Predictors of Pulmonary Hypertension and Right Ventricular Dysfunction in Patients with Hypersensitivity Pneumonitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behr, J.; Ryu, J.H. Pulmonary Hypertension in Interstitial Lung Disease. Eur. Respir. J. 2008, 31, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, M.; Church, A.C.; Johnson, M.K.; Peacock, A.J. Pulmonary Vascular and Cardiac Impairment in Interstitial Lung Disease. Eur. Respir. Rev. 2017, 26, 160053. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, 36–69. [Google Scholar] [CrossRef] [PubMed]
- Thomeer, M.J.; Costabe, U.; Rizzato, G.; Poletti, V.; Demedts, M. Comparison of Registries of Interstitial Lung Diseases in Three European Countries. Eur. Respir. J. Suppl. 2001, 32, 114–118. [Google Scholar]
- Papakosta, D.; Pitsiou, G.; Daniil, Z.; Dimadi, M.; Stagaki, E.; Rapti, A.; Antoniou, K.; Tzouvelekis, A.; Kontakiotis, T.; Tryfon, S.; et al. Prevalence of Pulmonary Hypertension in Patients with Idiopathic Pulmonary Fibrosis: Correlation with Physiological Parameters. Lung 2011, 189, 391–399. [Google Scholar] [CrossRef]
- Castria, D.; Refini, R.M.; Bargagli, E.; Mezzasalma, F.; Pierli, C.; Rottoli, P. Pulmonary Hypertension in Idiopathic Pulmonary Fibrosis: Prevalence and Clinical Progress. Int. J. Immunopathol. Pharm. 2012, 25, 681–689. [Google Scholar] [CrossRef]
- Kimura, M.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Nishiyama, O.; Aso, H.; Sakamoto, K.; Hasegawa, Y. Pulmonary Hypertension as a Prognostic Indicator at the Initial Evaluation in Idiopathic Pulmonary Fibrosis. Respiration 2013, 85, 456–463. [Google Scholar] [CrossRef]
- Dybowska, M.; Barańska, I.; Franczuk, M.; Skoczylas, A.; Szturmowicz, M. Echocardiographic Signs of Pulmonary Hypertension in Patients with Newly Recognized Hypersensitivity Pneumonitis, Prevalence and Clinical Predictors. J. Thorac. Dis. 2021, 13, 3988–3997. [Google Scholar] [CrossRef]
- Elnady, M.A.; Elkorashy, R.; Nabil, A.; Ibrahim, E.K. Predictors of Pulmonary Hypertension in Patients with Hypersensitivity Pneumonitis. BMC Pulm. Med. 2023, 23, 61. [Google Scholar] [CrossRef]
- Oliveira, R.K.F.; Pereira, C.A.C.; Ramos, R.P.; Ferreira, E.V.M.; Messina, C.M.S.; Kuranishi, L.T.; Gimenez, A.; Campos, O.; Silva, C.M.C.; Ota-Arakaki, J.S. A Haemodynamic Study of Pulmonary Hypertension in Chronic Hypersensitivity Pneumonitis. Eur. Respir. J. 2014, 44, 415–424. [Google Scholar] [CrossRef]
- Lettieri, C.J.; Nathan, S.D.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. Prevalence and Outcomes of Pulmonary Arterial Hypertension in Advanced Idiopathic Pulmonary Fibrosis. Chest 2006, 129, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.M.; Lederer, D.J.; Borczuk, A.C.; Kawut, S.M. Pulmonary Hypertension in Idiopathic Pulmonary Fibrosis. Chest 2007, 132, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Nadrous, H.F.; Pellikka, P.A.; Krowka, M.J.; Swanson, K.L.; Chaowalit, N.; Decker, P.A.; Ryu, J.H. Pulmonary