Right Ventricular Myocardial Involvement in Anderson–Fabry Disease at Diagnosis: Evaluation with Three-Dimensional Strain Imaging
Abstract
1. Introduction
2. Role of Echocardiography in Anderson–Fabry Disease
2.1. Left Ventricular Morphology, Systolic and Diastolic Function
2.2. Right Ventricular Morphology, Systolic and Diastolic Function
2.3. Speckle Tracking Echocardiography
3. Materials and Methods
3.1. Study Population
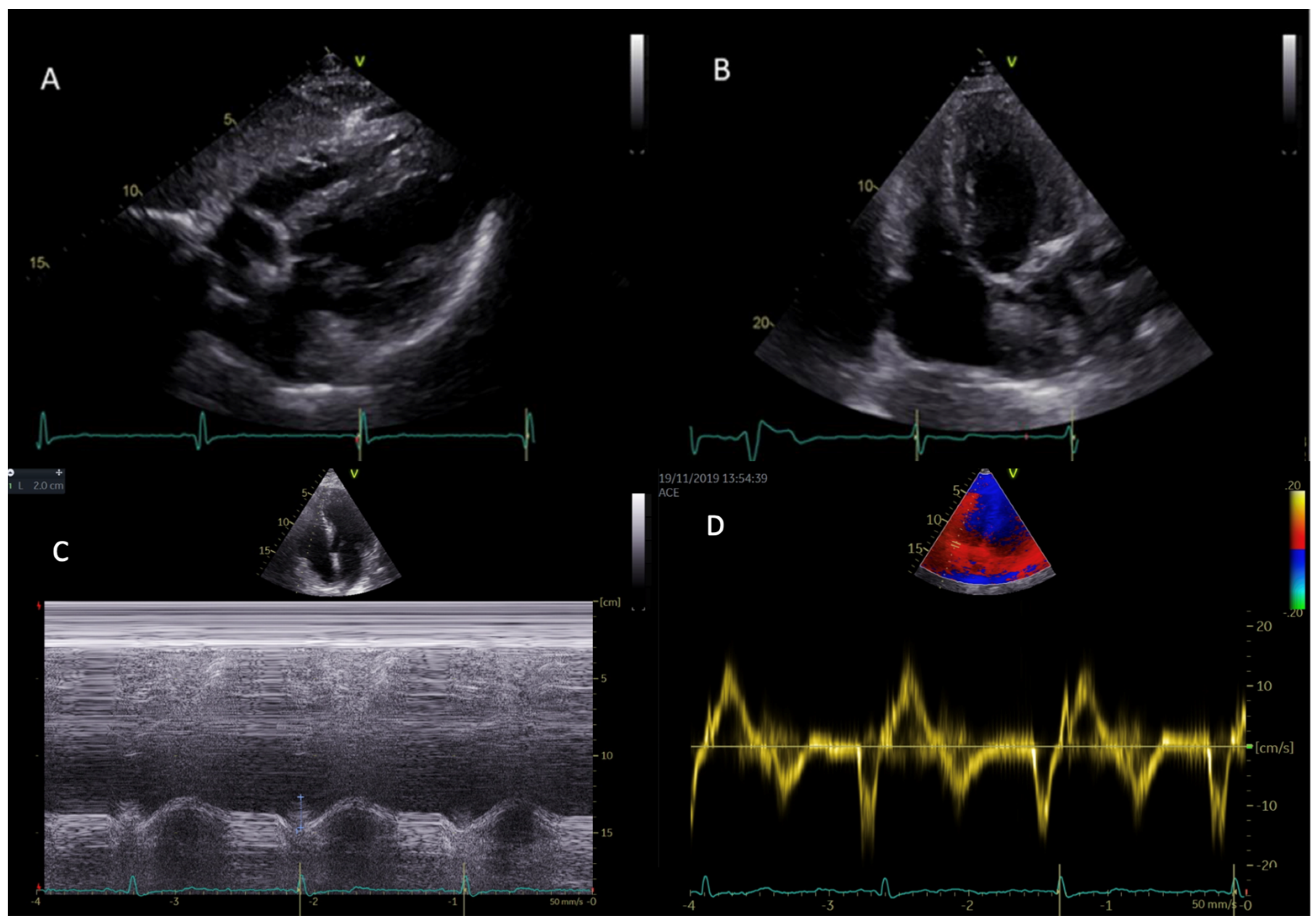
3.2. Echocardiography
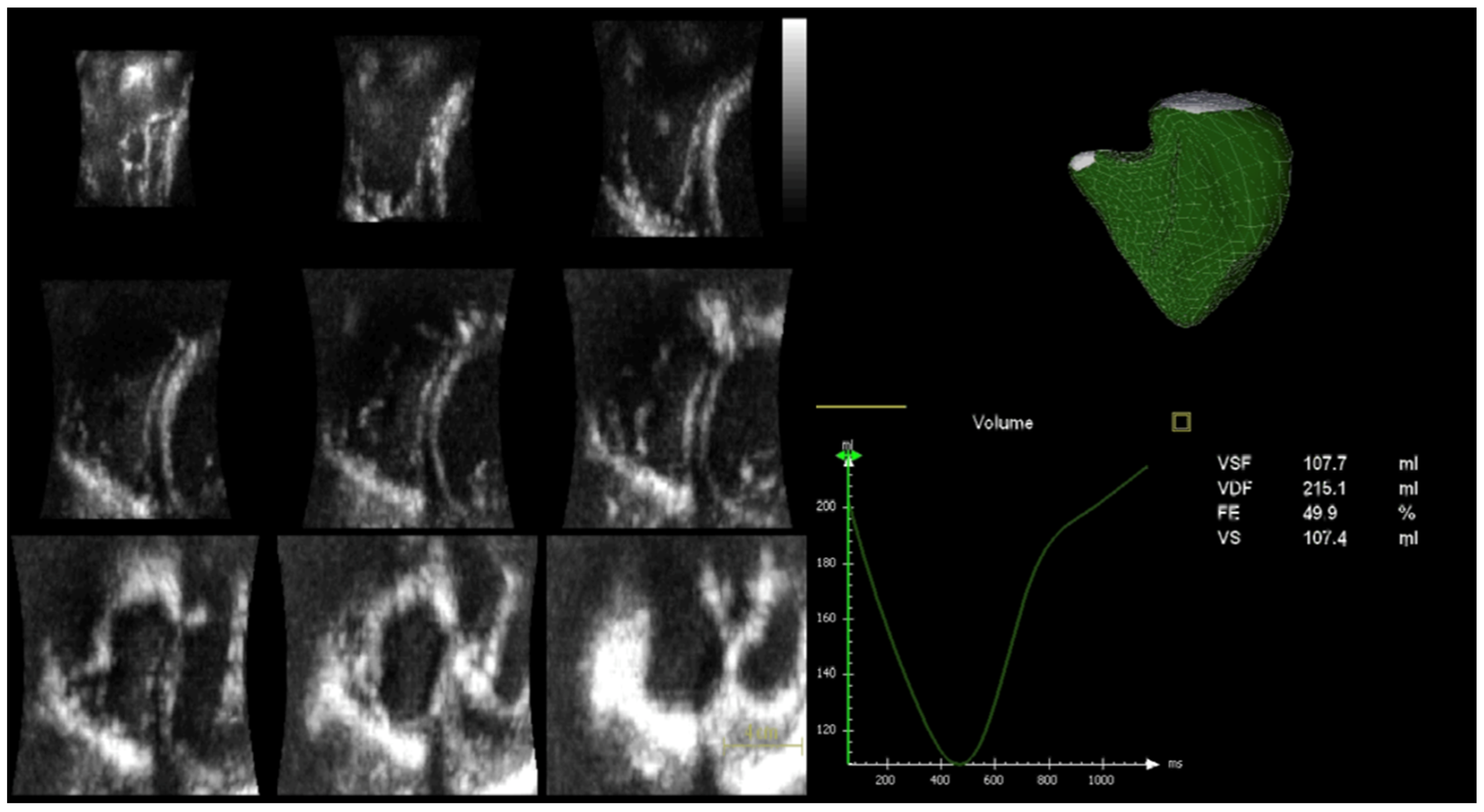
3.3. Speckle-Tracking Echocardiographic Analysis
3.4. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Toro, A.; Favalli, V.; Arbustini, E. Anderson-Fabry disease. J. Cardiovasc. Med. 2018, 19 (Suppl. S1), e1–e5. [Google Scholar] [CrossRef]
- Ramaswami, U.; Whybra, C.; Parini, R.; Pintos-Morell, G.; Mehta, A.; Sunder-Plassmann, G.; Widmer, U.; Beck, M.; FOS European Investigators. Clinical manifestations of Fabry disease in children: Data from the Fabry Outcome Survey. Acta Paediatr. 2006, 95, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Palecek, T.; Bultas, J.; Ferguson, J.J.; Hrudová, J.; Karetová, D.; Zeman, J.; Ledvinová, J.; Poupetová, H.; Elleder, M.; et al. New insights in cardiac structural changes in patients with Fabry’s disease. Am. Heart J. 2000, 139, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Kampmann, C.; Zamorano, J.L.; Sunder-Plassmann, G.; Beck, M.; Mehta, A.; Elliott, P.M.; European FOS Investigators. Cardiac manifestations of Anderson-Fabry disease: Results from the international Fabry outcome survey. Eur. Heart J. 2007, 28, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.E.; Cantor, R.; Schwartz, M.F.; Baker, M.; Desnick, R.J. Echocardiographic abnormalities and disease severity in Fabry’s disease. J. Am. Coll. Cardiol. 1986, 7, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C. Fabry disease model: A rational approach to the management of Fabry disease. Clin. Ther. 2007, 29, S2–S5, Erratum in Clin. Ther. 2007, 29, 2268. [Google Scholar] [CrossRef]
- Vedder, A.C.; Linthorst, G.E.; Houge, G.; Groener, J.E.; Ormel, E.E.; Bouma, B.J.; Aerts, J.M.; Hirth, A.; Hollak, C.E. Treatment of Fabry disease: Outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS ONE 2007, 2, e598. [Google Scholar] [CrossRef]
