ananyeva, N.; Selezneva, A.; Sushko, S.; Lepore, E.; Vasenev, V.; Demina, S.; Khabibullina, F.; Vaseneva, I.; Dolgikh, A.; et al. Assessing soil-like materials for ecosystem services provided by constructed technosols. Land 2021, 10, 11. [Google Scholar] [CrossRef]
- Perkins, W.T. Extreme selenium and tellurium contamination in soils—An eighty year-old industrial legacy surrounding a Ni refinery in the Swansea Valley. Sci. Total Environ. 2011, 412, 162–169. [Google Scholar] [CrossRef]
- Xing, G.; Zhu, J.; Xiong, Z. Ag, Ta, Ru, and Ir enrichment in surface soil: Evidence for land pollution of heavy metal from atmospheric deposition. Glob. Biogeochem. Cycles 2004, 18, 1–5. [Google Scholar] [CrossRef]
- Wiklund, J.A.; Kirk, J.L.; Muir, D.C.G.; Carrier, J.; Gleason, A.; Yang, F.; Evans, M.; Keating, J. Widespread Atmospheric Tellurium Contamination in Industrial and Remote Regions of Canada. Environ. Sci. Technol. 2018, 52, 6137–6145. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Crc Press: Boca Raton, FL, USA, 2010; p. 548. [Google Scholar]
- Yildirim, D.; Sasmaz, A. Phytoremediation of As, Ag, and Pb in contaminated soils using terrestrial plants grown on Gumuskoy mining area (Kutahya Turkey). J. Geochem. Explor. 2017, 182, 228–234. [Google Scholar] [CrossRef]
- Eivazi, F.; Afrasiabi, Z.; Jose, E. Pedosphere Effects of Silver Nanoparticles on the Activities of Soil Enzymes Involved in Carbon and Nutrient Cycling. Pedosphere 2018, 28, 209–214. [Google Scholar] [CrossRef]
- Druzhinin, A.V.; Karelina, E.V. The main types of technical silver deposits. Bull. Peoples’ Friendsh. Univ. Russ. A Ser. Eng. Stud. 2008, 1, 35–41. [Google Scholar]
- Lyapunov, M.Y. Patterns of the distribution of chemical elements in the soils of the Pioneer gold deposit of the Amur Region Geochemistry. Bull. Tomsk. Polytech. Univ. 2014, 325, 57–68. [Google Scholar]
- Elekes, C.C.; Busuioc, G. The mycoremediation of metals polluted soils using wild growing species of mushrooms. Latest Trends Eng. Educ. 2010, 1, 36–39. [Google Scholar]
- Grygoyć, K.; Jabłońska-Czapla, M. Development of a Tellurium Speciation Study Using IC-ICP-MS on Soil Samples Taken from an Area Associated with the Storage, Processing, and Recovery of Electrowaste. Molecules 2021, 26, 2651. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.M.; Ramos, N.A. Surficial geochemistry and bioaccessibility of tellurium in semiarid mine tailings. Environ. Chem. 2019, 16, 251–265. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640–659. [Google Scholar] [CrossRef]
- Grösslová, Z.; Vaněk, A.; Oborná, V.; Mihaljevič, M.; Ettler, V.; Trubač, J.; Drahota, P.; Penížek, V.; Pavlů, L.; Sracek, O.; et al. Thallium contamination of desert soil in Namibia: Chemical, mineralogical and isotopic insights. Environ. Pollut. 2018, 239, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Chen, Y.H.; Shen, C.C.; Jiang, X.Y.; Xie, X.F.; Chen, D.Y.; Lippold, H.; Wang, C.L. Thallium dispersal and contamination in surface sediments from South China and its source identification. Environ. Pollut. 2016, 213, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, E.I.; Volkova, O.O.; Turyanova, R.R. Enzymatic transformation of organic residues in soils contaminated with heavy metals. Ecol. Urban Territ. 2019, 1, 75–81. [Google Scholar] [CrossRef]
