A Ferroptosis-Related lncRNAs Signature Predicts Prognosis of Colon Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data Acquisition
2.2. Certification of frlncRNAs in COAD
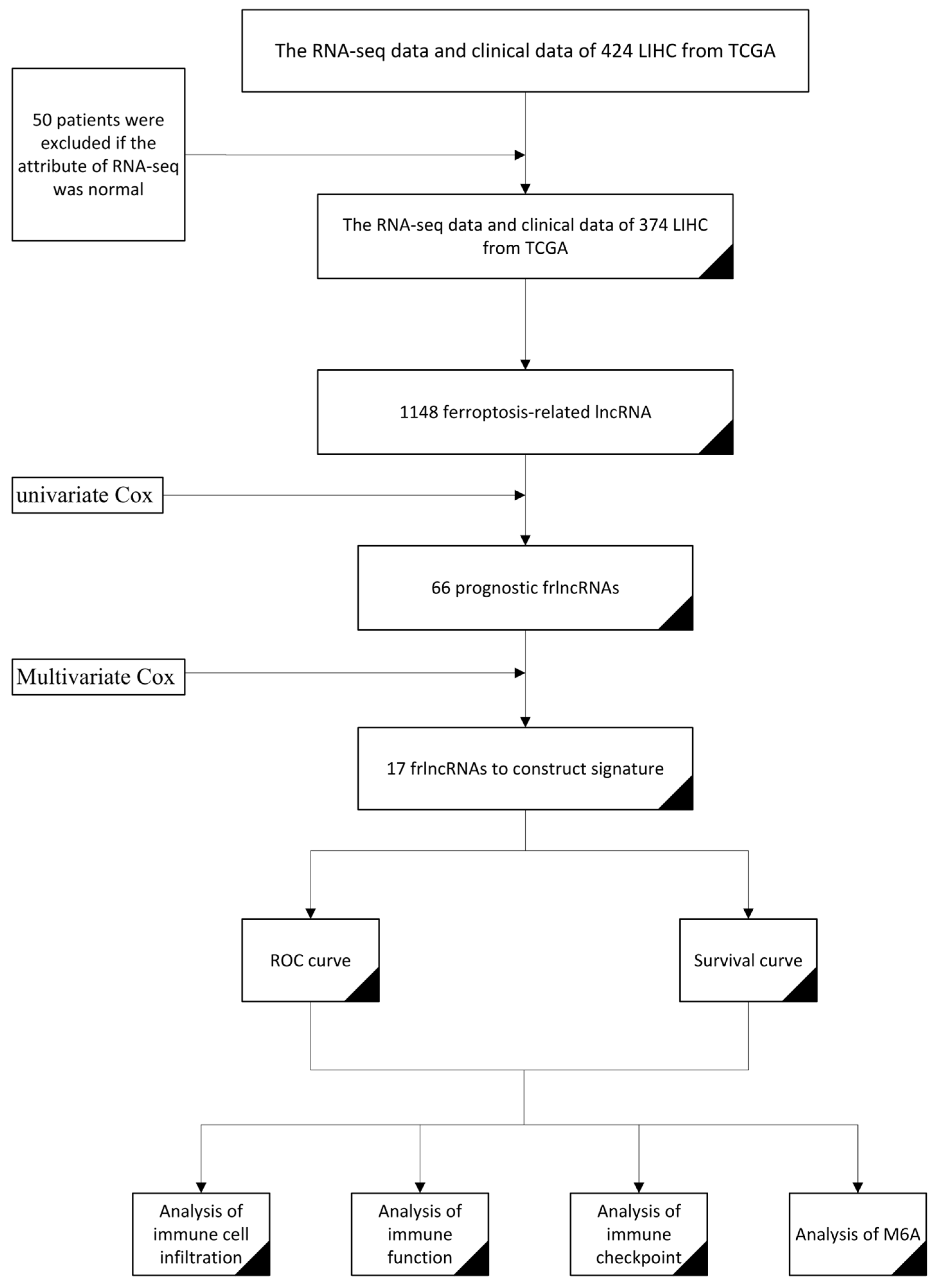
2.3. Construction of a Prognostic frlncRNAs Signature
2.4. Assessing the Prognostic Features of Five frlncRNAs
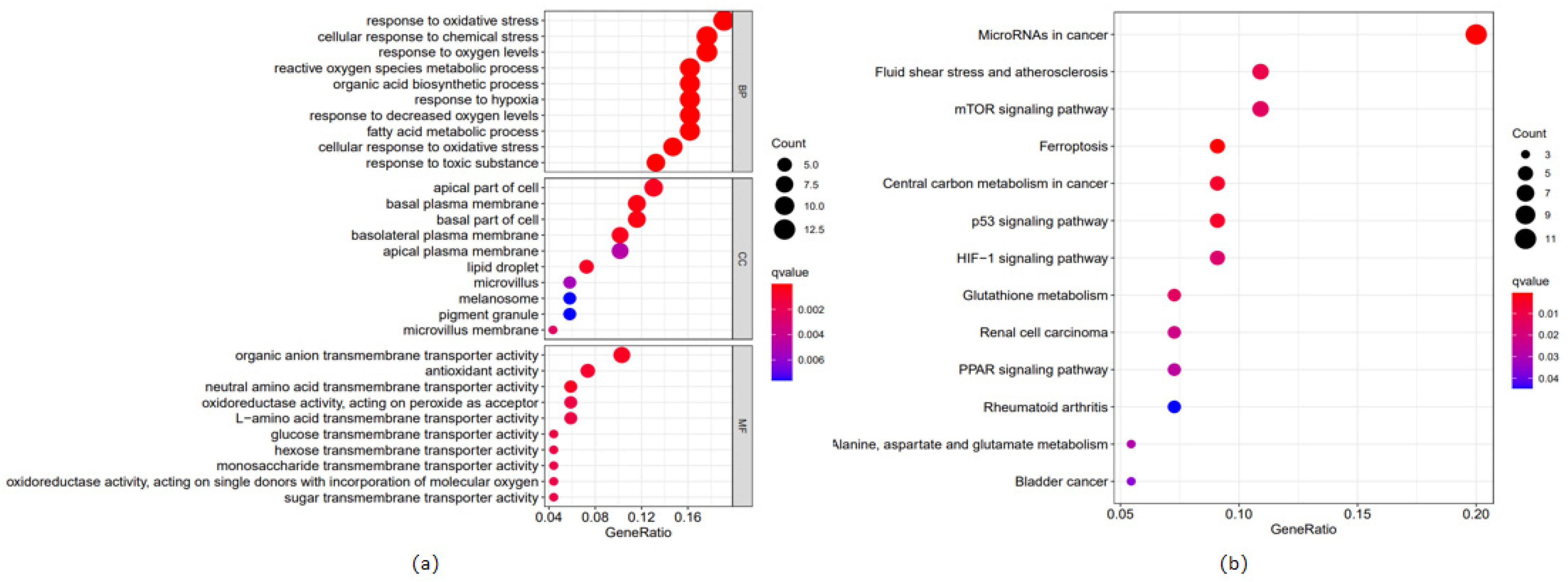
2.5. Gene Set Enrichment Analysis and Functional Enrichment Analysis
2.6. Immune Infiltration Level Analysis and N6-Methyladenosine Analysis
3. Results
3.1. Identification of Differentially Expressed frlncRNAs in Patients with Colon Cancer
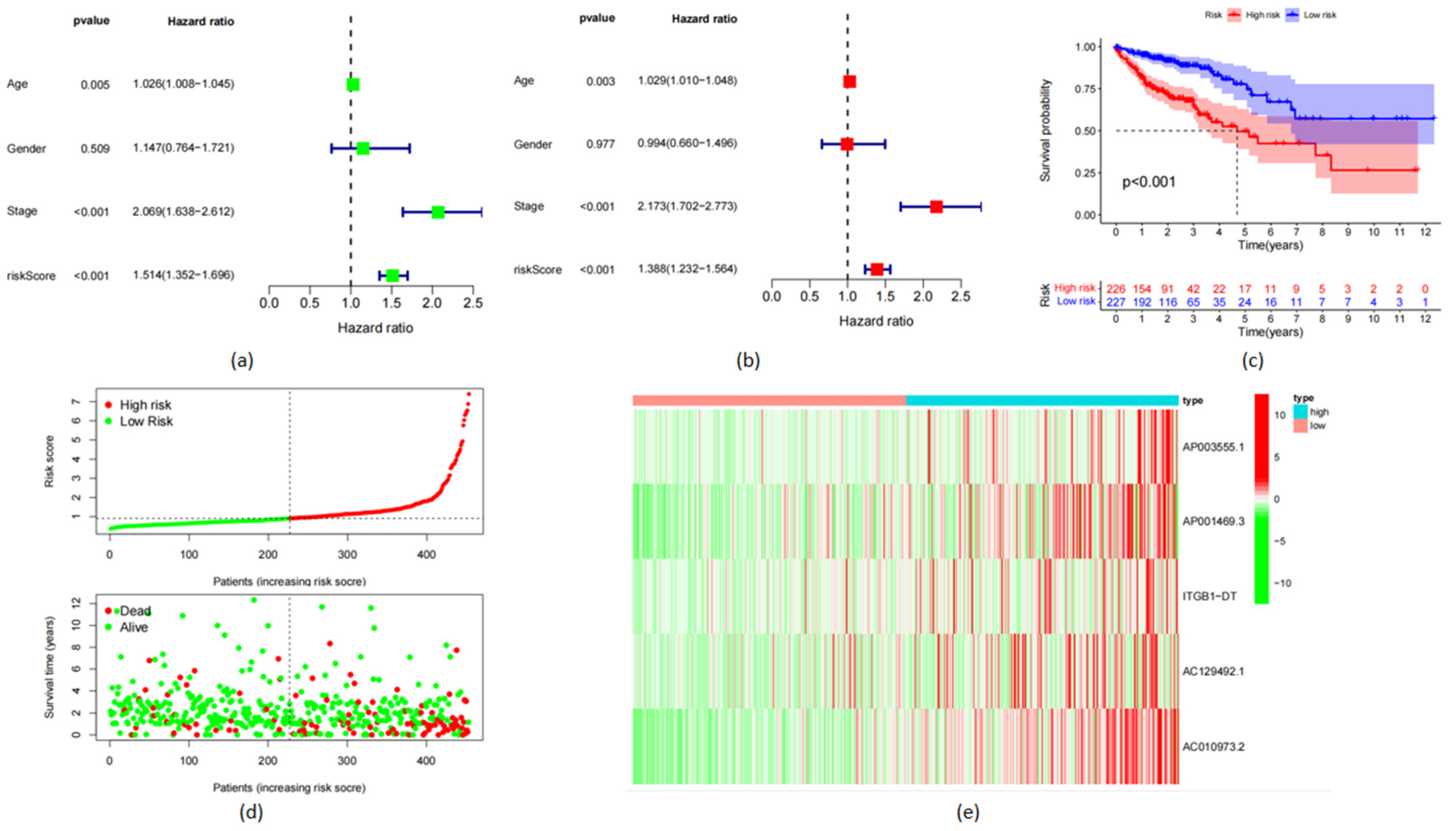
3.2. Construction of a Prognostic Model
3.3. Important Pathways Identified with Enrichment Analysis
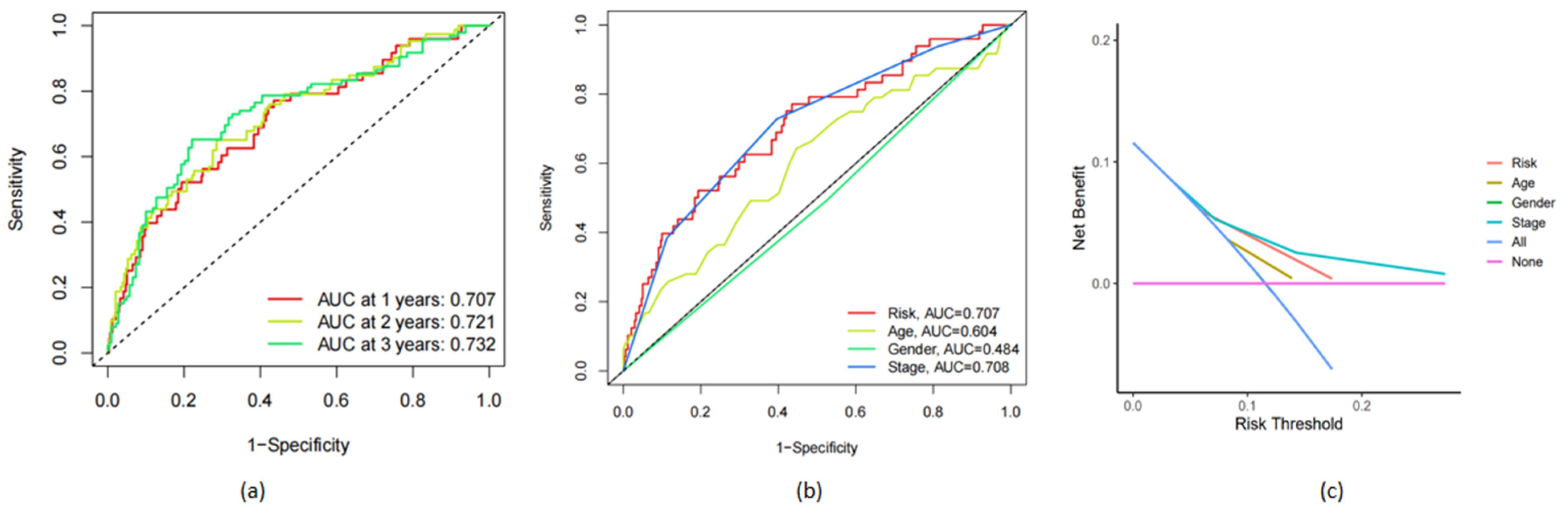
3.4. Verification of the Accuracy of the frlncRNAs Model
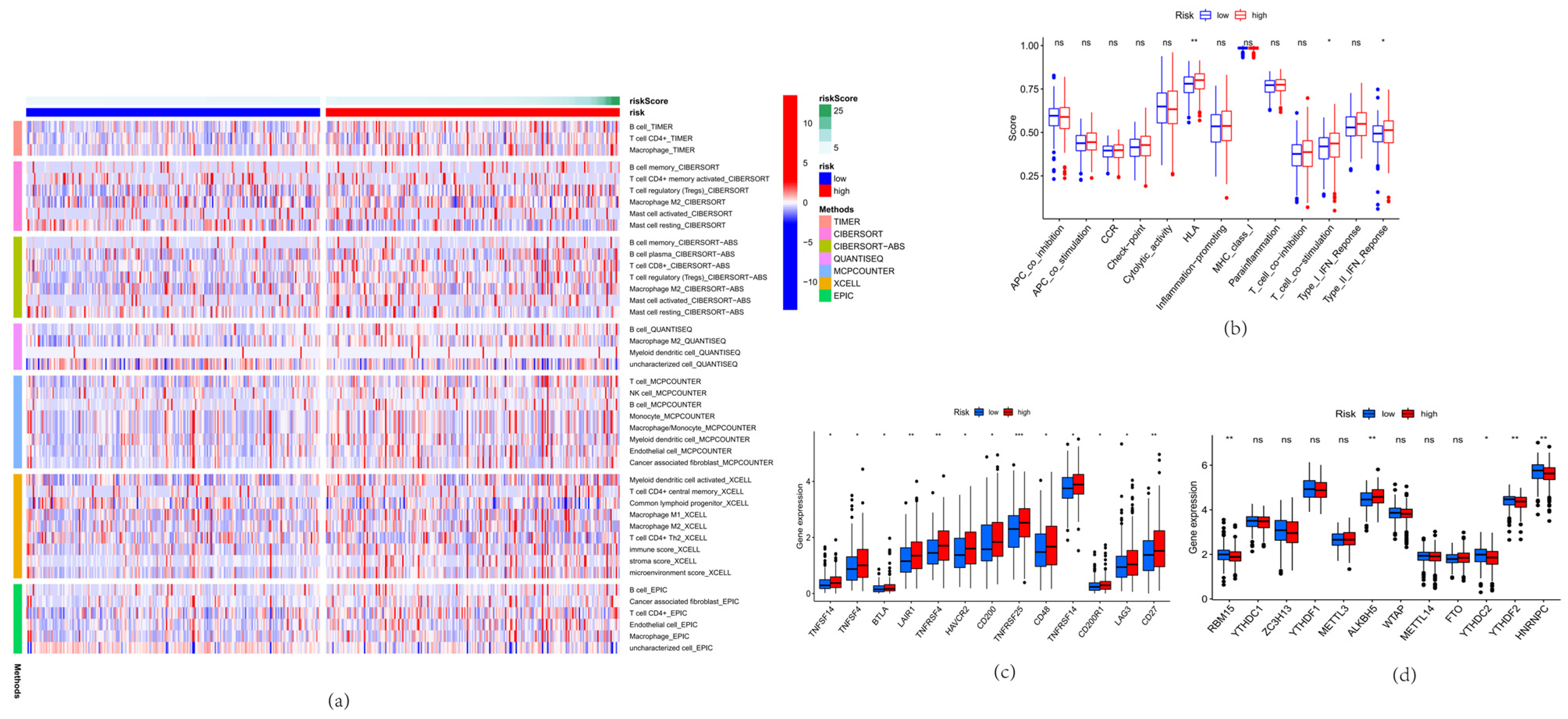
3.5. Immunoassay and m6A Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leow, C.C.; Polakis, P.; Gao, W.Q. A role for Hath1, a bHLH transcription factor, in colon adenocarcinoma. Ann. N. Y. Acad. Sci. 2005, 1059, 174–183. [Google Scholar] [CrossRef]
- Dunlop, M.G.; Tenesa, A.; Farrington, S.M.; Ballereau, S.; Brewster, D.H.; Koessler, T.; Pharoah, P.; Schafmayer, C.; Hampe, J.; Volzke, H.; et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42,103 individuals. Gut 2013, 62, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Aarons, C.B.; Shanmugan, S.; Bleier, J.I. Management of malignant colon polyps: Current status and controversies. World J. Gastroenterol. 2014, 20, 16178–16183. [Google Scholar] [CrossRef] [PubMed]