- Lee, K.; Jin, X.; Zhang, K.; Copertino, L.; Andrews, L.; Baker-Malcolm, J.; Geagan, L.; Qiu, H.; Seiger, K.; Barngrover, D.; et al. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology 2003, 13, 305–313. [Google Scholar] [CrossRef]
- Sakuraba, H.; Murata-Ohsawa, M.; Kawashima, I.; Tajima, Y.; Kotani, M.; Ohshima, T.; Chiba, Y.; Takashiba, M.; Jigami, Y.; Fukushige, T.; et al. Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice. J. Hum. Genet. 2006, 51, 180–188. [Google Scholar] [CrossRef]
- Mehta, A.; West, M.L.; Pintos-Morell, G.; Reisin, R.; Nicholls, K.; Figuera, L.E.; Parini, R.; Carvalho, L.R.; Kampmann, C.; Pastores, G.M.; et al. Therapeutic goals in the treatment of Fabry disease. Genet. Med. 2010, 12, 713–720. [Google Scholar] [CrossRef]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.M.; Tylki-Szymańska, A.; Hilz, M.J. Enzyme replacement therapy for Fabry disease: A systematic review of available evidence. Drugs 2009, 69, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, W.R.; Banikazemi, M.; Guffon, N.; Waldek, S.; Lee, P.; Linthorst, G.E.; Desnick, R.J.; Germain, D.P.; International Fabry Disease Study Group. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am. J. Hum. Genet. 2004, 75, 65–74. [Google Scholar] [CrossRef]
- Kampmann, C.; Linhart, A.; Devereux, R.B.; Schiffmann, R. Effect of agalsidase alfa replacement therapy on Fabry disease-related hypertrophic cardiomyopathy: A 12- to 36-month, retrospective, blinded echocardiographic pooled analysis. Clin. Ther. 2009, 31, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Kalvin, L.; Johnson, B.; Muthukumar, L.; Khandheria, B.K.; Tajik, A.J. Many Faces of Fabry’s Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 644–647. [Google Scholar] [CrossRef]
- Kampmann, C.; Linhart, A.; Baehner, F.; Palecek, T.; Wiethoff, C.M.; Miebach, E.; Whybra, C.; Gal, A.; Bultas, J.; Beck, M. Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int. J. Cardiol. 2008, 130, 367–373. [Google Scholar] [CrossRef]
- Wu, J.C.; Ho, C.Y.; Skali, H.; Abichandani, R.; Wilcox, W.R.; Banikazemi, M.; Packman, S.; Sims, K.; Solomon, S.D. Cardiovascular manifestations of Fabry disease: Relationships between left ventricular hypertrophy, disease severity, and alpha-galactosidase A activity. Eur. Heart J. 2010, 31, 1088–1097. [Google Scholar] [CrossRef]