- Novosyolova, E.I.; Volkova, O.O.; Khaziev, F.K.; Turyanova, R.R. Features of enzymatic dehydrogenation of organic substances in soils contaminated with heavy metals. Sci. Life 2020, 15, 1312–1320. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Sukharevich, V.I. Soil enzymes and soil pollution: Biodegradation, bioremediation, bioindication. Agrochemistry 2020, 3, 83–93. [Google Scholar] [CrossRef]
- Yakushev, A.V.; Zhuravleva, A.I.; Kuznetsova, I.N. Effect of long-term and short-term droughts on the hydrolytic enzymes in haplic luvisol. Eurasian Soil Sci. 2023, 6, 745–757. [Google Scholar] [CrossRef]
- Minnikova, T.V.; Mokrikov, G.V.; Kazeev, K.S.; Akimenko, Y.V.; Kolesnikov, S.I. Evaluation of dependencies between hydrothermal parameters and enzymatic activity of chernozems of the Rostov region using various agricultural technologies. Agrophysics 2018, 1, 9–17. [Google Scholar] [CrossRef]
- Minnikova, T.V.; Kolesnikov, S.I.; Denisova, T.V. Influence of nitrogen and humic fertilizers on the biochemical state of oil-contaminated chernozem. South Russ. Ecol. Dev. 2019, 14, 189–201. [Google Scholar] [CrossRef]
- Minnikova, T.; Kolesnikov, S.; Revina, S.; Ruseva, A.; Gaivoronsky, V. Enzymatic Assessment of the State of Oil-Contaminated Soils in the South of Russia after Bioremediation. Toxics 2023, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Mokrikov, G.; Minnikova, T.; Kazeev, K.; Kolesnikov, S. Use of soil enzyme activity in assessing the effect of No-Till in the South of Russia. Agron. Res. 2021, 19, 171–184. [Google Scholar] [CrossRef]
- Stangherlin, E.C.; Ardais, A.P.; Rocha, J.B.T.; Nogueira, C.W. Exposure to diphenyl ditelluride, via maternal milk, causes oxidative stress in cerebral cortex, hippocampus and striatum of young rats. Arch. Toxicol. 2009, 83, 485–491. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Yermiyahu, U. A scale of metal ion binding strengths correlating with ionic charge, pauling electronegativity, toxicity, and other physiological effects. J. Inorg. Biochem. 2007, 101, 1201–1213. [Google Scholar] [CrossRef]
- Najimi, S.; Shakibaie, M.; Jafari, E.; Ameri, A.; Rahimi, N.; Forootanfar, H.; Yazdanpanah, M.; Rahimiae, H.R. Acute and subacute toxicities of biogenic tellurium nanorods in mice. Regul. Toxicol. Pharmacol. 2017, 90, 222–230. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; Moustafa, A.A.; Mahmoud, H.K.; Abdel-Latife, H.M.R. Astragalus membranaceus polysaccharides modulate growth, hemato-biochemical indices, hepatic antioxidants, and expression of HSP70 and apoptosis-related genes in Oreochromis niloticus exposed to sub-lethal thallium toxicity. Fish Shellfish Immunol. 2021, 118, 251–260. [Google Scholar] [CrossRef]