Hypertension in Patients with Idiopathic Pulmonary Fibrosis. Chest 2005, 128, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Nagai, S.; Tanaka, S.; Handa, T.; Shigematsu, M.; Nagao, T.; Mishima, M.; Kitaichi, M.; Izumi, T. Significance of Pulmonary Arterial Pressure and Diffusion Capacity of the Lung as Prognosticator in Patients with Idiopathic Pulmonary Fibrosis. Chest 2007, 131, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Wälscher, J.; Gross, B.; Morisset, J.; Johannson, K.A.; Vasakova, M.; Bruhwyler, J.; Kreuter, M. Comorbidities and Survival in Patients with Chronic Hypersensitivity Pneumonitis. Respir. Res. 2020, 21, 12. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Cooper, B.G.; Stocks, J.; Hall, G.L.; Steenbruggen, I.; Carter, K.W.; Thompson, B.R.; Graham, B.L.; Miller, M.R.; Ruppel, G.; Henderson, J.; et al. The Global Lung Function Initiative (GLI) Network: Bringing the world’s respiratory reference values together. Breathe 2017, 13, 56–64. [Google Scholar] [CrossRef]
- Yock, P.G.; Popp, R.L. Noninvasive Estimation of Right Ventricular Systolic Pressure by Doppler Ultrasound in Patients with Tricuspid Regurgitation. Circulation 1984, 70, 657–662. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Colalillo, A.; Hoffmann-Vold, A.M.; Pellicano, C.; Romaniello, A.; Gabrielli, A.; Hachulla, E.; Smith, V.; Simeón-Aznar, C.P.; Castellví, I.; Airò, P.; et al. The Role of TAPSE/SPAP Ratio in Predicting Pulmonary Hypertension and Mortality in the Systemic Sclerosis EUSTAR Cohort. Autoimmun. Rev. 2023, 22, 103290. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [PubMed]
- Koschel, D.S.; Cardoso, C.; Wiedemann, B.; Höffken, G.; Halank, M. Pulmonary Hypertension in Chronic Hypersensitivity Pneumonitis. Lung 2012, 190, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Konstantinidis, I.; O’Sullivan, D.M.; Farber, H.W. Pulmonary Hypertension in Patients with Interstitial Lung Disease: A Tool for Early Detection. Pulm. Circ. 2022, 12, e12141. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champion, H.C.; Abraham, T.P.; et al. Tricuspid Annular Displacement Predicts Survival in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–1041. [Google Scholar] [CrossRef]
- Karatasakis, G.T.; Karagounis, L.A.; Kalyvas, P.A.; Manginas, A.; Athanassopoulos, G.D.; Aggelakas, S.A.; Cokkinos, D.V. Prognostic Significance of Echocardiographically Estimated Right Ventricular Shortening in Advanced Heart Failure. Am. J. Cardiol. 1998, 82, 329–334. [Google Scholar] [CrossRef]
- Ruocco, G.; Cekorja, B.; Rottoli, P.; Refini, R.M.; Pellegrini, M.; Di Tommaso, C.; Del Castillo, G.; Franci, B.; Nuti, R.; Palazzuoli, A. Role of BNP and Echo Measurement for Pulmonary Hypertension Recognition in Patients with Interstitial Lung Disease: An Algorithm Application Model. Respir. Med. 2015, 109, 406–415. [Google Scholar] [CrossRef]