- Calcagnino, M.; O’Mahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Janagarajan, K.; Mehta, A.; Hughes, D.; Murphy, E.; Lachmann, R.; et al. Exercise-induced left ventricular outflow tract obstruction in symptomatic patients with Anderson-Fabry disease. J. Am. Coll. Cardiol. 2011, 58, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Kozor, R.; Baig, S.; Abdel-Gadir, A.; Medina-Menacho, K.; Rosmini, S.; Captur, G.; Tchan, M.; Geberhiwot, T.; Murphy, E.; et al. Cardiac Phenotype of Prehypertrophic Fabry Disease. Circ. Cardiovasc. Imaging 2018, 11, e007168. [Google Scholar] [CrossRef]
- Patel, V.; O’Mahony, C.; Hughes, D.; Rahman, M.S.; Coats, C.; Murphy, E.; Lachmann, R.; Mehta, A.; Elliott, P.M. Clinical and genetic predictors of major cardiac events in patients with Anderson-Fabry Disease. Heart 2015, 101, 961–966. [Google Scholar] [CrossRef]
- Pieroni, M.; Chimenti, C.; De Cobelli, F.; Morgante, E.; Del Maschio, A.; Gaudio, C.; Russo, M.A.; Frustaci, A. Fabry’s disease cardiomyopathy: Echocardiographic detection of endomyocardial glycosphingolipid compartmentalization. J. Am. Coll. Cardiol. 2006, 47, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Kounas, S.; Demetrescu, C.; Pantazis, A.A.; Keren, A.; Lee, P.J.; Hughes, D.; Mehta, A.; Elliott, P.M. The binary endocardial appearance is a poor discriminator of Anderson-Fabry disease from familial hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2058–2061. [Google Scholar] [CrossRef] [PubMed]
- Mundigler, G.; Gaggl, M.; Heinze, G.; Graf, S.; Zehetgruber, M.; Lajic, N.; Voigtländer, T.; Mannhalter, C.; Sunder-Plassmann, R.; Paschke, E.; et al. The endocardial binary appearance (‘binary sign’) is an unreliable marker for echocardiographic detection of Fabry disease in patients with left ventricular hypertrophy. Eur. J. Echocardiogr. 2011, 12, 744–749. [Google Scholar] [CrossRef]
- Palecek, T.; Linhart, A.; Lubanda, J.C.; Magage, S.; Karetova, D.; Bultas, J.; Aschermann, M. Early diastolic mitral annular velocity and color M-mode flow propagation velocity in the evaluation of left ventricular diastolic function in patients with Fabry disease. Heart Vessel. 2006, 21, 13–19. [Google Scholar] [CrossRef]
- Pieroni, M.; Chimenti, C.; Ricci, R.; Sale, P.; Russo, M.A.; Frustaci, A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 2003, 107, 1978–1984. [Google Scholar] [CrossRef]
- Pieroni, M.; Chimenti, C.; Russo, A.; Russo, M.A.; Maseri, A.; Frustaci, A. Tissue Doppler imaging in Fabry disease. Curr. Opin. Cardiol. 2004, 19, 452–457. [Google Scholar] [CrossRef]