- Cao, C.; Huang, J.; Ca, W.; Yan, C.; Liu, J.; Jiang, Y. Effects of Silver Nanoparticles on Soil Enzyme Activity of Different Wetland Plant Soil Systems. Soil Sediment Contam. Int. J. 2017, 26, 558–567. [Google Scholar] [CrossRef]
- Kolesnikov, S.I. Impact of Contamination with Tellurium on Biological Properties of Ordinary Chernozem. Soil Sediment Contam. Int. J. 2019, 28, 792–800. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Tsepina, N.I.; Sudina, L.V.; Minnikova, T.V.; Kazeev, K.S.; Akimenko, Y.V. Silver Ecotoxicity Estimation by the Soil State Biological Indicators. Appl. Environ. Soil Sci. 2020, 2020, 1207210. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Tsepina, N.; Minnikova, T.; Kazeev, K.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Mazarji, M.; Singh, R.K.; Rajput, V.D. Influence of Silver Nanoparticles on the Biological Indicators of Haplic Chernozem. Plants 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Murata, T. Effects of bismuth contamination on the growth and activity of soil microorganisms using thiols as model compounds. J. Environ. Sci. Health Part A Tox Hazard. Subst. Env. Eng. 2006, 41, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ananyeva, N.D.; Ivashchenko, K.V.; Sushko, S.V. Microbial indicators of urban soils and their role in the assessment of ecosystem services (review). Soil Sci. 2021, 10, 1231–1246. [Google Scholar] [CrossRef]
- Baath, E. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Bogorodskaya, A.V.; Shishikin, A.S. Dynamics of microbial biomass, its structure and functional activity in soils during reforestation in clearings of fir forests of the Yenisei Ridge. Eurasian Soil Sci. 2020, 1, 119–130. [Google Scholar] [CrossRef]
- Gavrilenko, E.G.; Susyan, E.A.; Ananyeva, N.D.; Makarov, O.A. Spatial variation of the carbon content of microbial biomass and microbial respiration of soils in the southern Moscow region. Eurasian Soil Sci. 2011, 10, 1231–1245. [Google Scholar]
- Selezneva, A.E.; Ivashchenko, K.V.; Sushko, S.V.; Zhuravleva, A.I.; Ananyeva, N.D.; Blagodatsky, S.A. Respiratory activity of the microbial community of the soil and its functional diversity during the displacement of the upper forest boundary in the mountains of the North-Western Caucasus. Bull. Peoples’ Friendsh. Univ. Russ. Ser. Agron. Anim. Husb. 2021, 16, 226–237. [Google Scholar]
- Trofimov, S.Y.; Arzamazova, A.V.; Kinzhaev, R.R.; Avetov, N.A.; Karpukhin, M.M. Mineralization of organic matter in oil-contaminated and background soils of the Middle Ob region under laboratory conditions. Eurasian Soil Sci. 2022, 4, 511–518. [Google Scholar] [CrossRef]
- Zavyalova, N.E.; Vasbieva, M.T.; Fomin, D.S. Microbial biomass, respiratory activity, and nitrogen fixation in the soddy-podzolic soil of the Cis-Urals under various agricultural uses. Eurasian Soil Sci. 2020, 3, 372. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Sushko, S.V.; Ivashchenko, K.V.; Vasenev, V.I. Soil microbial respiration in Subtaiga and forest-steppe ecosystems of European Russia: Field and laboratory approaches. Eurasian Soil Sci. 2020, 53, 1492–1501. [Google Scholar] [CrossRef]