- Papadopoulos, C.E.; Pitsiou, G.; Karamitsos, T.D.; Karvounis, H.I.; Kontakiotis, T.; Giannakoulas, G.; Efthimiadis, G.K.; Argyropoulou, P.; Parharidis, G.E.; Bouros, D. Left Ventricular Diastolic Dysfunction in Idiopathic Pulmonary Fibrosis: A Tissue Doppler Echocardiographic [Corrected] Study. Eur. Respir. J. 2008, 31, 701–706. [Google Scholar] [CrossRef]
- Alkukhun, L.; Wang, X.F.; Ahmed, M.K.; Baumgartner, M.; Budev, M.M.; Dweik, R.A.; Tonelli, A.R. Non-Invasive Screening for Pulmonary Hypertension in Idiopathic Pulmonary Fibrosis. Respir. Med. 2016, 117, 65–72. [Google Scholar] [CrossRef]
- Tornyos, A.; Trinker, M.; Foris, V.; Pfeiffer, S.; Odler, B.; Douschan, P.; Avian, A.; Leitner, A.; Olschewski, A.; Kovacs, G.; et al. Pulmonary Hypertension in Hypersensitivity Pneumonitis. Eur. Respir. J. 2018, 52 (Suppl. S62), PA3100. [Google Scholar]
- Kazimierczyk, R.; Kazimierczyk, E.; Knapp, M.; Sobkowicz, B.; Malek, L.A.; Blaszczak, P.; Ptaszynska-Kopczynska, K.; Grzywna, R.; Kaminski, K.A. Echocardiographic Assessment of Right Ventricular-Arterial Coupling in Predicting Prognosis of Pulmonary Arterial Hypertension Patients. J. Clin. Med. 2021, 10, 2995. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP Ratio in Pulmonary Arterial Hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, C.; Raitiere, O.; Boucly, A.; De Groote, P.; Renard, S.; Bertona, J.; Lamblin, N.; Artaud-Macari, E.; Viacroze, C.; Schleifer, D.; et al. Interest of TAPSE/SPAP Ratio for Noninvasive Pulmonary Arterial Hypertension Risk Assessment. J. Heart Lung Transpl. 2022, 41, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.H.; Maron, B.A.; French, J.; Huang, S.; Thayer, T.; Farber-Eger, E.H.; Wells, Q.S.; Choudhary, G.; Hemnes, A.R.; Brittain, E.L. Association of Mild Echocardiographic Pulmonary Hypertension with Mortality and Right Ventricular Function. JAMA Cardiol. 2019, 4, 1112–1121. [Google Scholar] [CrossRef]
- Farkas, L.; Gauldie, J.; Voelkel, N.F.; Kolb, M. Pulmonary Hypertension and Idiopathic Pulmonary Fibrosis: A Tale of Angiogenesis, Apoptosis, and Growth Factors. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 1–15. [Google Scholar] [CrossRef]
- Ruffenach, G.; Hong, J.; Vaillancourt, M.; Medzikovic, L.; Eghbali, M. Pulmonary Hypertension Secondary to Pulmonary Fibrosis: Clinical Data, Histopathology and Molecular Insights. Respir. Res. 2020, 21, 303. [Google Scholar] [CrossRef]
- Nathan, S.D.; Shlobin, O.A.; Ahmad, S.; Urbanek, S.; Barnett, S.D. Pulmonary Hypertension and Pulmonary Function Testing in Idiopathic Pulmonary Fibrosis. Chest 2007, 131, 657–663. [Google Scholar] [CrossRef]
- Steen, V.D. The Lung in Systemic Sclerosis. J. Clin. Rheumatol. 2005, 11, 40–46. [Google Scholar] [CrossRef]
- Trad, S.; Amoura, Z.; Beigelman, C.; Haroche, J.; Costedoat, N.; Boutin, L.T.H.D.; Cacoub, P.; Frances, C.; Wechsler, B.; Grenier, P.; et al. Pulmonary Arterial Hypertension Is a Major Mortality Factor in Diffuse Systemic Sclerosis, Independent of Interstitial Lung Disease. Arthritis Rheum. 2006, 54, 184–191. [Google Scholar] [CrossRef]