- Cappell, M.S. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol. Clin. N. Am. 2008, 37, 1–24. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Lu, Y.F.; Zhou, J.P.; Yang, X.Y.; Wang, X.J.; Yu, J.N.; Du, Y.Z.; Yu, R.S. Self-amplification of oxidative stress with tumour microenvironment-activatable iron-doped nanoplatform for targeting hepatocellular carcinoma synergistic cascade therapy and diagnosis. J. Nanobiotechnol. 2021, 19, 361. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C.; Chen, W.P.; Li, Z.L.; Cheng, H. Development and Validation of a Novel Ferroptosis-Related Gene Signature for Predicting Prognosis and the Immune Microenvironment in Gastric Cancer. Biomed. Res. Int. 2021, 2021, 6014202. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Wang, F.; Zhou, X.; Fu, Q.; Yang, X.; Lin, J.; Jin, X. A Novel Assessment Model Based on Molecular Subtypes of Hypoxia-Related LncRNAs for Prognosis of Bladder Cancer. Front. Cell Dev. Biol. 2021, 9, 718991. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Z.; Yin, H. Long Non-Coding RNA CCAT2 Activates RAB14 and Acts as an Oncogene in Colorectal Cancer. Front. Oncol. 2021, 11, 751903. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Li, Z.; Zheng, Y.; Shi, Z.; Wang, G. Long noncoding RNA CRNDE functions as a diagnostic and prognostic biomarker in osteosarcoma, as well as promotes its progression via inhibition of miR-335-3p. J. Biochem. Mol. Toxicol. 2021, 35, e22734. [Google Scholar] [CrossRef]