- Gorobtsova, O.N.; Gedgafova, F.V.; Uligova, T.S.; Tembotov, R.K. Ecophysiological indicators of microbial biomass status in chernozem soils of the central Caucasus (in the territory of Kabardino-Balkaria with the Terek variant of altitudinal zonation). Russ. J. Ecol. 2016, 47, 19–25. [Google Scholar] [CrossRef]
- Prikhodko, V.E.; Sizemskaya, M.L. Basal respiration and composition of microbial biomass in virgin, agro- and forest-ameliorated semi-desert soils of the Northern Caspian region. Eurasian Soil Sci. 2015, 8, 974–983. [Google Scholar] [CrossRef]
- Petrov, A.M.; Vershinin, A.A.; Akaikin, D.V.; Yuranets-Luzhaeva, R.C. Changes in the toxicological properties and respiratory activity of soddy-podzolic soils under conditions of long-term oil pollution. Ecol. Ind. Russ. 2015, 19, 50–53. [Google Scholar]
- Skachkova, A.D.; Pozdnyakov, L.A.; Selitskaya, O.V.; Weiss, F. Evaluation of the effect of ethandinitrile on the microbiological activity of soddy-podzolic soil. Agrochem. Bull. 2022, 1, 31–35. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Polyanskaya, L.M.; Susyan, E.A.; Vasenkina, I.V.; Wirth, S.; Zvyagintsev, D.G. Comparative assessment of soil microbial biomass determined by direct microscopy and substrate-induced respiration. Microbiology 2008, 77, 404–412. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Evdokimov, I.V. Methods for determining the biomass of soil microorganisms. Russ. J. Ecosyst. Ecol. 2018, 3. [Google Scholar] [CrossRef]
- Slukovskaya, M.; Dolgikh, A.V.; Novikov, A.I.; Mosendz, I.A.; Kremenetskaya, I.P.; Ran, L.P.; Ran, I.G. Soil respiration as an indicator of the toxicity of technozems. Proc. Fersman Sci. Sess. GI KSC RAS 2019, 16, 529–533. [Google Scholar]
- Ananyeva, N.D.; Susyan, E.A.; Gavrilenko, E.G. Peculiarities of determining the carbon of soil microbial biomass by the method of substrate-induced respiration. Eurasian Soil Sci. 2011, 11, 1327–1333. [Google Scholar]
- Evdokimova, G.A.; Mozgova, N.P.; Korneikova, M.V. The content and toxicity of heavy metals in the soils of the zone affected by gas-air emissions from the Pechenganickel plant. Eurasian Soil Sci. 2014, 5, 625–631. [Google Scholar] [CrossRef]
- Ivashchenko, K.V.; Ananyeva, N.D.; Vasenev, V.I.; Kudeyarov, V.N.; Valentini, R. Biomass and respiratory activity of soil microorganisms in anthropogenically modified ecosystems (Moscow region). Eurasian Soil Sci. 2014, 9, 1077. [Google Scholar] [CrossRef]
- Kadulin, M.S.; Koptsik, G.N. Emission of CO2 by soils in the zone of influence of MMC Severonickel in the Kola Subarctic. Eurasian Soil Sci. 2013, 11, 1387–1396. [Google Scholar] [CrossRef]