- Zamorano, J.; Serra, V.; Pérez de Isla, L.; Feltes, G.; Calli, A.; Barbado, F.J.; Torras, J.; Hernandez, S.; Herrera, J.; Herrero, J.A.; et al. Usefulness of tissue Doppler on early detection of cardiac disease in Fabry patients and potential role of enzyme replacement therapy (ERT) for avoiding progression of disease. Eur. J. Echocardiogr. 2011, 12, 671–677. [Google Scholar] [CrossRef]
- Pieroni, M. Echocardiographic assessment of fabry cardiomyopathy: Early diagnosis and follow-up. J. Am. Soc. Echocardiogr. 2011, 24, 1033–1036. [Google Scholar] [CrossRef]
- Barbey, F.; Qanadli, S.D.; Juli, C.; Brakch, N.; Palacek, T.; Rizzo, E.; Jeanrenaud, X.; Eckhardt, B.; Linhart, A. Aortic remodelling in Fabry disease. Eur. Heart J. 2010, 31, 347–353. [Google Scholar] [CrossRef]
- Linhart, A.; Lubanda, J.C.; Palecek, T.; Bultas, J.; Karetová, D.; Ledvinová, J.; Elleder, M.; Aschermann, M. Cardiac manifestations in Fabry disease. J. Inherit. Metab. Dis. 2001, 24 (Suppl. S2), 75–83; discussion 65. [Google Scholar] [CrossRef]
- Boyd, A.C.; Lo, Q.; Devine, K.; Tchan, M.C.; Sillence, D.O.; Sadick, N.; Richards, D.A.; Thomas, L. Left atrial enlargement and reduced atrial compliance occurs early in Fabry cardiomyopathy. J. Am. Soc. Echocardiogr. 2013, 26, 1415–1423. [Google Scholar] [CrossRef]
- Chimenti, C.; Russo, M.A.; Frustaci, A. Atrial biopsy evidence of Fabry disease causing lone atrial fibrillation. Heart 2010, 96, 1782–1783. [Google Scholar] [CrossRef]
- Liu, D.; Oder, D.; Salinger, T.; Hu, K.; Müntze, J.; Weidemann, F.; Herrmann, S.; Ertl, G.; Wanner, C.; Frantz, S.; et al. Association and diagnostic utility of diastolic dysfunction and myocardial fibrosis in patients with Fabry disease. Open Heart 2018, 5, e000803. [Google Scholar] [CrossRef] [PubMed]
- Ferrans, V.J.; Hibbs, R.G.; Burda, C.D. The heart in Fabry’s disease. A histochemical and electron microscopic study. Am. J. Cardiol. 1969, 24, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Niemann, M.; Liu, D.; Hu, K.; Machann, W.; Beer, M.; Wanner, C.; Ertl, G.; Weidemann, F. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur. Heart J. 2013, 34, 1587–1596. [Google Scholar] [CrossRef]
- Desnick, R.J.; Blieden, L.C.; Sharp, H.L.; Hofschire, P.J.; Moller, J.H. Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies. Circulation 1976, 54, 818–825. [Google Scholar] [CrossRef]
