An Overview of Rhodoliths: Ecological Importance and Conservation Emergency
Abstract
1. Introduction
2. Geographical Distribution
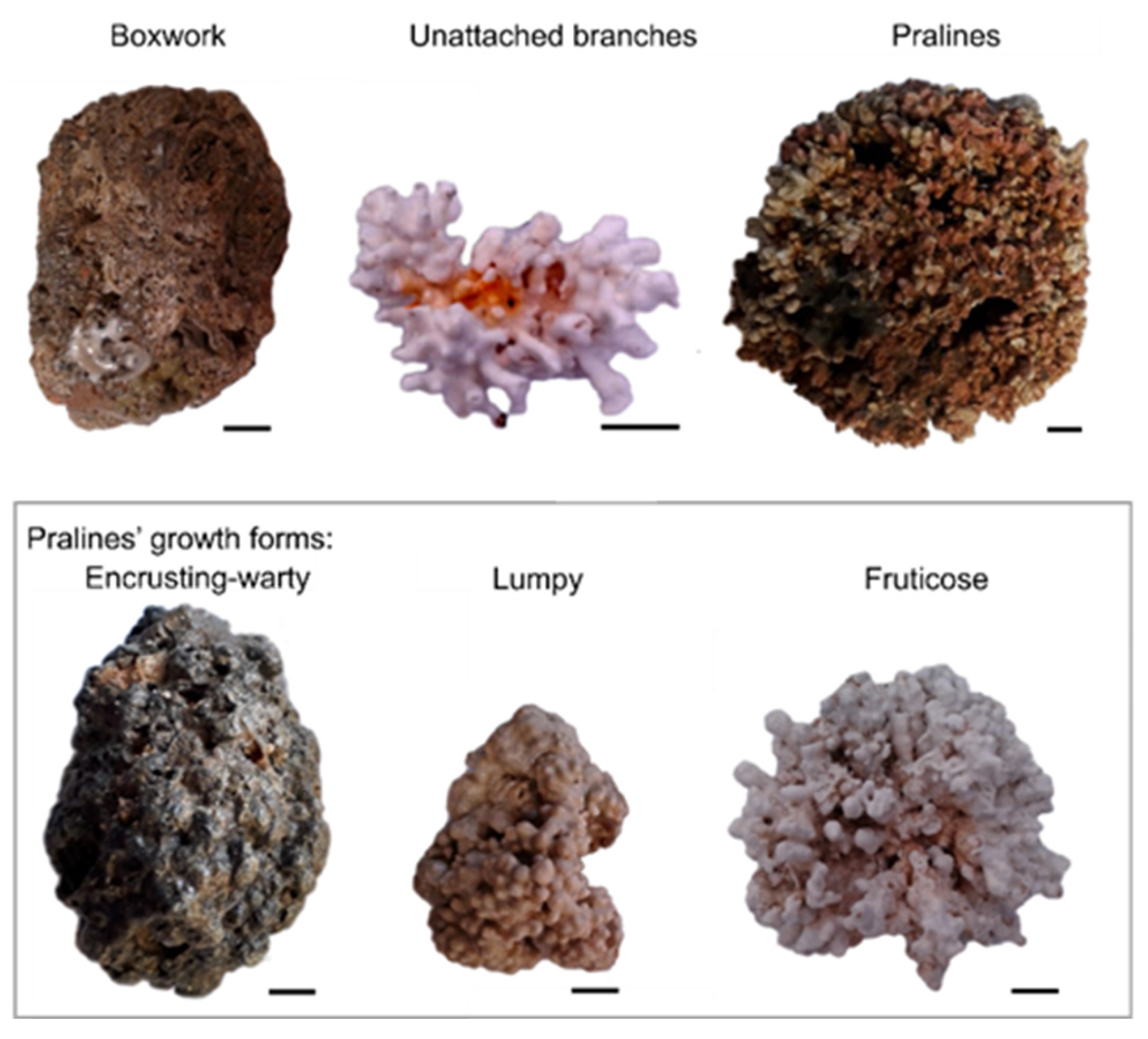
3. Rhodoliths’ General Morphological Characteristics
- (a)
- Boxwork: multi-specific and irregular nodules with inside spaces filled by sediment and a core containing a small pebble or biogenic remnant;
- (b)
- Unattached branches: rhodoliths lacking a macroscopic nucleus and potentially characterized by a high degree of protuberance;
- (c)
- Pralines: compact mono (oligo)specific nodules, with a lytic or biogenic nucleus, with protuberances that have developed strongly on the surface.
- (a)
- Warty growth: branches can be cylindrical or compact, and are typically organized radially;
- (b)
- Lumpy growth: protuberances are enlarged, numerous and contiguous and rarely branched;
- (c)
- Fruticose growth: branches are remarkably detached from each other.