- Hon, K.W.; Zainal Abidin, S.A.; Othman, I.; Naidu, R. The Crosstalk Between Signaling Pathways and Cancer Metabolism in Colorectal Cancer. Front. Pharmacol. 2021, 12, 768861. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Lao, Y.; Yu, W.; Zhang, X.; Jiang, M.; Zhu, C. Progress in the Application of Immune Checkpoint Inhibitor-Based Immunotherapy for Targeting Different Types of Colorectal Cancer. Front. Oncol. 2021, 11, 764618. [Google Scholar] [CrossRef]
- Chang, R.; Xiao, X.; Fu, Y.; Zhang, C.; Zhu, X.; Gao, Y. ITGB1-DT Facilitates Lung Adenocarcinoma Progression via Forming a Positive Feedback Loop with ITGB1/Wnt/beta-Catenin/MYC. Front. Cell Dev. Biol. 2021, 9, 631259. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Jiang, N.; Ke, D.; Guo, Q.; Zhai, K.; Han, H.; Xiao, X.; Fan, T. Interfering with ITGB1-DT expression delays cancer progression and promotes cell sensitivity of NSCLC to cisplatin by inhibiting the MAPK/ERK pathway. Am. J. Cancer Res. 2022, 12, 2966–2988. [Google Scholar]
- Li, N.; Shen, J.; Qiao, X.; Gao, Y.; Su, H.B.; Zhang, S. Long Non-Coding RNA Signatures Associated with Ferroptosis Predict Prognosis in Colorectal Cancer. Int. J. Gen. Med. 2022, 15, 33–43. [Google Scholar] [CrossRef]
- Andorfer, C.A.; Necela, B.M.; Thompson, E.A.; Perez, E.A. MicroRNA signatures: Clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef]
- Huang, Q.; Li, S.; Hu, X.; Sun, M.; Wu, Q.; Dai, H.; Tan, Y.; Sun, F.; Wang, C.; Rong, X.; et al. Shear stress activates ATOH8 via autocrine VEGF promoting glycolysis dependent-survival of colorectal cancer cells in the circulation. J. Exp. Clin. Cancer Res. 2020, 39, 25. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, L.; Li, F.; Yang, J.; Wan, X.; Ouyang, M. Silencing of RHEB inhibits cell proliferation and promotes apoptosis in colorectal cancer cells via inhibition of the mTOR signaling pathway. J. Cell Physiol. 2020, 235, 442–453. [Google Scholar] [CrossRef]
- Tasaka, Y.; Honda, T.; Nishiyama, N.; Tsutsui, T.; Saito, H.; Watabe, H.; Shimaya, K.; Mochizuki, A.; Tsuyuki, S.; Kawahara, T.; et al. Non-inferior clinical outcomes of immune checkpoint inhibitors in non-small cell lung cancer patients with interstitial lung disease. Lung Cancer 2021, 155, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Moller, K.; Blessin, N.C.; Hoflmayer, D.; Buscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Zalewski, K.; Hinsch, A.; Neipp, M.; et al. High density of cytotoxic T-lymphocytes is linked to tumoral PD-L1 expression regardless of the mismatch repair status in colorectal cancer. Acta Oncol. 2021, 60, 1210–1217. [Google Scholar] [CrossRef]
- Bai, Z.; Zhou, Y.; Ye, Z.; Xiong, J.; Lan, H.; Wang, F. Tumor-Infiltrating Lymphocytes in Colorectal Cancer: The Fundamental Indication and Application on Immunotherapy. Front. Immunol. 2021, 12, 808964. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kochin, V.; Kanaseki, T.; Hongo, A.; Tokita, S.; Kikuchi, Y.; Takaya, A.; Hirohashi, Y.; Tsukahara, T.; Terui, T.; et al. The Antigen ASB4 on Cancer Stem Cells Serves as a Target for CTL Immunotherapy of Colorectal Cancer. Cancer Immunol. Res. 2018, 6, 358–369. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, W.; Cui, S.; Li, J.; Jiang, Y.; Chen, X.; Peng, L. Modulation of miR-146b by N6-methyladenosine modification remodels tumor-associated macrophages and enhances anti-PD-1 therapy in colorectal cancer. Cell Oncol. 