- Putko, B.N.; Wen, K.; Thompson, R.B.; Mullen, J.; Shanks, M.; Yogasundaram, H.; Sergi, C.; Oudit, G.Y. Anderson-Fabry cardiomyopathy: Prevalence, pathophysiology, diagnosis and treatment. Heart Fail. Rev. 2015, 20, 179–191. [Google Scholar] [CrossRef]
- Niemann, M.; Breunig, F.; Beer, M.; Herrmann, S.; Strotmann, J.; Hu, K.; Emmert, A.; Voelker, W.; Ertl, G.; Wanner, C.; et al. The right ventricle in Fabry disease: Natural history and impact of enzyme replacement therapy. Heart 2010, 96, 1915–1919. [Google Scholar] [CrossRef]
- Kampmann, C.; Baehner, F.A.; Whybra, C.; Bajbouj, M.; Baron, K.; Knuf, M.; Wiethoff, C.M.; Trübel, H.; Beck, M. The right ventricle in Fabry disease. Acta Paediatr. Suppl. 2005, 94, 15–18; discussion 9–10. [Google Scholar] [CrossRef]
- Palecek, T.; Dostalova, G.; Kuchynka, P.; Karetova, D.; Bultas, J.; Elleder, M.; Linhart, A. Right ventricular involvement in Fabry disease. J. Am. Soc. Echocardiogr. 2008, 21, 1265–1268. [Google Scholar] [CrossRef]
- Graziani, F.; Laurito, M.; Pieroni, M.; Pennestrì, F.; Lanza, G.A.; Coluccia, V.; Camporeale, A.; Pedicino, D.; Verrecchia, E.; Manna, R.; et al. Right Ventricular Hypertrophy, Systolic Function, and Disease Severity in Anderson-Fabry Disease: An Echocardiographic Study. J. Am. Soc. Echocardiogr. 2017, 30, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Poon, J.; Leung, J.T.; Leung, D.Y. 3D Echo in Routine Clinical Practice—State of the Art in 2019. Hear. Lung Circ. 2019, 28, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Marsan, N.A.; Gripari, P.; Maffessanti, F.; Brusoni, D.; Muratori, M.; Caiani, E.G.; Fiorentini, C.; Pepi, M. Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: Evaluation in a large series of normal subjects. J. Am. Soc. Echocardiogr. 2010, 23, 109–115. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Radmilovic, J.; Ballo, P.; Mele, D.; Agricola, E.; Cameli, M.; Rossi, A.; Esposito, R.; Novo, G.; Mondillo, S.; et al. Left ventricular hypertrophy or storage disease? the incremental value of speckle tracking strain bull’s-eye. Echocardiography 2017, 34, 746–759. [Google Scholar] [CrossRef]
- Shanks, M.; Thompson, R.B.; Paterson, I.D.; Putko, B.; Khan, A.; Chan, A.; Becher, H.; Oudit, G.Y. Systolic and diastolic function assessment in fabry disease patients using speckle-tracking imaging and comparison with conventional echocardiographic measurements. J. Am. Soc. Echocardiogr. 2013, 26, 1407–1414. [Google Scholar] [CrossRef]