- Kadulin, M.S.; Koptsik, G.N. Changes in the flux of carbon dioxide from the soils of forest ecosystems under the influence of technogenic pollution in the Kola Subarctic. Eurasian Soil Sci. 2021, 10, 1281–1292. [Google Scholar] [CrossRef]
- Koptsik, G.N.; Kadulin, M.S.; Zakharova, A.I. Influence of technogenic pollution on carbon dioxide emission by soils in the Kola Subarctic. J. Gen. Biol. 2015, 76, 48–62. [Google Scholar]
- Vasenev, V.I.; Prokofieva, T.V.; Makarov, O.A. Development of an approach to assessing the reserves of soil organic carbon in a metropolis and a small settlement. Eurasian Soil Sci. 2013, 6, 725–736. [Google Scholar]
- Ananyeva, N.D.; Blagodatskaya, E.V.; Demkina, T.S. Influence of drying-wetting and freezing-thawing on the stability of soil microbial communities. Soil Sci. 1997, 9, 1132–1137. [Google Scholar]
- Petrov, A.M.; Zainulgabidinov, E.R.; Shagidullin, R.R.; Ivanov, D.V.; Kuznetsova, T.V.; Karimullin, L.K. Development of standards for the permissible residual content of oil and its transformation products in soils for the lands of the forest fund of the Republic of Tatarstan. Bull. Kazan. Technol. Univ. 2013, 16, 265–270. [Google Scholar]
- Vershinin, A.A.; Petrov, A.M.; Akaikin, D.V.; Ignatiev, Y.A. Evaluation of the biological activity of soddy-podzolic soils of different granulometric composition under conditions of oil pollution. Eurasian Soil Sci. 2014, 2, 250–256. [Google Scholar]
- Vershinin, A.A.; Petrov, A.M.; Yuranets-Luzhaeva, R.C.; Kuznetsova, T.V.; Khabibullin, R.E. Coefficient of microbial respiration of various types of soils under conditions of oil pollution. Vestn. Tekhnologicheskogo Univ. 2017, 20, 103–106. [Google Scholar]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J.; Turner, A. An evaluation of the toxicity and bioaccumulation of bismuth in the coastal environment using three species of macroalga. Environ. Pollut. 2016, 208, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.I.; Sudina, L.V.; Kuzina, A.A.; Minnikova, T.V.; Tsepina, N.I.; Kazeev, K.S.; Akimenko, Y.V. The effect of bismuth contamination on the soil biological properties. Agric. Nat. Resour. 2022, 56, 417–428. [Google Scholar] [CrossRef]
- Minnikova, T.; Kolesnikov, S.; Evstegneeva, N.; Timoshenko, A.; Tsepina, N. Estimation of the Enzymatic Activity of Haplic Chernozem under Contamination with Oxides and Nitrates of Ag, Bi, Te and Tl. Agronomy 2022, 12, 2183. [Google Scholar] [CrossRef]
- Lin, Z.H.; Lee, C.H.; Chang, H.Y.; Chang, H.T. Antibacterial activities of tellurium nanomaterials. Chem. Asian J. 2012, 5, 930–934. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Minnikova, T.; Minkina, T.; Rajput, V.D.; Tsepina, N.; Kazeev, K.; Zhadobin, A.; Nevedomaya, E.; Ter-Misakyants, T.; Akimenko, Y.; et al. Toxic Effects of Thallium on Biological Indicators of Haplic Chernozem Health: A Case Study. Environments 2021, 8, 119. [Google Scholar] [CrossRef]