2023. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Y.; Shan, M.; Wang, S.; Chen, J.; Liu, Z.; Xu, Q. Targeting crosstalk of STAT3 between tumor-associated M2 macrophages and Tregs in colorectal cancer. Cancer Biol. Ther. 2023, 24, 2226418. [Google Scholar] [CrossRef]
- Safarzadeh, A.; Alizadeh, M.; Beyranvand, F.; Falavand Jozaaee, R.; Hajiasgharzadeh, K.; Baghbanzadeh, A.; Derakhshani, A.; Argentiero, A.; Baradaran, B.; Silvestris, N. Varied functions of immune checkpoints during cancer metastasis. Cancer Immunol. Immunother. 2021, 70, 569–588. [Google Scholar] [CrossRef]
- Schmitz, F.; Wolf, D.; Holderried, T.A.W. The Role of Immune Checkpoints after Cellular Therapy. Int. J. Mol. Sci. 2020, 21, 3650. [Google Scholar] [CrossRef] [PubMed]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386.e317. [Google Scholar] [CrossRef]
- Qiao, G.; Kone, L.B.; Phillips, E.H.; Lee, S.S.; Brown, G.E.; Khetani, S.R.; Thakur, A.; Lum, L.G.; Prabhakar, B.S.; Maker, A.V. LIGHT enhanced bispecific antibody armed T-cells to treat immunotherapy resistant colon cancer. Oncogene 2022, 41, 2054–2068. [Google Scholar] [CrossRef] [PubMed]
- Ming, S.; Zhang, M.; Liang, Z.; Li, C.; He, J.; Chen, P.; Zhang, S.; Niu, X.; Deng, S.; Geng, L.; et al. OX40L/OX40 Signal Promotes IL-9 Production by Mucosal MAIT Cells During Helicobacter pylori Infection. Front. Immunol. 2021, 12, 626017. [Google Scholar] [CrossRef]