- Esposito, R.; Galderisi, M.; Santoro, C.; Imbriaco, M.; Riccio, E.; Maria Pellegrino, A.; Sorrentino, R.; Lembo, M.; Citro, R.; Angela Losi, M.; et al. Prominent longitudinal strain reduction of left ventricular basal segments in treatment-naïve Anderson-Fabry disease patients. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 438–445. [Google Scholar] [CrossRef]
- Esposito, R.; Santoro, C.; Sorrentino, R.; Riccio, E.; Citro, R.; Buonauro, A.; Di Risi, T.; Imbriaco, M.; Trimarco, B.; Pisani, A.; et al. Layer-specific longitudinal strain in Anderson-Fabry disease at diagnosis: A speckle tracking echocardiography analysis. Echocardiography 2019, 36, 1273–1281. [Google Scholar] [CrossRef]
- Liu, D.; Hu, K.; Nordbeck, P.; Ertl, G.; Störk, S.; Weidemann, F. Longitudinal strain bull’s eye plot patterns in patients with cardiomyopathy and concentric left ventricular hypertrophy. Eur. J. Med. Res. 2016, 21, 21. [Google Scholar] [CrossRef]
- Sheppard, M.N.; Cane, P.; Florio, R.; Kavantzas, N.; Close, L.; Shah, J.; Lee, P.; Elliott, P. A detailed pathologic examination of heart tissue from three older patients with Anderson-Fabry disease on enzyme replacement therapy. Cardiovasc. Pathol. 2010, 19, 293–301. [Google Scholar] [CrossRef]
- Pichette, M.; Serri, K.; Pagé, M.; Di, L.Z.; Bichet, D.G.; Poulin, F. Impaired Left Atrial Function in Fabry Disease: A Longitudinal Speckle-Tracking Echocardiography Study. J. Am. Soc. Echocardiogr. 2017, 30, 170–179.e2. [Google Scholar] [CrossRef]
- Morris, D.A.; Blaschke, D.; Canaan-Kühl, S.; Krebs, A.; Knobloch, G.; Walter, T.C.; Haverkamp, W. Global cardiac alterations detected by speckle-tracking echocardiography in Fabry disease: Left ventricular, right ventricular, and left atrial dysfunction are common and linked to worse symptomatic status. Int. J. Cardiovasc. Imaging 2015, 31, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Lillo, R.; Graziani, F.; Panaioli, E.; Mencarelli, E.; Pieroni, M.; Camporeale, A.; Manna, R.; Sicignano, L.L.; Verrecchia, E.; Lombardo, A.; et al. Right ventricular strain in Anderson-Fabry disease. Int. J. Cardiol. 2021, 330, 84–90. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–788. [Google Scholar] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270, Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412; Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 969. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600, Erratum in Eur. Heart J. Cardiovasc. Imaging 2018, 19, 830–833. [Google Scholar] [CrossRef]