- Sobiecka, M.; Lewandowska, K.; Kober, J.; Franczuk, M.; Skoczylas, A.; Tomkowski, W.; Kuś, J.; Szturmowicz, M. Can a New Scoring System Improve Prediction of Pulmonary Hypertension in Newly Recognised Interstitial Lung Diseases? Lung 2020, 198, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Sonti, R.; Gersten, R.A.; Barnett, S.; Brown, A.W.; Nathan, S.D. Multimodal Noninvasive Prediction of Pulmonary Hypertension in IPF. Clin. Respir. J. 2019, 13, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Waxman, A.B.; Elia, D.; Adir, Y.; Humbert, M.; Harari, S. Recent Advances in the Management of Pulmonary Hypertension with Interstitial Lung Disease. Eur. Respir. Rev. 2022, 31, 210220. [Google Scholar] [CrossRef] [PubMed]
- Zisman, D.A.; Ross, D.J.; Belperio, J.A.; Saggar, R.; Lynch, J.P.; Ardehali, A.; Karlamangla, A.S. Prediction of Pulmonary Hypertension in Idiopathic Pulmonary Fibrosis. Respir. Med. 2007, 101, 2153–2159. [Google Scholar] [CrossRef]
- Nathan, S.D. Hypersensitivity Pneumonitis and Pulmonary Hypertension: How the Breeze Affects the Squeeze. Eur. Respir. J. 2014, 44, 287–288. [Google Scholar] [CrossRef] [PubMed]
- McGoon, M.; Gutterman, D.; Steen, V.; Barst, R.; McCrory, D.C.; Fortin, T.A.; Loyd, J.E. American College of Chest Physicians. Screening, Early Detection, and Diagnosis of Pulmonary Arterial Hypertension: ACCP Evidence-Based Clinical Practice Guidelines. Chest 2004, 126 (Suppl. S1), 14S–34S. [Google Scholar] [CrossRef]
- Nathan, S.D.; Barbera, J.A.; Gaine, S.P.; Harari, S.; Martinez, F.J.; Olschewski, H.; Olsson, K.M.; Peacock, A.J.; Pepke-Zaba, J.; Provencher, S.; et al. Pulmonary Hypertension in Chronic Lung Disease and Hypoxia. Eur. Respir. J. 2019, 53, 1801914. [Google Scholar] [CrossRef]

| Parameters | Fibrotic HPMedian (IQR)/Frequency | Nonfibrotic HP Median (IQR)/Frequency | p Value |
|---|---|---|---|
| Age, years | 65.0 (56.5–69.0) | 49.0 (41.0–59.5) | 0.0001 |
| Disease duration, months | 12.0 (2.0–36.5) | 5.0 (0.0–36.0) | 0.153 |
| Gender (m/f), % | 39.3/60.7 | 20.8/79.2 | 0.105 |
| BMI, kg/m2 | 28.4 (24.3–32.3) | 27.3 (22.7–29.2) | 0.250 |
| Smoking history, pack/years | 0.0 (0.0–7.0 | 0.0 (0.0–10.5) | 0.879 |
| GAP stages | 1 (1–2) | 1 (1–1) | 0.003 |
| EQ-5D-5L: | |||
| mobility | 3 (2–4) | 3 (2–3) | 0.485 |
| self-care | 2 (1–4) | 1 (1–2) | 0.016 |
| usual activities | 3 (2–4) | 2 (1–3) | 0.027 |
| pain/discomfort | 2 (1–3) | 2 (1–2) | 0.363 |
| anxiety/depression | 2 (2–3) | 2 (1–2) | 0.043 |
| self-rated health | 50 (30–70) | 75 (60–80) | 0.001 |
| K-BILD, points | 54 (45–75) | 61 (49–82) | 0.188 |
| mMRC, points | 3 (2–4) | 2 (1–3) | 0.048 |
| Cough, VAS, points | 5 (2–7) | 2 (1–7) | 0.755 |
| Cyanosis, % | 11.5 | 4.2 | 0.322 |
| Peripheral edema, % | 11.5 | 4.2 | 0.322 |
| Finger clubbing, % | 29.5 | 12.5 | 0.099 |