4. Associated Biota
Functional Approach
5. Ecological Importance as Regulation Services
6. Climate Change and Anthropogenic Impacts
Environmental Stressors
7. Rhodolith Bed Preservation and Protection Initiatives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basso, D. Carbonate production by calcareous red algae and global change. Geodiversitas 2012, 34, 13–33. [Google Scholar] [CrossRef]
- Hernández-Kantún, J.J.; Riosmena-Rodriguez, R.; Hall-Spencer, J.M.; Peña, V.; Maggs, C.A.; Rindi, F. Phylogenetic analysis of rhodolith formation in the Corallinales (Rhodophyta). Eur. J. Phycol. 2015, 50, 46–61. [Google Scholar] [CrossRef]
- Riosmena-Rodríguez, R.; Nelson, W.; Aguirre, J. Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Coastal Research Library; Springer International Publishing: Cham, Switzerland, 2017; Volume 15, ISBN 978-3-319-29313-4. [Google Scholar]
- Costa, D.A.; Massei, K.; Moura, A.; Christoffersen, M.L.; Dolbeth, M. Rhodoliths: Our “rock-and-rolling” underwater friends. Front. Young Minds 2022, 10, 675695. [Google Scholar] [CrossRef]
- Bosence, D.W.J. The occurrence and ecology of recent rhodoliths—A review. In Coated Grains; Peryt, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 225–242. ISBN 9783642688690. [Google Scholar]
- Darrenougue, N.; De Deckker, P.; Payri, C.; Eggins, S.; Fallon, S. Growth and chronology of the rhodolith-forming, coralline red alga Sporolithon durum. Mar. Ecol. Prog. Ser. 2013, 474, 105–119. [Google Scholar] [CrossRef]
- Foster, M.S. Rhodoliths: Between rocks and soft places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Sañé, E.; Chiocci, F.L.; Basso, D.; Martorelli, E. Environmental factors controlling the distribution of rhodoliths: An integrated study based on seafloor sampling, ROV and side scan sonar data, offshore the W-Pontine Archipelago. Cont. Shelf Res. 2016, 129, 10–22. [Google Scholar] [CrossRef]
- Steneck, R.S. The ecology of coralline algal crusts: Convergent patterns and adaptative strategies. Annu. Rev. Ecol. Syst. 1986, 17, 273–303. [Google Scholar] [CrossRef]
- Krayesky-Self, S.; Schmidt, W.E.; Phung, D.; Henry, C.; Sauvage, T.; Camacho, O.; Felgenhauer, B.E.; Fredericq, S. Eukaryotic life inhabits rhodolith-forming coralline algae (Hapalidiales, Rhodophyta), remarkable marine benthic microhabitats. Sci. Rep. 2017, 7, 45850. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Pereira-Filho, G.H.; Bahia, R.G.; Abrantes, D.P.; Veras, P.C.; Matheus, Z. Occurrence and distribution of rhodolith beds on the Fernando de Noronha Archipelago of Brazil. Aquat. Bot. 2012, 101, 41–45. [Google Scholar] [CrossRef]
- Grall, J.; Hall-Spencer, J.M. Problems facing maerl conservation in Brittany. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S55–S64. [Google Scholar] [CrossRef]
- Lemoine, M.D.B. Répartition et Mode de Vie du Maërl (“Lithothamnium calcareum”) Aux Environs de Concarneau (Finistère), par Mme Paul Lemoine; Lemoine, M.D.B., Ed.; Institut Océanographique: Paris, France, 1910. [Google Scholar]
- Bosellini, A.; Ginsburg, R.N. Form and internal structure of recent algal nodules (Rhodolites) from Bermuda. J. Geol. 1971, 79, 669–682. [Google Scholar] [CrossRef]
- Peña, V.; Bárbara, I. Los fondos de maërl en Galicia. In Proceedings of the Boletín de la Sociedad Española de Ficología; Congresso Europeo de Ficología; Sociedad Española de Ficología: Barcelona, Spain, 2007; pp. 11–17. [Google Scholar]
- Leeder, M. Sedimentology and Sedimentary Basins, 2nd ed.; Leeder, M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2011; ISBN 978-1-405-17783-2. [Google Scholar]
- Riosmena-Rodríguez, R. Natural history of rhodolith/maërl beds: Their role in near-shore biodiversity and management. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–26. ISBN 978-3-319-29313-4. [Google Scholar]
- Forever-Príncipe. Assess the Importance of Príncipe’s Rhodolith Beds (as Soon as Possible). Available online: https://forever-principe.com/conservation-projects/rhodolith-beds/ (accessed on 20 June 2022).
- Mazzullo, S.J.; Cys, J.M. Unusual algal-crystalline carbonate coated grains from the Capitan Reef (Permian, Guadalupian), New Mexico, USA. In Coated Grains; Peryt, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 599–608. [Google Scholar]
- McCoy, S.J.; Kamenos, N.A. Coralline algae (Rhodophyta) in a changing world: Integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 2015, 51, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Birkett, D.A.; Maggs, C.; Dring, M.J. An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs; Birkett, D.A., Maggs, C., Dring, M.J., Eds.; UK Marine SACs Project: Oban, Scotland, 1998; Volume 5. [Google Scholar]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith bed diversity in the Gulf of California: The importance of rhodolith structure and consequences of disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S5–S20. [Google Scholar] [CrossRef]
- Riosmena-Rodríguez, R.; Steller, D.L.; Hinojosa-Arango, G.; Foster, M.S. Reefs that rock and roll: Biology and conservation of rhodolith beds in the Gulf of California. In The Gulf of California: Biodiversity and Conservation; Brusca, R.C., Ed.; Arizona University Press and the Arizona Sonora Desert Museum: Tucson, AZ, USA, 2010; pp. 49–71. ISBN 9780816527397. [Google Scholar]
- Rendina, F.; Buonocore, E.; di Montanara, A.C.; Russo, G.F. The scientific research on rhodolith beds: A review through bibliometric network analysis. Ecol. Inform. 2022, 70, 101738. [Google Scholar] [CrossRef]
- Montefalcone, M.; Tunesi, L.; Ouerghi, A. A review of the classification systems for marine benthic habitats and the new updated Barcelona Convention classification for the Mediterranean. Mar. Environ. Res. 2021, 169, 105387. [Google Scholar] [CrossRef]
- Peña, V. Estudio Ficológico de Los Fondos de Maërl y Cascajo en el Noroeste de la Península Ibérica; Universidade da Coruña: A Coruña, Spain, 2010. [Google Scholar]
- Blake, C.; Maggs, C.A. Comparative growth rates and internal banding periodicity of maerl species (Corallinales, Rhodophyta) from northern Europe. Phycologia 2003, 42, 606–612. [Google Scholar] [CrossRef]