- Pavoni, E.; Petranich, E.; Adami, G.; Baracchini, E.; Crosera, M.; Emili, A.; Lenaz, D.; Higueras, P.; Covelli, S. Bioaccumulation of thallium and other trace metals in Biscutella laevigata nearby a decommisioned zinc-lead mine (Northeastern Italian Alps). J. Environ. Manag. 2017, 186, 214–224. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; 234p. [Google Scholar]
- Kolesnikov, S.I.; Kazeev, K.S.; Akimenko, Y.V. Development of regional standards for pollutants in the soil using biological parameters. Environ. Monit. Assess. 2019, 191, 544. [Google Scholar] [CrossRef]
- Nikitin, B.A. Refinement to the method for determining humus in soil. Agrochemistry 1983, 8, 18–26. [Google Scholar]
- Ponomareva, V.V.; Plotnikova, T.A. Humus and Soil Formation; Nauka Publishing House: Saint Petersburg, Russia, 1980; 222p. [Google Scholar]
- Tyurin, I.V. A new modification of the volumetric method for determining humus using chromic acid. Soil Sci. 1931, 6, 36–47. [Google Scholar]
- Vadyunina, A.F.; Korchagina, Z.A. Methods for Studying the Physical Properties of Soils, 3rd ed.; Vadyunina, A.F., Korchagin, Z.A., Eds.; Agropromizdat: Moscow, Russia, 1986; 416p. [Google Scholar]
- Blagodatskaya, E.V.; Ananyeva, N.D.; Myakshina, T.N. Characterization of the state of the microbial community of soils by the value of the metabolic coefficient. Soil Sci. 1995, 2, 2015–2210. [Google Scholar]
- Anderson, T.; Domsch, K. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Terekhova, V.A.; Prudnikova, E.V.; Kulachkova, S.A.; Gorlenko, M.V.; Uchanov, P.V.; Sushko, S.V.; Ananyeva, N.D. Microbiological indicators of agro-soddy-podzolic soils of different humus content upon the introduction of heavy metals and carbon-containing preparations. Soil Sci. 2021, 3, 372–384. [Google Scholar] [CrossRef]
- Dilly, O. Microbial energetics in soils. In Microorganisms in Soils: Roles in Genesis and Functions; Buscot, F., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 123–138. [Google Scholar]
- Chander, K.; Dyckmans, J.; Joergensen, R.G.; Meyer, B.; Raubuch, M. Different sources of heavy metals and their long-term effects on soil microbial properties. Biol. Fertil. Soils. 2001, 34, 241–247. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Ivashchenko, K.; Ananyeva, N.; Sushko, S.; Seleznyova, A.; Kudeyarov, V.; Vasenev, V. Microbial C-availability and organic matter decomposition in urban soils of megapolis depend on functional zoning. Soil Environ. 2019, 38, 31–41. [Google Scholar] [CrossRef]
- Vasenev, V.I.; Ananyeva, N.D.; Ivashchenko, K.V. Influence of pollutants (heavy metals, diesel fuel) on the respiratory activity of constructozems. Ecology 2013, 6, 436. [Google Scholar] [CrossRef]
- Khaziev, F.K. Functional role of enzymes in soil processes. Bull. Acad. Sci. Repub. Bashkortostan 2015, 20, 14–24. [Google Scholar]
- Khaziev, F.K. Ecological connections of enzymatic activity of soils. Ecobiotech 2018, 1, 80–92. [Google Scholar]