| Cardiovascular diseases, % | 45.9% | 16.7% | 0.007 |
| Charlson comorbidity index, points | 4 (4–5) | 3 (2–5) | 0.012 |
| 6-min walking test: | |||
| distance, m | 332 (225–468) | 443 (400–520) | 0.006 |
| pre-SpO2, % | 94 (92–96) | 95 (93–97) | 0.239 |
| post-SpO2, % | 85 (79–88) | 88 (84–92) | 0.016 |
| Hemoglobin, g/L | 136 (126–145) | 142 (130–152) | 0.338 |
| Erythrocytes, 1012/L | 4.6 (4.2–5.2) | 4.9 (4.5–5.2) | 0.165 |
| Leukocytes, 109/L | 8.1 (6.7–10.3) | 7.4 (6.03–11.4) | 0.421 |
| Monocytes, 109/L | 0.6 (0.4–0.7) | 0.5 (0.4–0.6) | 0.931 |
| Neutrophils to lymphocytes ratio | 1.9 (1.4–2.5) | 1.8 (1.4–2.5) | 0.821 |
| CRP, mg/L | 6.1 (3.5–10.8) | 5.9 (1.9–10.9) | 0.650 |
| FVC, % pred | 65.5 (52.0–79.0) | 68.5 (52.0–81.0) | 0.862 |
| FEV1, % pred | 70.0 (55.0–84.5) | 71.5 (57.0–83.5) | 0.838 |
| TLC, % pred | 67.0 (51.0–77.0) | 67.0 (58.0–85.0) | 0.147 |
| DLco, % pred | 37.5 (29.0–55.0) | 55.5 (43.5–61.0) | 0.010 |
| FVC/DLCO ratio | 1.6 (1.6–2.1) | 1.3 (1.1–1.5) | 0.005 |
| CPI | 47.7 (39.7–58.9) | 50.8 (36.9–61.2) | 0.901 |
| PaO2, mmHg | 63.5 (58.8–72.8) | 74.9 (58.8–86.3) | 0.312 |
| PaCO2, mmHg | 37.9 (34.6–41.5) | 36.5 (32.6–41.3) | 0.381 |
| RV basal diameter, mm | 38.6 (35.5–39.4) | 37.2 (35.6–38.5) | 0.112 |
| LV line size, mm | 43.4 (43.0–47.1) | 43.0 (43.0–46.4) | 0.867 |
| RV/LV | 0.8 (0.8–0.9) | 0.8 (0.8–0.9) | 0.120 |
| RA area, cm2 | 15.3 (13.4–18.5) | 15.2 (13.1–16.5) | 0.522 |
| LA volume, mL | 51.2 (45.8–57.3) | 48.5 (44.5–54.5) | 0.595 |
| TV E/A ratio | 0.7 (0.7–0.9) | 1.6 (1.0–1.6) | 0.001 |
| Pulmonary artery diameter > 25 mm, % | 11.5 | 0.0 | 0.052 |
| Inferior vena cava diameter > 21 mm, % | 1.6 | 12.5 | 0.020 |
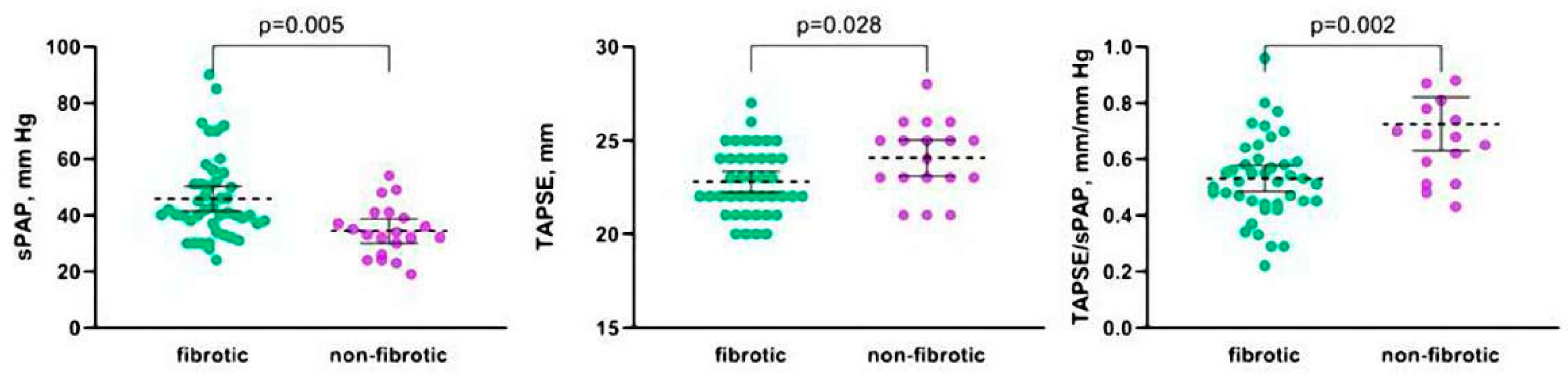
| sPAP, mmHg | 40 (34–51) | 33 (25–41) | 0.005 |
| TAPSE, mm | 23 (21–24) | 25 (23–26) | 0.028 |
| TAPSE/sPAP, mm/mm Hg | 0.5 (0.5–0.6) | 0.7 (0.5–0.9) | 0.002 |
| TAPSE/sPAP < 0.55, % | 37.7 | 16.7 | 0.105 |
| Pulmonary hypertension, % | 57.4 | 25 | 0.014 |
| EF, % | 62 (61–63) | 65 (63–67) | 0.001 |
| Parameters | PH Median (IQR)/Frequency | No PH Median (IQR)/Frequency | p Value |
|---|---|---|---|
| Fibrotic type, % | 83.3 | 45.5 | 0.002 |
| Age, years | 66.5 (58.0–69.5) | 56.0 (46.0–64.0) | 0.006 |
| Disease duration, months | 10.0 (1.5–24.0) | 6.0 (0.0–26.0) | 0.227 |
| Gender (m/f), % | 40.0/60.0 | 33.3/ 66.7 | 0.610 |
| BMI, kg/m2 | 29.2 (27.3–32.4) | 26.2 (23.2–28.4) | 0.010 |
| Smoking history, pack/years | 0.0 (0.0–7.5) | 0.0 (0.0–13.0) | 0.991 |
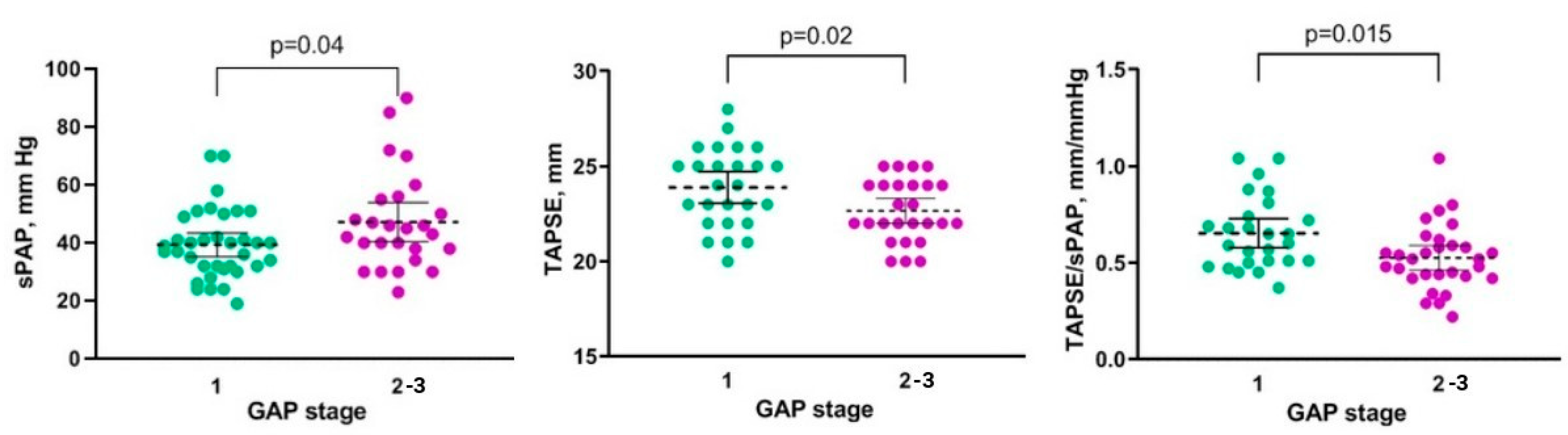
| GAP, stage | 2 (1–2) | 1 (1–2) | 0.235 |
| EQ5D5L self-rated health, points | 55 (35–73) | 80 (50–80) | 0.086 |
| K-BILD, points | 58 (50–75) | 50 (46–86) | 0.812 |
| mMRC, points | 3 (2–3) | 3 (2–4) | 0.776 |
| Cough, VAS, points | 5 (3–7) | 2 (1–3) | 0.075 |
| Cough, % | 84.2 | 60.0 | 0.041 |
| Cyanosis, % | 21.2 | 5.0 | 0.110 |
| Peripheral edema, % | 22.0 | 0 | 0.021 |
| Finger clubbing, % | 36.8 | 10.0 | 0.031 |