- Bosence, D.; Wilson, J. Maerl growth, carbonate production rates and accumulation rates in the ne atlantic. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S21–S31. [Google Scholar] [CrossRef]
- Rivera, M.G.; Riosmena-Rodríguez, R.; Foster, M.S. Age and growth of Lithothamnion muelleri (Corallinales, Rhodophyta) in the southwestern Gulf of California, Mexico. Ciencias Mar. 2004, 30, 235–249. [Google Scholar] [CrossRef]
- Steller, D.L.; Hernández-Ayón, J.M.; Riosmena-Rodríguez, R.; Cabello-Pasini, A. Effect of temperature on photosynthesis, growth and calcification rates of the free-living coralline alga Lithophyllum margaritae. Ciencias Mar. 2007, 33, 441–456. [Google Scholar] [CrossRef]
- Bassi, D.; Nebelsick, J.H.; Checconi, A.; Hohenegger, J.; Iryu, Y. Present-day and fossil rhodolith pavements compared: Their potential for analysing shallow-water carbonate deposits. Sediment. Geol. 2009, 214, 74–84. [Google Scholar] [CrossRef]
- Braga, J.C.; Bassi, D. Neogene history of Sporolithon Heydrich (Corallinales, Rhodophyta) in the Mediterranean region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 243, 189–203. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Cusack, M.; Huthwelker, T.; Lagarde, P.; Scheibling, R.E. Mg-lattice associations in red coralline algae. Geochim. Cosmochim. Acta 2009, 73, 1901–1907. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Cusack, M.; Moore, P.G. Coralline algae are global palaeothermometers with bi-weekly resolution. Geochim. Cosmochim. Acta 2008, 72, 771–779. [Google Scholar] [CrossRef]
- Erickson-Davis, M. Revealed for the First Time: The Surprising Biodiversity of Algae ‘Reefs’. Available online: https://news.mongabay.com/2014/03/revealed-for-the-first-time-the-surprising-biodiversity-of-algae-reefs/ (accessed on 20 June 2022).
- Aguado-Giménez, F.; Ruiz-Fernández, J.M. Influence of an experimental fish farm on the spatio-temporal dynamic of a Mediterranean maërl algae community. Mar. Environ. Res. 2012, 74, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, A.B.; Tâmega, F.T.D.S.; Andrade, M.; Coutinho, R.; Figueiredo, M.A.D.O. Experimental effects of sediment burial and light attenuation on two coralline algae of a deep water rhodolith bed in Rio de Janeiro, Brazil. Cryptogam. Algol. 2014, 35, 67–76. [Google Scholar] [CrossRef]
- Aguirre, J.; Braga, J.C.; Martín, J.M.; Betzler, C. Palaeoenvironmental and stratigraphic significance of Pliocene rhodolith beds and coralline algal bioconstructions from the Carboneras Basin (SE Spain). Geodiversitas 2012, 34, 115–136. [Google Scholar] [CrossRef]
- Prager, E.J.; Ginsburg, R.N. Carbonate nodule growth on Florida’s outer shelf and its implications for fossil interpretations. Palaios 1989, 4, 310–312. [Google Scholar] [CrossRef]
- Aguirre, J.; Braga, J.; Martín, J. Algal nodules in the Upper Pliocene deposits at the coast of Cadiz (S Spain). In Studies on Fossil Benthic Algae; Barattolo, F., De Castro, P., Parente, M., Eds.; Bollettino della Società paleontologica italiana: Modena, Italy, 1993; Volume 1, pp. 1–7. ISBN 9788870002171. [Google Scholar]
- Costa, D.A. Environmental Education and the Ecological-Taxonomic Study of Marine Invertebrates Associated with Rhodoliths/Maërl Beds in Tropical Coast. Ph.D. Thesis, Federal University of Paraíba (UFPB), João Pessoa, Paraíba, Brazil, 2020. [Google Scholar]
- Littler, M.M.; Littler, D.S. Models of tropical reefs biogenesis: The contribution of algae. In Progress in Phycological Research; Round, F.E., Chapman, V.J., Eds.; Biopress: London, UK, 1984; pp. 323–364. ISBN 9780948737091. [Google Scholar]
- Adey, W.H.; Steneck, R.S. Thermogeography over time creates biogeographic regions: A temperature/space/time-integrated model and an abundance-weighted test for benthic marine algae. J. Phycol. 2001, 37, 677–698. [Google Scholar] [CrossRef]
- Hernandez-Kantun, J.J.; Hall-Spencer, J.M.; Grall, J.; Adey, W.; Rindi, F.; Maggs, C.A.; Bárbara, I.; Peña, V. North Atlantic rhodolith beds. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 265–279. ISBN 9783319293158. [Google Scholar]
- Konar, B.; Riosmena-Rodriguez, R.; Iken, K. Rhodolith bed: A newly discovered habitat in the North Pacific Ocean. Bot. Mar. 2006, 49, 355–359. [Google Scholar] [CrossRef]
- Fredericq, S.; Krayesky-Self, S.; Sauvage, T.; Richards, J.; Kittle, R.; Arakaki, N.; Hickerson, E.; Schmidt, W.E. The critical importance of rhodoliths in the life cycle completion of both macro- and microalgae, and as holobionts for the establishment and maintenance of marine biodiversity. Front. Mar. Sci. 2019, 5, 502. [Google Scholar] [CrossRef]
- Henriques, M.C.; Villas-Boas, A.; Rodriguez, R.R.; Figueiredo, M.A.O. New records of rhodolith-forming species (Corallinales, Rhodophyta) from deep water in Espírito Santo State, Brazil. Helgol. Mar. Res. 2012, 66, 219–231. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Moura, R.L.; Bastos, A.C.; Salgado, L.T.; Sumida, P.Y.; Guth, A.Z.; Francini-Filho, R.B.; Pereira-Filho, G.H.; Abrantes, D.P.; Brasileiro, P.S.; et al. Rhodolith beds are major CaCO3 bio-factories in the Tropical South West Atlantic. PLoS ONE 2012, 7, e35171. [Google Scholar] [CrossRef] [PubMed]
- Milliman, J.D.; Amaral, C.A.B. Economic potential of Brazilian continental margin sediments. In Proceedings of the Anais do XXVIII Congresso Brasileiro de Geologia; Sociedade Brasileira de Geologia: Porto Alegre, Brazil, 1974; pp. 335–344. [Google Scholar]
- Moura, R.L.; Amado-Filho, G.M.; Moraes, F.C.; Brasileiro, P.S.; Salomon, P.S.; Mahiques, M.M.; Bastos, A.C.; Almeida, M.G.; Silva, J.M.; Araujo, B.F.; et al. An extensive reef system at the Amazon River mouth. Sci. Adv. 2016, 2, e1501252. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.; Antunes, C.; Costa, D.A. Insights into the migration routes and historical dispersion of species surviving the Messinian Crisis: The case of Patella ulyssiponensis and epizoic rhodolith Lithophyllum hibernicum. Hydrobiology 2022, 1, 10–38. [Google Scholar] [CrossRef]
- OBIS. Corallinales P.C. Silva & H.W. Johansen. 1986. Available online: https://obis.org/taxon/15308 (accessed on 17 January 2023).