- Sugeng, L.; Mor-Avi, V.; Weinert, L.; Niel, J.; Ebner, C.; Steringer-Mascherbauer, R.; Bartolles, R.; Baumann, R.; Schummers, G.; Lang, R.M.; et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc. Imaging 2010, 3, 10–18. [Google Scholar] [CrossRef]
- Jenkins, C.; Chan, J.; Bricknell, K.; Strudwick, M.; Marwick, T.H. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: Comparison with cardiac MRI. Chest 2007, 131, 1844–1851. [Google Scholar] [CrossRef]
- van der Zwaan, H.B.; Helbing, W.A.; McGhie, J.S.; Geleijnse, M.L.; Luijnenburg, S.E.; Roos-Hesselink, J.W.; Meijboom, F.J. Clinical value of real-time three-dimensional echocardiography for right ventricular quantification in congenital heart disease: Validation with cardiac magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2010, 23, 134–140. [Google Scholar] [CrossRef]
- Meucci, M.C.; Lillo, R.; Lombardo, A.; Lanza, G.A.; Bootsma, M.; Butcher, S.C.; Massetti, M.; Manna, R.; Bax, J.J.; Crea, F.; et al. Comparative analysis of right ventricular strain in Fabry cardiomyopathy and sarcomeric hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 542–551. [Google Scholar] [CrossRef]
- Meucci, M.C.; Lillo, R.; Mango, F.; Lombardo, A.; Lanza, G.A.; Parisi, V.; Grandinetti, M.; Massetti, M.; Ajmone Marsan, N.; Crea, F.; et al. Right ventricular strain in Fabry disease: Prognostic implications. Int. J. Cardiol. 2023, 374, 79–82. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | AFD (n = 23) | Controls (n = 15) | p-Value |
|---|---|---|---|
| Gender (M/F) | 15/8 | 10/5 | |
| Age (years) | 37.3 ± 14.3 | 38.5 ± 11.9 | 0.78 |
| BMI (Kg/m2) | 26.1 ± 4.3 | 23.6 ± 2.5 | 0.05 |
| SBP (mmHg) | 126.9 ± 20.9 | 119.3 ± 9.4 | 0.20 |
| DBP (mmHg) | 77.3 ± 12.1 | 74.0 ± 9.6 | 0.37 |
| MBP (mmHg) | 93.8 ± 14.4 | 89.1 ± 8.5 | 0.25 |
| HR (bpm) | 71.4 ± 9.8 | 73.2 ± 7.6 | 0.56 |
| Variables | AFD (n = 23) | Controls (n = 15) | p-Value |
|---|---|---|---|
| RV basal diameter (mm) | 36.2 ± 4.6 | 37.0 ± 5.1 | 0.44 |
| TAPSE (mm) | 23.7 ± 3.7 | 23.8 ± 3.3 | 0.93 |
| RV E/e’ ratio | 1.45 ± 0.48 | 1.64 ± 0.25 | 0.17 |
| TDI s’ vel (cm/s) | 14.6 ± 2.3 | 14.4 ± 1.8 | 0.73 |
| TDI e’ vel (cm/s) | 14.4 ± 3.1 | 14.3 ± 3.4 | 0.91 |
| RV wall thickness | 5.85 ± 1.43 | 3.93 ± 0.73 | <0.0001 |
| Variables | AFD (n = 18) | Controls (n = 14) | p-Value |
|---|---|---|---|