| Cardiovascular diseases, % | 61.8 | 30.0 | 0.024 |
| Charlson comorbidity index, points | 4 (4–5) | 3 (2–5) | 0.186 |
| 6-min walking test: | |||
| distance, m | 318 (220–400) | 475 (400–520) | <0.001 |
| pre-SpO2, % | 94 (92–95) | 95 (93–97) | 0.135 |
| post-SpO2, % | 84 (78–88) | 88 (86–93) | 0.007 |
| FVC, % pred | 70.5 (55.8–79.5) | 64.5 (48.0–77.5) | 0.249 |
| FEV1, % pred | 72.5 (58.5–85.0) | 62.5 (48.3–77.5) | 0.196 |
| TLC, % pred | 67.0 (54.5–76.0) | 65.0 (51.8–85.8) | 0.854 |
| DLco, % pred | 38.3 (30.5–54.0) | 50.2 (31.0–61.0) | 0.363 |
| FVC/DLco ratio | 1.6 (1.3–2.2) | 1.4 (1.3–1.5) | 0.021 |
| CPI | 49.2 (38.8–60.9) | 47/1 (37.6–60.2) | 0.745 |
| PaO2, mm Hg | 62.0 (54.2–70.0) | 70.0 (61.0–77.0) | 0.220 |
| PaCO2, mm Hg | 35.0 (32.3–42.2) | 36.0 (33.0–38.0) | 0.919 |
| RV basal diameter, mm | 38.0 (36.0–39.0) | 36.0 (35.0–38.0) | 0.080 |
| RA area, cm2 | 15.5 (14.2–18.0) | 14.0 (11.8–15.6) | 0.132 |
| LV line size, mm | 43.0 (43.0–47.5) | 43.0 (42.3–43.0) | 0.061 |
| LA volume, mL | 51.0 (45.0–57.0) | 46.0 (44.0–54.0) | 0.439 |
| TV E/A ratio | 0.77 (0.70–0.96) | 1.30 (0.70–1.60) | 0.209 |
| sPAP, mm Hg | 46 (40–54) | 30 (26–32) | 0.0001 |
| TAPSE, mm | 23 (21–25) | 23 (22–25) | 0.549 |
| TAPSE/sPAP, mm/mm Hg | 0.51 (0.44–0.56) | 0.77 (0.70–0.88) | 0.0001 |
| TAPSE/sPAP <0.55, % | 64.3 | 0.0 | 0.0001 |
| UIP-pattern on HRCT, % | 40.5 | 22.7 | 0.169 |
| Long-term oxygen, % | 28.6 | 10.5 | 0.128 |
| Nintedanib treatment, % | 11.8 | 5 | 0.408 |
| SCS treatment, % | 62.9 | 70 | 0.592 |
| MMF treatment, % | 5.7 | 15.0 | 0.249 |
| Parameters | WHO-FC I and II Median (IQR)/Frequency | WHO-FC III and IV Median (IQR)/Frequency | p Value |
|---|---|---|---|
| Fibrotic type, % | 76.9 | 92.9 | 0.244 |
| Age, years | 60.0 (50.0–68.0) | 67.5 (61.0–72.0) | 0.049 |
| Disease duration, months | 6 (1–24) | 11 (1–36) | 0.639 |
| Gender (m/f), % | 46.2/53.8 | 35.7/64.3 | 0.581 |
| BMI, kg/m2 | 28.6 (27.7–31.1) | 28.9 (25.4–32.4) | 0.923 |
| Smoking history, pack/years | 0.0 (0.0–20.0) | 0.0 (0.0–10.0) | 0.341 |
| GAP, points | 3 (2–4) | 4 (3–5) | 0.039 |
| GAP, stage | 1 (1–2) | 2 (1–2) | 0.060 |
| EQ5D5L self-rated health, points | 60 (50–70) | 55 (25–77) | 0.958 |
| K-BILD, points | 60 (50–79) | 50 (42–75) | 0.292 |
| mMRC, points | 2 (1–3) | 3 (3–4) | 0.013 |
| Cough, VAS, points | 5 (2–8) | 5 (4–7) | 0.882 |
| Cough, % | 53.8 | 71.4 | 0.345 |
| Cyanosis, % | 15.4 | 35.7 | 0.332 |
| Peripheral edema, % | 15.4 | 28.6 | 0.473 |
| Finger clubbing, % | 23.1 | 50.0 | 0.148 |
| Cardiovascular diseases, % | 30.8 | 71.4 | 0.052 |
| Charlson comorbidity index, points | 4 (2–5) | 4 (4–6) | 0.190 |
| 6-min walking test: | |||
| distance, m | 400 (345–448) | 220 (115–285) | 0.0001 |
| pre-SpO2, % | 94 (92–95) | 95 (92–96) | 0.244 |
| post-SpO2, % | 85 (77–88) | 84 (80–88) | 1.000 |
| FVC, % pred | 74.0 (54.0–80.0) | 67.5 (56.8–75.8) | 0.846 |
| FEV1, % pred | 73.0 (58.0–82.5) | 75.0 (59.3–85.0) | 0.716 |