- Yarwood, S.A. Microbial ecology. In Principles and Applications of Soil Microbiology, 3rd ed.; Baishideng Publishing Group: Pleasanton, CA, USA, 2021. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Minnikova, T.; Tsepina, N.; Evstegneeva, N.; Timoshenko, A. Assessment of the Ecotoxicity of Ag, Bi, Te and Tl According to the Biological Indicators of Haplic Chernozem. Appl. Sci. 2022, 12, 12854. [Google Scholar] [CrossRef]

| Element | Compound Formula | Background Element Content, mg/kg | Specific Permissible Concentration (SPC), mg/kg |
|---|---|---|---|
| Silver | Ag2O | 0.10 | 0.21 |
| AgNO3 | 0.10 | 0.16 | |
| Bismuth | Bi2O3 | 0.27 | 0.60 |
| Bi(NO3)3 × 5H2O | 0.27 | 0.62 | |
| Thallium | Tl2O3 | 0.47 | 1.05 |
| TlNO3 | 0.40 | 0.61 | |
| Tellurium | TeO2 | 0.50 | 0.62 |
| Te2O3(OH)NO3 | 0.50 | 1.49 |
| Variants | Haplic Chernozem | Haplic Arenosols | Haplic Cambisols | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmiv/ Corg | qR | qCO2 | Cmiv/ Corg | qR | qCO2 | Cmiv/ Corg | qR | qCO2 | ||
| silver oxide | 0.5 SPC | 0.32 | 0.05 | 0.66 | 1.46 | 0.06 | 0.84 | 0.34 | 0.24 | 3.11 |
| 1 SPC | 0.34 | 0.04 | 0.51 | 1.35 | 0.07 | 0.94 | 0.31 | 0.29 | 3.81 | |
| 3 SPC | 0.26 | 0.05 | 0.59 | 1.25 | 0.06 | 0.84 | 0.31 | 0.33 | 4.34 | |
| 10 SPC | 0.26 | 0.07 | 0.92 | 1.23 | 0.09 | 1.15 | 0.28 | 0.30 | 3.93 | |
| 30 SPC | 0.18 | 0.08 | 1.10 | 0.16 | 0.33 | 3.91 | 0.33 | 0.29 | 3.76 | |
| bismuth oxide | 0.5 SPC | 0.36 | 0.05 | 0.67 | 1.62 | 0.07 | 0.91 | 0.36 | 0.26 | 3.46 |
| 1 SPC | 0.32 | 0.03 | 0.42 | 1.40 | 0.06 | 0.82 | 0.30 | 0.29 | 3.78 | |
| 3 SPC | 0.34 | 0.04 | 0.57 | 1.39 | 0.06 | 0.75 | 0.31 | 0.24 | 3.17 | |
| 10 SPC | 0.36 | 0.04 | 0.56 | 1.42 | 0.05 | 0.72 | 0.29 | 0.25 | 3.26 | |
| 30 SPC | 0.34 | 0.04 | 0.53 | 1.14 | 0.06 | 0.75 | 0.29 | 0.26 | 3.46 | |
| thallium oxide | 0.5 SPC | 0.35 | 0.06 | 0.73 | 1.46 | 0.06 | 0.74 | 0.29 | 0.30 | 3.96 |
| 1 SPC | 0.35 | 0.05 | 0.69 | 1.19 | 0.06 | 0.79 | 0.30 | 0.25 | 3.25 | |
| 3 SPC | 0.32 | 0.04 | 0.59 | 1.11 | 0.07 | 0.86 | 0.37 | 0.31 | 4.10 | |
| 10 SPC | 0.35 | 0.05 | 0.64 | 1.19 | 0.06 | 0.74 | 0.29 | 0.28 | 3.73 | |
| 30 SPC | 0.25 | 0.04 | 0.55 | 1.11 | 0.06 | 0.76 | 0.29 | 0.29 | 3.82 | |
| tellurium oxide | 0.5 SPC | 0.37 | 0.04 | 0.51 | 1.18 | 0.06 | 0.75 | 0.32 | 0.27 | 3.51 |
| 1 SPC | 0.30 | 0.05 | 0.60 | 1.02 | 0.06 | 0.80 | 0.31 | 0.31 | 4.03 | |
| 3 SPC | 0.44 | 0.05 | 0.60 | 0.94 | 0.05 | 0.69 | 0.29 | 0.27 | 3.60 | |
| 10 SPC | 0.53 | 0.04 | 0.56 | 1.20 | 0.06 | 0.81 | 0.31 | 0.29 | 3.82 | |
| 30 SPC | 0.46 | 0.04 | 0.58 | 1.06 | 0.05 | 0.64 | 0.32 | 0.29 | 3.78 | |
| silver nitrate | 0.5 SPC | 0.39 | 0.04 | 0.50 | 1.13 | 0.06 | 0.82 | 0.28 | 0.29 | 3.78 |
| 1 SPC | 0.37 | 0.04 | 0.57 | 1.11 | 0.06 | 0.85 | 0.30 | 0.26 | 3.37 | |
| 3 SPC | 0.47 | 0.03 | 0.45 | 1.00 | 0.06 | 0.86 | 0.26 | 0.34 | 4.44 | |
| 10 SPC | 0.49 | 0.04 | 0.47 | 0.84 | 0.06 | 0.85 | 0.26 | 0.27 | 3.57 | |
| 30 SPC | 0.44 | 0.05 | 0.61 | 0.98 | 0.08 | 1.07 | 0.16 | 0.35 | 4.48 | |
| bismuth nitrate | 0.5 SPC | 0.55 | 0.03 | 0.43 | 1.55 | 0.06 | 0.74 | 0.35 | 0.24 | 3.21 |
| 1 SPC | 0.50 | 0.04 | 0.48 | 1.56 | 0.06 | 0.76 | 0.36 | 0.26 | 3.44 | |