- BIOMAERL Team. BIOMAERL: Maerl Biodiversity; Functional Structure and Anthropogenic Impacts; BIOMAERL: Millport, Scotland, 1999. [Google Scholar]
- Hernandez-Kantun, J.J.; Rindi, F.; Adey, W.H.; Heesch, S.; Peña, V.; Le Gall, L.; Gabrielson, P.W. Sequencing type material resolves the identity and distribution of the generitype Lithophyllum incrustans, and related European species L. hibernicum and L. bathyporum (Corallinales, Rhodophyta). J. Phycol. 2015, 51, 791–807. [Google Scholar] [CrossRef]
- European Red List of Habitats. Rhodolith Beds in the Mediterranean; European Environment Agency: Copenhagen, Denmark, 2016. [Google Scholar]
- NatureScot. Scotland’s Nature Agency. Available online: https://www.nature.scot/ (accessed on 17 January 2023).
- Maneveldt, G.W.; Van der Merwe, E.; Keats, D.W. Updated keys to the non-geniculate coralline red algae (Corallinophycidae, Rhodophyta) of South Africa. S. Afr. J. Bot. 2016, 106, 158–164. [Google Scholar] [CrossRef]
- Peña, V.; Rousseau, F.; De Reviers, B.; Le Gall, L. First assessment of the diversity of coralline species forming maerl and rhodoliths in Guadeloupe, Caribbean using an integrative systematic approach. Phytotaxa 2014, 190, 190–215. [Google Scholar] [CrossRef]
- Braga, J.; Jaramillo-Vogel, D.; Foubert, A.; Atnafu, B.; Kidane, T.; Negga, H. Coralline algae in Pleistocene reefs in the Danakil Depression (Afar Triangle, Ethiopia). In Proceedings of the Abstract Book of the VI International Rhodolith Workshop; Marine Station of Roscoff: Roscoff, France, 2018; p. 32. [Google Scholar]
- Johnson, M.E.; Ledesma-Vázquez, J.; Ramalho, R.S.; da Silva, C.M.; Rebelo, A.C.; Santos, A.; Baarli, B.G.; Mayoral, E.; Cachão, M. Taphonomic range and sedimentary dynamics of modern and fossil rhodolith beds: Macaronesian realm (North Atlantic Ocean). In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 221–261. ISBN 978-3-319-29313-4. [Google Scholar]
- Kato, A.; Baba, M.; Matsuda, S.; Iryu, Y. Western Pacific. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 335–347. ISBN 978-3-319-29313-4. [Google Scholar]
- Rösler, A.; Pretkovic, V.; Novak, V.; Renema, W.; Braga, J.C. Coralline algae from the Miocene Mahakam Delta (East Kalimantan, Southeast Asia). Palaios 2015, 30, 83–93. [Google Scholar] [CrossRef]
- Teichert, S. Rhodoliths (Corallinales, Rhodophyta) as a Biosedimentary System in Arctic Environments (Svalbard Archipelago, Norway). Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2013. [Google Scholar]
- Teichert, S.; Woelkerling, W.; Rüggeberg, A.; Wisshak, M.; Piepenburg, D.; Meyerhöfer, M.; Form, A.; Büdenbender, J.; Freiwald, A. Rhodolith beds (Corallinales, Rhodophyta) and their physical and biological environment at 80°31′N in Nordkappbukta (Nordaustlandet, Svalbard Archipelago, Norway). Phycologia 2012, 51, 371–390. [Google Scholar] [CrossRef]
- Matsuda, S.; Iryu, Y. Rhodoliths from deep fore-reef to shelf areas around Okinawa-jima, Ryukyu Islands, Japan. Mar. Geol. 2011, 282, 215–230. [Google Scholar] [CrossRef]
- Ryan, D.A.; Brooke, B.P.; Collins, L.B.; Kendrick, G.A.; Baxter, K.J.; Bickers, A.N.; Siwabessy, P.J.W.; Pattiaratchi, C.B. The influence of geomorphology and sedimentary processes on shallow-water benthic habitat distribution: Esperance Bay, Western Australia. Estuar. Coast. Shelf Sci. 2007, 72, 379–386. [Google Scholar] [CrossRef]
- Harvey, A.S.; Harvey, R.M.; Merton, E. The distribution, significance and vulnerability of Australian rhodolith beds: A review. Mar. Freshw. Res. 2016, 68, 411–428. [Google Scholar] [CrossRef]
- Pereira-Filho, G.H.; Francini-Filho, R.B.; Pierozzi-Jr, I.; Pinheiro, H.T.; Bastos, A.C.; de Moura, R.L.; Moraes, F.C.; Matheus, Z.; da Gama Bahia, R.; Amado-Filho, G.M. Sponges and fish facilitate succession from rhodolith beds to reefs. Bull. Mar. Sci. 2015, 91, 45–46. [Google Scholar] [CrossRef]
- Perry, C.T.; Edinger, E.N.; Kench, P.S.; Murphy, G.N.; Smithers, S.G.; Steneck, R.S.; Mumby, P.J. Estimating rates of biologically driven coral reef framework production and erosion: A new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 2012, 31, 853–868. [Google Scholar] [CrossRef]
- Nalin, R.; Nelson, C.S.; Basso, D.; Massari, F. Rhodolith-bearing limestones as transgressive marker beds: Fossil and modern examples from North Island, New Zealand. Sedimentology 2007, 55, 249–274. [Google Scholar] [CrossRef]