| TLC, % pred | 68.0 (53.0–83.5) | 61.0 (53.0–71.8) | 0.423 |
| DLco, % pred | 41 (37–59) | 33 (23–47) | 0.040 |
| FVC/DLco ratio | 1.47 (1.20–1.80) | 2.20 (1.62–2.27) | 0.033 |
| CPI | 55.6 (37.1–63.7) | 58.63 (41.5–61.7) | 0.885 |
| PaO2, mm Hg | 64.0 (62.0–86.0) | 44.0 (41.5–76.5) | 0.153 |
| PaCO2, mm Hg | 33.4 (31.3–41.0) | 34.5 (31.8–42.3) | 0.816 |
| UIP-pattern on HRCT, % | 38.5 | 57.1 | 0.239 |
| RV basal diameter, mm | 38.0 (36.5–39.0) | 37.0 (36.0–42.5) | 0.856 |
| RA area, cm2 | 16.0 (15.0–17.4) | 15.7 (13.8–20.9) | 0.616 |
| LV line size, mm | 44.0 (43.0–47.5) | 43.0 (43.0–46.0) | 0.616 |
| LA volume, mL | 50.0 (45.0–63.0) | 48.0 (43.0–54.8) | 0.268 |
| TV E/A ratio | 0.93 (0.72–1.00) | 0.70 (0.64–0.92) | 0.103 |
| sPAP, mm Hg | 41.0 (39.5–49.5) | 46.0 (39.5–59.5) | 0.253 |
| TAPSE, mm | 23 (22–25) | 24 (22–25) | 0.832 |
| TAPSE/sPAP, mm/mm Hg | 0.55 (0.51–0.59) | 0.49 (0.40–0.57) | 0.110 |
| TAPSE/sPAP < 0.55, % | 38.5 | 64.3 | 0.249 |
| Parameters | Univariate Analysis OR (95% CI) | Univariate Analysis p-Value | Multivariate Analysis OR (95% CI) | Multivariate Analysis p-Value |
|---|---|---|---|---|
| HRCT signs of fibrosis | 5.5 (1.7–17.6) | 0.004 | 6.4 (1.6–24.7) | 0.008 |
| Finger clubbing | 5.3 (1.1–26.1) | 0.040 | ||
| FVC/DLco ratio | 3.9 (1.1–14.9) | 0.040 | ||
| Cardiovascular diseases | 3.8 (1.1–12.9) | 0.030 | 3.7 (1.0–13.6) | 0.050 |
| Age | 1.1 (1.0–1.1) | 0.005 | ||
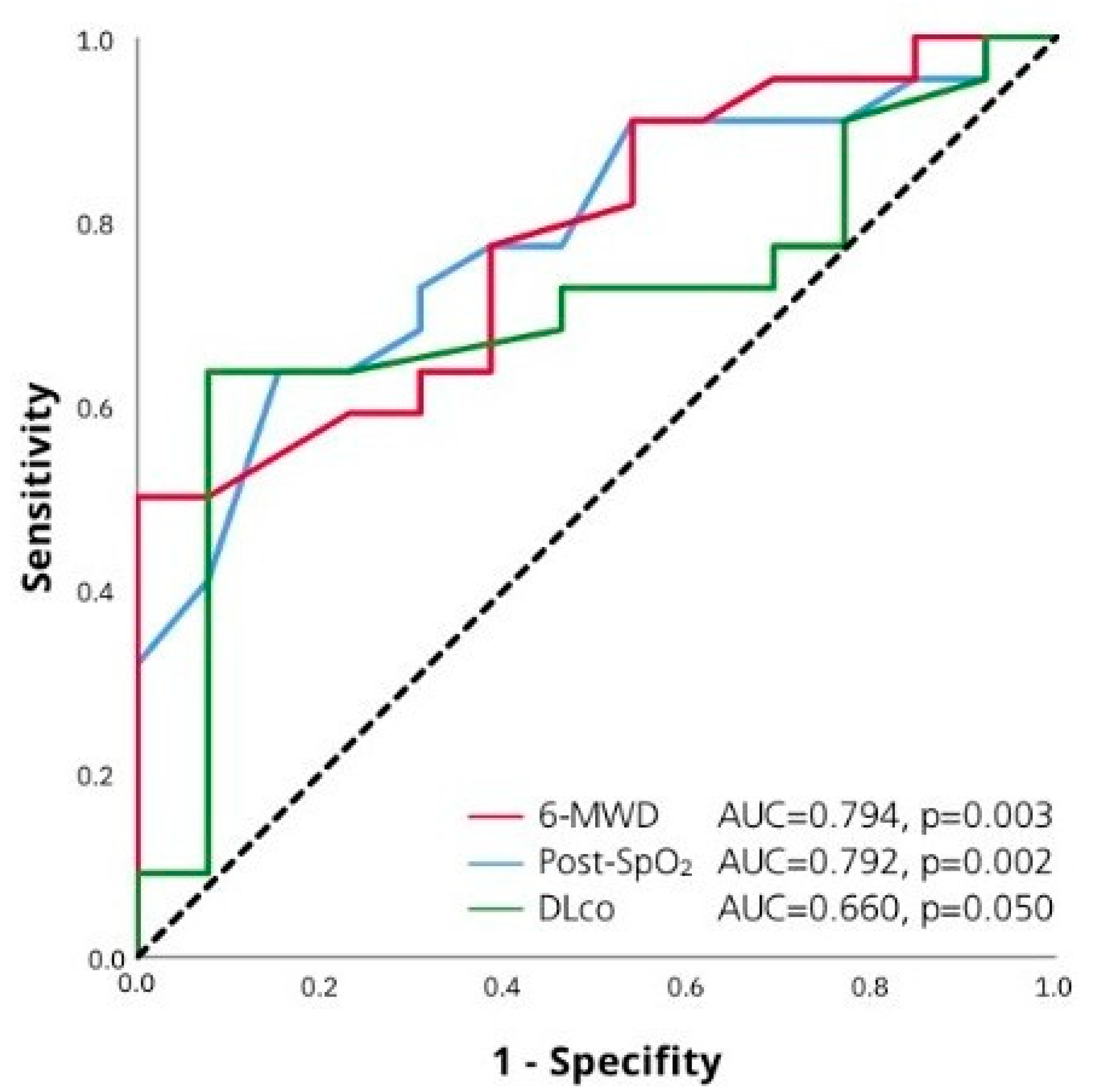
| Distance walked in 6-MWT | 0.99 (0.98–0.99) | 0.007 | ||
| Post-SpO2 6-MWT | 0.80 (0.70–0.96) | 0.010 |
| Parameters | TAPSE/sPAP ≥ 0.55 Median (IQR)/Frequency | TAPSE/sPAP < 0.55 Median (IQR)/Frequency | p Value |
|---|---|---|---|
| Fibrotic type, % | 56.3 | 85.28 | 0.016 |
| Age, years | 66 (57–69) | 60 (46–68) | 0.177 |
| Disease duration, months | 1.5 (0.0–17.0) | 23.0 (5.0–36.0) | 0.013 |
| Gender (m/f), % | 37.5/62.5 | 40.7/59.3 | 0.799 |
| BMI, kg/m2 | 29.2 (27.0–32.4) | 28.1 (25.2–30.6) | 0.278 |
| Smoking history, pack/years | 0.0 (0.0–13.0) | 0.0 (0.0–10.0) | 0.333 |
| GAP, stage | 2 (1–2) | 1 (1–2) | 0.129 |
| EQ5D5L self-rated health, points | 65 (30–80) | 60 (40–75) | 0.442 |
| K-BILD, points | 52.0 (46.5–82.0) | 58.5 (49.5–73.5) | 0.835 |
| mMRC, points | 2 (2–3) | 3 (3–4) | 0.073 |
| Cough, VAS, points | 2.5 (1.5–5.0) | 5.0 (4.0–7.0) | 0.114 |
| Cough, % | 70.6 | 73.3 | 0.863 |
| Cyanosis, % | 7.1 | 30.0 | 0.036 |
| Peripheral edema, % | 10.3 | 14.3 | 0.672 |
| Finger clubbing, % | 13.8 | 45.8 | 0.010 |
| Cardiovascular diseases, 5 | 37.9 | 70.0 | 0.027 |
| Charlson comorbidity index, points | 4.0 (2.0–5.0) | 4.0 (3.5–5.0) | 0.531 |
| 6-MWT | |||
| distance, m | 418 (318–480) | 270 (120–380) | 0.002 |
| pre-SpO2, % | 95.0 (93.0–96.5) | 94.0 (92.0–94.5) | 0.191 |
| post-SpO2, % | 88.0 (84.5–90.5) | 81.5 (77.0–86.0) | 0.002 |
| FVC, % pred | 68.0 (55.0–79.0) | 61.5 (54.5–74.0) | 0.353 |
| FEV1, % pred | 72.0 (61.0–78.0) | 67.7 (57.0–83.0) | 0.809 |