| RV EDV (mL) | 87.8 ± 27.6 | 62.8 ± 10.4 | 0.003 |
| RV ESV (mL) | 39.4 ± 11.3 | 24.5 ± 6.7 | <0.0001 |
| RV SV (L/min) | 48.2 ± 19.6 | 37.8 ± 6.9 | 0.07 |
| RV EF (%) | 54.3 ± 6.5 | 60.8 ± 6.6 | 0.009 |
| RV Septal LS (%) | 17.1 ± 2.9 | 24.2 ± 5.1 | <0.0001 |
| RV free wall LS (%) | 25.3 ± 3.5 | 32.3 ± 4.8 | <0.0001 |
| RV GLS (%) | 21.2 ± 2.2 | 28.2 ± 4.7 | <0.0001 |
| Variables | R Coefficient | p-Value | |
|---|---|---|---|
| RV wall thickness | TAPSE | −0.19 | 0.24 |
| RV E/a ratio | −0.47 | 0.003 | |
| TDI s’ vel | −0.21 | 0.19 | |
| TDI e’ vel | −0.24 | 0.15 | |
| PAPs | 0.31 | 0.06 | |
| 3D RV EDV | 0.43 | 0.01 | |
| 3D RV ESV | 0.43 | 0.01 | |
| 3D RV EF | −0.21 | 0.24 | |
| 3D RV Septal LS | 0.39 | 0.25 | |
| 3D RV Free wall LS | 0.35 | 0.05 | |
| 3D RV GLS | 0.40 | 0.02 |
| Dependent Variables | Covariate | β-Coefficient | p-Value |
|---|---|---|---|
| RV GLS | Age | −0.39 | 0.03 |
| Heart rate | −0.90 | 0.581 | |
| RV wall thickness | 0.57 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, M.; Iadevaia, V.; Gammaldi, V.; Iervolino, A.; Capece, L.M.; Sciascia, D.; Cuomo, V.; Iacono, M.; Paoletta, D.; Santoro, C.; et al. Right Ventricular Myocardial Involvement in Anderson–Fabry Disease at Diagnosis: Evaluation with Three-Dimensional Strain Imaging. Life 2023, 13, 1571. https://doi.org/10.3390/life13071571
Pucci M, Iadevaia V, Gammaldi V, Iervolino A, Capece LM, Sciascia D, Cuomo V, Iacono M, Paoletta D, Santoro C, et al. Right Ventricular Myocardial Involvement in Anderson–Fabry Disease at Diagnosis: Evaluation with Three-Dimensional Strain Imaging. Life. 2023; 13(7):1571. https://doi.org/10.3390/life13071571
Chicago/Turabian StylePucci, Martina, Velia Iadevaia, Vittoria Gammaldi, Adelaide Iervolino, Luca Maria Capece, Domenico Sciascia, Vittoria Cuomo, Marina Iacono, Daniele Paoletta, Ciro Santoro, and et al. 2023. "Right Ventricular Myocardial Involvement in Anderson–Fabry Disease at Diagnosis: Evaluation with Three-Dimensional Strain Imaging" Life 13, no. 7: 1571. https://doi.org/10.3390/life13071571
APA StylePucci, M., Iadevaia, V., Gammaldi, V., Iervolino, A., Capece, L. M., Sciascia, D., Cuomo, V., Iacono, M., Paoletta, D., Santoro, C., & Esposito, R. (2023). Right Ventricular Myocardial Involvement in Anderson–Fabry Disease at Diagnosis: Evaluation with Three-Dimensional Strain Imaging. Life, 13(7), 1571. https://doi.org/10.3390/life13071571