- Marshall, J.F.; Tsuji, Y.; Matsuda, H.; Davies, P.J.; Iryu, Y.; Honda, N.; Satoh, Y. Quaternary and Tertiary subtropical carbonate platform development on the continental margin of southern Queensland, Australia. In Reefs and Carbonate Platforms in the Pacific and Indian Oceans; Camoin, G.F., Davies, P.J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 1998; pp. 163–195. [Google Scholar]
- Martin, J.M.; Braga, J.C.; Konishi, K.; Pigram, C.J. A model for the development of rhodoliths on platforms influenced by storms: The Middle Miocene carbonates of the Marion Plateau (Northeastern Australia). In Proceedings of the Ocean Drilling Program, Scientific Results; McKenzie, J.A., Davies, P.J., Palmer-Julson, A., Betzler, C.G., Brachert, T.C., Chen, M.-P.P., Crumière, J.-P., Dix, G.R., Droxler, A.W., Feary, D.A., et al., Eds.; Ocean Drilling Program: Townsville, Australia, 1993; Volume 133, pp. 455–460. [Google Scholar]
- Brodie, J.; Zuccarello, G.C. Systematics of the species rich algae: Red algal classification, phylogeny and speciation. In Reconstructing the Tree of Life: Taxonomy and Systematics of Species Rich Taxa; Hodkinson, T.R., Parnell, J.A.N., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2007; pp. 317–330. ISBN 139780849395796. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. Order Corallinales. Available online: https://www.algaebase.org/browse/taxonomy/#4621 (accessed on 25 May 2023).
- Horta, P.A.; Riul, P.; Amado Filho, G.M.; Gurgel, C.F.D.; Berchez, F.; Nunes, J.M.d.C.; Scherner, F.; Pereira, S.; Lotufo, T.; Peres, L.; et al. Rhodoliths in Brazil: Current knowledge and potential impacts of climate change. Braz. J. Oceanogr. 2016, 64, 117–136. [Google Scholar] [CrossRef]
- Sciberras, M.; Rizzo, M.; Mifsud, J.R.; Camilleri, K.; Borg, J.A.; Lanfranco, E.; Schembri, P.J. Habitat structure and biological characteristics of a maerl bed off the northeastern coast of the Maltese Islands (central Mediterranean). Mar. Biodivers. 2009, 39, 251–264. [Google Scholar] [CrossRef]
- Dias, G.T.M. Marine bioclasts: Calcareous algae. Rev. Bras. Geofísica 2000, 18, 307–318. [Google Scholar] [CrossRef]
- García-Sanz, S.; Navarro, P.G.; Landeira, J.M.; Tuya, F. Colonization patterns of decapods into artificial collectors: Seasonality between habitat patches. J. Crustac. Biol. 2014, 34, 431–441. [Google Scholar] [CrossRef]
- Basso, D. Deep rhodolith distribution in the Pontian Islands, Italy: A model for the paleoecology of a temperate sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 137, 173–187. [Google Scholar] [CrossRef]
- Woelkerling, W.J.; Irvine, L.M.; Harvey, A.S. Growth-forms in non-geniculate coralline red algae (Coralliinales, Rhodophyta). Aust. Syst. Bot. 1993, 6, 277–293. [Google Scholar] [CrossRef]
- Costa, D.A.; Dolbeth, M.; Prata, J.; Silva, F.A.; Silva, G.M.B.; Freitas, P.R.S.; Christoffersen, M.L.; Lima, S.F.B.; Massei, K.; Lucena, R.F.P. Marine invertebrates associated with rhodoliths/maërl beds from northeast Brazil (State of Paraíba). Biodivers. Data J. 2021, 9, e62736. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.A.O.; Santos de Menezes, K.; Costa-Paiva, E.M.; Paiva, P.C.; Ventura, C.R.R. Experimental evaluation of rhodoliths as living substrata for infauna at the Abrolhos Bank, Brazil. Ciencias Mar. 2007, 33, 427–440. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; McGlathery, K.J.; Holmer, M.; Silliman, B.R. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 2010, 50, 158–175. [Google Scholar] [CrossRef]
- Basso, D.; Babbini, L.; Kaleb, S.; Bracchi, V.A.; Falace, A. Monitoring deep Mediterranean rhodolith beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 549–561. [Google Scholar] [CrossRef]
- Prata, J.; Costa, D.A.; Manso, C.L.C.; Crispim, M.C.; Christoffersen, M.L. Echinodermata associated to rhodoliths from Seixas Beach, State of Paraíba, Northeast Brazil. Biota Neotrop. 2017, 17, e20170363. [Google Scholar] [CrossRef]
- Costa, D.A. Assembleias de Poliquetas (Annelida), Associados aos Rodolitos (Corallinophycidae, Rhodophyta), na Praia do Seixas, João Pessoa, Paraíba, Brasil. Master’s Thesis, Federal University of Paraíba (UFPB), João Pessoa, Paraíba, Brazil, 2016. [Google Scholar]
- Hinojosa-Arango, G.; Riosmena-Rodriguez, R. Influence of rhodolith-forming species and growth-form on associated fauna of rhodolith beds in the Central-West Gulf of California, Mexico. Mar. Ecol. 2004, 25, 109–127. [Google Scholar] [CrossRef]
- Riul, P.; Lacouth, P.; Pagliosa, P.R.; Christoffersen, M.L.; Horta, P.A. Rhodolith beds at the easternmost extreme of South America: Community structure of an endangered environment. Aquat. Bot. 2009, 90, 315–320. [Google Scholar] [CrossRef]