| TLC, % pred | 67.0 (53.0–84.0) | 64.0 (55.0–73.0) | 0.268 |
| DLco, % pred | 52.5 (32.5–61.0) | 37.0 (29.5–49.0) | 0.050 |
| FVC/DLco ratio | 1.41 (1.31–1.78) | 1.60 (1.29–2.21) | 0.190 |
| CPI | 47.4 (39.1–63.7) | 49.0 (38.6–58.8) | 0.558 |
| PaO2, mm Hg | 70.0 (61.5–79.0) | 62.0 (45.0–67.0) | 0.037 |
| PaCO2, mm Hg | 35.4 (32.9–39.5) | 37.5 (34.1–42.3) | 0.377 |
| UIP-pattern by HRCT,% | 21.9 | 48.1 | 0.050 |
| TV E/A ratio | 0.89 (0.70–1.08) | 0.73 (0.67–0.92) | 0.374 |
| sPAP, mm Hg | 34 (30–39) | 51 (45–60) | 0.0001 |
| TAPSE, mm | 23 (22–25) | 23 (21–25) | 0.528 |
| LV line size, mm | 43.0 (43.0–47.5) | 43.0 (43.0–46.0) | 0.580 |
| LA volume, mL | 50.5 (45.0–55.5) | 49.5 (45.0–58.8) | 0.691 |
| RV basal diameter, mm | 37.0 (36.0–39.0) | 38.0 (36.0–39.0) | 0.580 |
| RA area, cm2 | 15.3 (14.5–16.0) | 16.5 (13.5–20.0) | 0.544 |
| Parameters | Univariate Analysis OR (95% CI) | Univariate Analysis p-Value | Multivariate Analysis OR (95% CI) | Multivariate Analysis p-Value |
|---|---|---|---|---|
| Finger clubbing | 5.3 (1.4–19.9) | 0.010 | ||
| HRCT signs of fibrosis | 3.85 (1.15–12.9) | 0.028 | ||
| Cardiovascular diseases | 3.82 (1.1–12.9) | 0.030 | 4.29 (0.85–21.5) | 0.078 |
| Post-SpO2 6-MWT | 0.84 (0.73–0.96) | 0.008 | 0.83 (0.72–0.95) | 0.008 |
| DLco, % pred | 0.96 (0.93–0.99 | 0.050 | ||
| Distance walked in 6-MWT | 0.99 (0.98–0.99) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trushenko, N.V.; Suvorova, O.A.; Nekludova, G.V.; Levina, I.A.; Chikina, S.Y.; Nikolenko, A.M.; Tsareva, N.A.; Volkov, A.V.; Yaroshetskiy, A.I.; Merzhoeva, Z.M.; et al. Predictors of Pulmonary Hypertension and Right Ventricular Dysfunction in Patients with Hypersensitivity Pneumonitis. Life 2023, 13, 1348. https://doi.org/10.3390/life13061348
Trushenko NV, Suvorova OA, Nekludova GV, Levina IA, Chikina SY, Nikolenko AM, Tsareva NA, Volkov AV, Yaroshetskiy AI, Merzhoeva ZM, et al. Predictors of Pulmonary Hypertension and Right Ventricular Dysfunction in Patients with Hypersensitivity Pneumonitis. Life. 2023; 13(6):1348. https://doi.org/10.3390/life13061348
Chicago/Turabian StyleTrushenko, Natalia V., Olga A. Suvorova, Galina V. Nekludova, Iuliia A. Levina, Svetlana Y. Chikina, Alexandra M. Nikolenko, Natalia A. Tsareva, Alexandr V. Volkov, Andrey I. Yaroshetskiy, Zamira M. Merzhoeva, and et al. 2023. "Predictors of Pulmonary Hypertension and Right Ventricular Dysfunction in Patients with Hypersensitivity Pneumonitis" Life 13, no. 6: 1348. https://doi.org/10.3390/life13061348
APA StyleTrushenko, N. V., Suvorova, O. A., Nekludova, G. V., Levina, I. A., Chikina, S. Y., Nikolenko, A. M., Tsareva, N. A., Volkov, A. V., Yaroshetskiy, A. I., Merzhoeva, Z. M., Nuralieva, G. S., & Avdeev, S. N. (2023). Predictors of Pulmonary Hypertension and Right Ventricular Dysfunction in Patients with Hypersensitivity Pneumonitis. Life, 13(6), 1348. https://doi.org/10.3390/life13061348