- Demerle, C.; Gorvel, L.; Mello, M.; Pastor, S.; Degos, C.; Zarubica, A.; Angelis, F.; Fiore, F.; Nunes, J.A.; Malissen, B.; et al. Anti-HVEM mAb therapy improves antitumoral immunity both in vitro and in vivo, in a novel transgenic mouse model expressing human HVEM and BTLA molecules challenged with HVEM expressing tumors. J. Immunother. Cancer 2023, 11, e006348. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zi, J.; Han, Q.; Song, C.; Ge, Z. Elevated TNFRSF4 gene expression is a predictor of poor prognosis in non-M3 acute myeloid leukemia. Cancer Cell Int. 2020, 20, 146. [Google Scholar] [CrossRef]
- Wu, Q.; Tian, P.; He, D.; Jia, Z.; He, Y.; Luo, W.; Lv, X.; Wang, Y.; Zhang, P.; Liang, Y.; et al. SCUBE2 mediates bone metastasis of luminal breast cancer by modulating immune-suppressive osteoblastic niches. Cell Res. 2023, 33, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.K.; He, K.; Corless, C.L.; Vetto, J.T.; Weinberg, A.D. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134). Am. J. Surg. 2002, 183, 512–518. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, Y.; Xu, S.; Jin, L.; Shen, Y.; Rajan, K.C.; Bhandari, A.; Xia, E. Construction and Analysis of a Colorectal Cancer Prognostic Model Based on N6-Methyladenosine-Related lncRNAs. Front. Cell Dev. Biol. 2021, 9, 698388. [Google Scholar] [CrossRef]
- Yan, G.; An, Y.; Xu, B.; Wang, N.; Sun, X.; Sun, M. Potential Impact of ALKBH5 and YTHDF1 on Tumor Immunity in Colon Adenocarcinoma. Front. Oncol. 2021, 11, 670490. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Dong, Z.; Li, J.; Yu, Y.; Chen, X.; Ren, F.; Cui, G.; Sun, R. Expression patterns and prognostic value of m(6)A-related genes in colorectal cancer. Am. J. Transl. Res. 2019, 11, 3972–3991. [Google Scholar]