- Scherner, F.; Riul, P.; Bastos, E.; Bouzon, Z.L.; Pagliosa, P.R.; Blankensteyn, A.; Oliveira, E.C.; Horta, P.A. Herbivory in a rhodolith bed: A structuring factor? Panam. J. Aquat. Sci. 2010, 5, 358–366. [Google Scholar]
- Costa, D.A.; Lucena, R.F.P.; Silva, F.A.; Silva, G.M.B.; Massei, K.; Christoffersen, M.L.; Dolbeth, M. Importance of rhodoliths as habitats for benthic communities in impacted environments. Reg. Stud. Mar. Sci. 2021, 48, 102055. [Google Scholar] [CrossRef]
- Gagnon, P.; Matheson, K.; Stapleton, M. Variation in rhodolith morphology and biogenic potential of newly discovered rhodolith beds in Newfoundland and Labrador (Canada). Bot. Mar. 2012, 55, 85–99. [Google Scholar] [CrossRef]
- Perry, F.; Tyler-Walters, H.; Garrard, S.L. Lithothamnion corallioides Maerl Beds on Infralittoral Muddy Gravel. Available online: https://www.marlin.ac.uk/habitats/detail/219/lithothamnion_corallioides_maerl_beds_on_infralittoral_muddy_gravel (accessed on 31 May 2023).
- Berlandi, R.M.; Figueiredo, M.A.O.; Paiva, P.C. Rhodolith Morphology and the Diversity of Polychaetes Off the Southeastern Brazilian Coast. J. Coast. Res. 2012, 28, 280–287. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Maneveldt, G.W.; Pereira-Filho, G.H.; Manso, R.C.C.; Bahia, R.G.; Barros-Barreto, M.B.; Guimarães, S.M.P.B. Seaweed diversity associated with a Brazilian tropical rhodolith bed. Ciencias Mar. 2010, 36, 371–391. [Google Scholar] [CrossRef]
- Klein, J.C.; Verlaque, M. Macroalgal assemblages of disturbed coastal detritic bottoms subject to invasive species. Estuar. Coast. Shelf Sci. 2009, 82, 461–468. [Google Scholar] [CrossRef]
- Joher, S.; Ballesteros, E.; Rodríguez-Prieto, C. Contribution to the study of deep coastal detritic bottoms: The algal communities of the continental shelf off the Balearic Islands, Western Mediterranean. Mediterr. Mar. Sci. 2015, 16, 573–590. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Cosme, M.; Tuya, F.; Espino, F.; Haroun, R. Effect of depth and seasonality on the functioning of rhodolith seabeds. Estuar. Coast. Shelf Sci. 2020, 235, 106579. [Google Scholar] [CrossRef]
- De Grave, S. The influence of sedimentary heterogeneity on within maerl bed differences in infaunal crustacean community. Estuar. Coast. Shelf Sci. 1999, 49, 153–163. [Google Scholar] [CrossRef]
- Hall-Spencer, J. Conservation issues relating to maerl beds as habitats for molluscs. J. Conchol. 1998, 36, 271–286. [Google Scholar]
- Kamenos, N.A.; Moore, P.G.; Hall-Spencer, J.M. Maerl grounds provide both refuge and high growth potential for juvenile queen scallops (Aequipecten opercularis L.). J. Exp. Mar. Bio. Ecol. 2004, 313, 241–254. [Google Scholar] [CrossRef]
- Steller, D.L.; Cáceres-Martínez, C. Coralline algal rhodoliths enhance larval settlement and early growth of the Pacific calico scallop Argopecten ventricosus. Mar. Ecol. Prog. Ser. 2009, 396, 49–60. [Google Scholar] [CrossRef]
- Otero-Ferrer, F.; Mannarà, E.; Cosme, M.; Falace, A.; Montiel-Nelson, J.A.; Espino, F.; Haroun, R.; Tuya, F. Early-faunal colonization patterns of discrete habitat units: A case study with rhodolith-associated vagile macrofauna. Estuar. Coast. Shelf Sci. 2019, 218, 9–22. [Google Scholar] [CrossRef]
- Lueder, S.; Narasimhan, K.; Olivo, J.; Cabrera, D.; Jurado, J.G.; Greenstein, L.; Karubian, J. Functional traits, species diversity and species composition of a neotropical palm community vary in relation to forest age. Front. Ecol. Evol. 2022, 10, 678125. [Google Scholar] [CrossRef]
- Martin, S.; Clavier, J.; Chauvaud, L.; Thouzeau, G. Community metabolism in temperate maerl beds. II. Nutrient fluxes. Mar. Ecol. Prog. Ser. 2007, 335, 31–41. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Strong, S.C.; Shenoy, D.M.; Wilson, S.T.; Hatton, A.D.; Moore, P.G. Red coralline algae as a source of marine biogenic dimethylsulphoniopropionate. Mar. Ecol. Prog. Ser. 2008, 372, 61–66. [Google Scholar] [CrossRef]
- Mao, J.; Burdett, H.L.; McGill, R.A.R.; Newton, J.; Gulliver, P.; Kamenos, N.A. Carbon burial over the last four millennia is regulated by both climatic and land use change. Glob. Chang. Biol. 2020, 26, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, L.H.; Kamenos, N.A. Reviews and syntheses: Calculating the global contribution of coralline algae to total carbon burial. Biogeosciences 2015, 12, 6429–6441. [Google Scholar] [CrossRef]
- Hester, E.R.; Barott, K.L.; Nulton, J.; Vermeij, M.J.; Rohwer, F.L. Stable and sporadic symbiotic communities of coral and algal holobionts. ISME J. 2016, 10, 1157–1169. [Google Scholar] [CrossRef]