| 3 SPC | 0.61 | 0.04 | 0.47 | 0.88 | 0.05 | 0.70 | 0.29 | 0.24 | 3.15 | |
| 10 SPC | 0.59 | 0.03 | 0.41 | 0.76 | 0.07 | 0.92 | 0.30 | 0.35 | 4.55 | |
| 30 SPC | 0.57 | 0.03 | 0.43 | 0.94 | 0.05 | 0.64 | 0.37 | 0.27 | 3.62 | |
| thallium nitrate | 0.5 SPC | 0.53 | 0.04 | 0.55 | 1.25 | 0.07 | 0.89 | 0.27 | 0.31 | 4.09 |
| 1 SPC | 0.59 | 0.04 | 0.51 | 0.62 | 0.07 | 0.99 | 0.21 | 0.38 | 4.99 | |
| 3 SPC | 0.47 | 0.04 | 0.58 | 0.38 | 0.12 | 1.57 | 0.20 | 0.34 | 4.50 | |
| 10 SPC | 0.24 | 0.10 | 1.34 | 0.27 | 0.23 | 2.84 | 0.11 | 0.47 | 6.05 | |
| 30 SPC | 0.15 | 0.19 | 2.34 | 0.02 | −0.56 | 2.52 | 0.16 | 0.27 | 3.53 | |
| tellurium nitrate | 0.5 SPC | 0.53 | 0.04 | 0.50 | 1.23 | 0.06 | 0.76 | 0.14 | 0.43 | 5.55 |
| 1 SPC | 0.53 | 0.03 | 0.40 | 0.47 | 0.07 | 0.92 | 0.13 | 0.43 | 5.60 | |
| 3 SPC | 0.38 | 0.07 | 0.98 | 0.39 | 0.11 | 1.49 | 0.12 | 0.44 | 5.69 | |
| 10 SPC | 0.11 | 0.28 | 3.40 | 0.15 | 0.12 | 1.46 | 0.21 | 0.28 | 3.70 | |
| 30 SPC | 0.02 | 0.37 | 1.77 | 0.01 | 1.09 | 2.72 | 0.01 | 1.97 | 11.65 | |
| Control | 0.48 | 0.04 | 0.58 | 0.82 | 0.06 | 0.75 | 0.40 | 0.24 | 3.23 | |
| r | Haplic Chernozem | Haplic Arenosols | Haplic Cambisols | |||
|---|---|---|---|---|---|---|
| Vbasal | Cmic | Vbasal | Cmic | Vbasal | Cmic | |
| oxides | ||||||
| Ag | 0.35 | −0.95 ** | −0.91 ** | −1.00 ** | −0.13 | −0.87 ** |
| Bi | −0.04 | 0.49 * | −0.54 * | −0.84 ** | 0.02 | 0.34 |
| Te | −0.78 * | −0.88 ** | −0.42 * | −0.69 * | −0.14 | −0.31 |
| Tl | 0.67 * | 0.90 ** | −0.68 * | −0.85 ** | 0.64 * | 0.52 * |
| nitrates | ||||||
| Ag | −0.17 | −0.99 ** | −0.65 * | −0.95 ** | −0.88 ** | −0.98 ** |
| Bi | −0.59 * | −0.85 ** | −0.52 * | −0.83 ** | 0.36 | 0.03 |
| Te | 0.53 * | −0.92 ** | −0.97 ** | −0.81 ** | −0.89 ** | −0.54 * |
| Tl | −0.76 * | −0.88 ** | −0.97 ** | −0.83 ** | −0.96 ** | −0.75 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnikova, T.; Kolesnikov, S.; Khoroshaev, D.; Tsepina, N.; Evstegneeva, N.; Timoshenko, A. Assessment of the Health of Soils Contaminated with Ag, Bi, Tl, and Te by the Intensity of Microbiological Activity. Life 2023, 13, 1592. https://doi.org/10.3390/life13071592
Minnikova T, Kolesnikov S, Khoroshaev D, Tsepina N, Evstegneeva N, Timoshenko A. Assessment of the Health of Soils Contaminated with Ag, Bi, Tl, and Te by the Intensity of Microbiological Activity. Life. 2023; 13(7):1592. https://doi.org/10.3390/life13071592
Chicago/Turabian StyleMinnikova, Tatiana, Sergei Kolesnikov, Dmitry Khoroshaev, Natalia Tsepina, Natalia Evstegneeva, and Alena Timoshenko. 2023. "Assessment of the Health of Soils Contaminated with Ag, Bi, Tl, and Te by the Intensity of Microbiological Activity" Life 13, no. 7: 1592. https://doi.org/10.3390/life13071592
APA StyleMinnikova, T., Kolesnikov, S., Khoroshaev, D., Tsepina, N., Evstegneeva, N., & Timoshenko, A. (2023). Assessment of the Health of Soils Contaminated with Ag, Bi, Tl, and Te by the Intensity of Microbiological Activity. Life, 13(7), 1592. https://doi.org/10.3390/life13071592