- Zhu, J.; Tong, H.; Sun, Y.; Li, T.; Yang, G.; He, W. YTHDF1 Promotes Bladder Cancer Cell Proliferation via the METTL3/YTHDF1-RPN2-PI3K/AKT/mTOR Axis. Int. J. Mol. Sci. 2023, 24, 6905. [Google Scholar] [CrossRef]
- Chen, M.; Tian, B.; Hu, G.; Guo, Y. METTL3-Modulated circUHRF2 Promotes Colorectal Cancer Stemness and Metastasis through Increasing DDX27 mRNA Stability by Recruiting IGF2BP1. Cancers 2023, 15, 3148. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Wang, Q.; Bao, L.; He, X.; Wang, Z.; Liu, L.; Wu, L.; Zhao, Y.; Zhou, J.; Xie, Y. Effects of Helicobacter pylori on the expression of the FTO gene and its biological role in gastric cancer. Oncol. Lett. 2023, 25, 143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, Z.; Tian, Y.; Li, L.; Dong, J. A Ferroptosis-Related lncRNAs Signature Predicts Prognosis of Colon Adenocarcinoma. Life 2023, 13, 1557. https://doi.org/10.3390/life13071557
Guo Y, Wang Z, Tian Y, Li L, Dong J. A Ferroptosis-Related lncRNAs Signature Predicts Prognosis of Colon Adenocarcinoma. Life. 2023; 13(7):1557. https://doi.org/10.3390/life13071557
Chicago/Turabian StyleGuo, Ying, Zehao Wang, Ye Tian, Lin Li, and Jing Dong. 2023. "A Ferroptosis-Related lncRNAs Signature Predicts Prognosis of Colon Adenocarcinoma" Life 13, no. 7: 1557. https://doi.org/10.3390/life13071557
APA StyleGuo, Y., Wang, Z., Tian, Y., Li, L., & Dong, J. (2023). A Ferroptosis-Related lncRNAs Signature Predicts Prognosis of Colon Adenocarcinoma. Life, 13(7), 1557. https://doi.org/10.3390/life13071557