- Cavalcanti, G.S.; Shukla, P.; Morris, M.; Ribeiro, B.; Foley, M.; Doane, M.P.; Thompson, C.C.; Edwards, M.S.; Dinsdale, E.A.; Thompson, F.L. Rhodoliths holobionts in a changing ocean: Host-microbes interactions mediate coralline algae resilience under ocean acidification. BMC Genom. 2018, 19, 701. [Google Scholar] [CrossRef]
- Webster, N.S.; Reusch, T.B.H. Microbial contributions to the persistence of coral reefs. ISME J. 2017, 11, 2167–2174. [Google Scholar] [CrossRef]
- Gherardi, D.F.M.; Bosence, D.W.J. Modeling of the ecological succession of encrusting organisms in recent coralline-algal frameworks from Atol Das Rocas, Brazil. Palaios 1999, 14, 145–158. [Google Scholar] [CrossRef]
- Tierney, P.W.; Johnson, M.E. Stabilization role of crustose coralline algae during Late Pleistocene reef development on Isla Cerralvo, Baja California Sur (Mexico). J. Coast. Res. 2012, 28, 244–254. [Google Scholar] [CrossRef]
- UK Marine SACS Project. Nature and Importance of Maerl Beds. Available online: http://www.ukmarinesac.org.uk/communities/maerl/m1_1.htm (accessed on 27 August 2022).
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; Intergovernmental Panel on Climate Change: New York, NY, USA, 2014; ISBN 9781107641655. [Google Scholar]
- Kelaher, B.P.; Mamo, L.T.; Provost, E.; Litchfield, S.G.; Giles, A.; Butcherine, P. Influence of ocean warming and acidification on habitat-forming coralline algae and their associated molluscan assemblages. Glob. Ecol. Conserv. 2022, 35, e02081. [Google Scholar] [CrossRef]
- Millero, F.J. The marine inorganic carbon cycle. Chem. Rev. 2007, 107, 308–341. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Hall-Spencer, J.M. Effects of ocean warming and acidification on rhodolith/maërl beds. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 55–85. ISBN 978-3-319-29313-4. [Google Scholar]
- Martone, P.T.; Alyono, M.; Stites, S. Bleaching of an intertidal coralline alga: Untangling the effects of light, temperature, and desiccation. Mar. Ecol. Prog. Ser. 2010, 416, 57–67. [Google Scholar] [CrossRef]
- Fredericq, S.; Arakaki, N.; Camacho, O.; Gabriel, D.; Krayesky, D.; Self-Krayesky, S.; Rees, G.; Richards, J.; Sauvage, T.; Venera-Ponton, D.; et al. A dynamic approach to the study of rhodoliths: A case study for the Northwestern Gulf of Mexico. Cryptogam. Algol. 2014, 35, 77–98. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef]
- Martin, S.; Gattuso, J.-P. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob. Chang. Biol. 2009, 15, 2089–2100. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Hepburn, C.D.; Pilditch, C.A.; Hurd, C.L. Concentration boundary layers around complex assemblages of macroalgae: Implications for the effects of ocean acidification on understory coralline algae. Limnol. Oceanogr. 2013, 58, 121–130. [Google Scholar] [CrossRef]
- Hurd, C.L.; Cornwall, C.E.; Currie, K.; Hepburn, C.D.; McGraw, C.M.; Hunter, K.A.; Boyd, P.W. Metabolically induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: A mechanism for differential susceptibility? Glob. Chang. Biol. 2011, 17, 3254–3262. [Google Scholar] [CrossRef]
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 2009, 37, 1131–1134. [Google Scholar] [CrossRef]
- Martin, S.; Cohu, S.; Vignot, C.; Zimmerman, G.; Gattuso, J.-P. One-year experiment on the physiological response of the Mediterranean crustose coralline alga, Lithophyllum cabiochae, to elevated pCO2 and temperature. Ecol. Evol. 2013, 3, 676–693. [Google Scholar] [CrossRef] [PubMed]
- Horta, P.A.; Berchez, F.A.S.; Nunes, J.M.D.C.; Scherner, F.; Pereira, S.M.B.; Riul, P.; Lotufo, T.M.C.; Peres, L.M.C.; Sissini, M.N.; Rosa, J.; et al. Monitoramento de banco de rodolitos. In Protocolos Para o Monitoramento de Habitats Bentônicos Costeiros; Turra, A., Denadai, M.R., Eds.; Instituto Oceanográfico da Universidade de São Paulo: São Paulo, Brazil, 2015; pp. 48–61. ISBN 978-85-98729-25-1. [Google Scholar]
- Hall-Spencer, J.; Bamber, R. Effects of salmon farming on benthic Crustacea. Ciencias Mar. 2007, 33, 353–366. [Google Scholar] [CrossRef]
- Peña, V.; Bárbara, I. Maërl community in the north-western Iberian Peninsula: A review of floristic studies and long-term changes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 339–366. [Google Scholar] [CrossRef]
- Riul, P.; Targino, C.H.; Farias, J.N.; Visscher, P.T.; Horta, P.A. Decrease in Lithothamnion sp. (Rhodophyta) primary production due to the deposition of a thin sediment layer. J. Mar. Biol. Assoc. United Kingd. 2008, 88, 17–19. [Google Scholar] [CrossRef]
- Hilmi, N.; Chami, R.; Sutherland, M.D.; Hall-Spencer, J.M.; Lebleu, L.; Benitez, M.B.; Levin, L.A. The role of blue carbon in climate change mitigation and carbon stock conservation. Front. Clim. 2021, 3, 710546. [Google Scholar] [CrossRef]
- Bordehore, C.; Ramos-Esplá, A.A.; Riosmena-Rodríguez, R. Comparative study of two maerl beds with different otter trawling history, southeast Iberian Peninsula. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S43–S54. [Google Scholar] [CrossRef]
- Peña, V.; Bárbara, I.; Grall, J.; Maggs, C.A.; Hall-Spencer, J.M. The diversity of seaweeds on maerl in the NE Atlantic. Mar. Biodivers. 2014, 44, 533–551. [Google Scholar] [CrossRef]
- Sanz-Lázaro, C.; Belando, M.D.; Marín-Guirao, L.; Navarrete-Mier, F.; Marín, A. Relationship between sedimentation rates and benthic impact on maërl beds derived from fish farming in the Mediterranean. Mar. Environ. Res. 2011, 71, 22–30. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Bahia, R.G.; Pereira-Filho, G.H.; Longo, L.L. South Atlantic rhodolith beds: Latitudinal distribution, species composition, structure and ecosystem functions, threats and conservation status. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 299–317. ISBN 978-3-319-29313-4. [Google Scholar]
- Figueiredo, M.A.O.; Coutinho, R.; Villas-Boas, A.B.; Tâmega, F.T.S.; Mariath, R. Deep-water rhodolith productivity and growth in the southwestern Atlantic. J. Appl. Phycol. 2012, 24, 487–493. [Google Scholar] [CrossRef]
- Costa, D.A.; Silva, F.A.; Silva, J.M.L.; Pereira, A.R.; Dolbeth, M.; Christoffersen, M.L.; Lucena, R.F.P. Is tourism affecting polychaete assemblages associated with rhodolith beds in Northeastern Brazil? Rev. Biol. Trop. 2019, 67, S1–S15. [Google Scholar] [CrossRef]
- Lavenère-Wanderley, A.A.; Asp, N.E.; Thompson, F.L.; Siegle, E. Rhodolith mobility potential from seasonal and extreme waves. Cont. Shelf Res. 2021, 228, 104527. [Google Scholar] [CrossRef]
- Carvalho, V.F.; Assis, J.; Serrão, E.A.; Nunes, J.M.; Anderson, A.B.; Batista, M.B.; Barufi, J.B.; Silva, J.; Pereira, S.M.B.; Horta, P.A. Environmental drivers of rhodolith beds and epiphytes community along the South Western Atlantic coast. Mar. Environ. Res. 2020, 154, 104827. [Google Scholar] [CrossRef]
- OSPAR Commission. Maerl Beds. Available online: https://www.ospar.org/ (accessed on 3 April 2023).
- Adey, W.H.; Adey, P.J. Studies on the biosystematics and ecology of the epilithic crustose Corallinaceae of the British Isles. Br. Phycol. J. 1973, 8, 343–407. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M. Biological Studies on Nongeniculate Corallinaceae; University of London: London, UK, 1994. [Google Scholar]
- Barbera, C.; Bordehore, C.; Borg, J.A.; Glémarec, M.; Grall, J.; Hall-Spencer, J.M.; de la Huz, C.; Lanfranco, E.; Lastra, M.; Moore, P.G.; et al. Conservation and management of northeast Atlantic and Mediterranean maerl beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S65–S76. [Google Scholar] [CrossRef]
- Council of the European Union. Council Decision 82/72/EEC of 3 December 1981 Concerning the Conclusion of the Convention on the Conservation of European Wildlife and Natural Habitats (Bern Convention). Available online: https://eur-lex.europa.eu/EN/legal-content/summary/bern-convention.html (accessed on 25 May 2023).
- Council of the European Union. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31992L0043 (accessed on 20 June 2022).
- European Commission. Natura 2000. Available online: http://ec.europa.eu/environment/nature/natura2000/index_en.htm (accessed on 23 March 2023).
- Council of the European Union. Council Regulation (EC) No. 1967/2006 Concerning Management Measures for the Sustainable Exploitation of Fishery Resources in the Mediterranean Sea, Amending Regulation (EEC) No. 2847/93 and Repealing Regulation (EC) No. 1626/94. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1967 (accessed on 25 May 2023).
- Robinson, N.M.; Fernández-García, C.; Riosmena-Rodríguez, R.; Rosas-Alquicira, E.F.; Konar, B.; Chenelot, H.; Jewett, S.C.; Melzer, R.R.; Meyer, R.; Försterra, G.; et al. Eastern Pacific. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 319–333. ISBN 978-3-319-29313-4. [Google Scholar]
- Global Geoparks Network. Global Geoparks Network. Available online: https://globalgeoparksnetwork.org/ (accessed on 20 January 2023).
- Ministry of the Environment of Japan. National Parks & Important Biodiversity Areas of Japan. Available online: http://www.env.go.jp/park/topics/review/attach/pamph1/en_full.pdf (accessed on 23 August 2022).
- Turra, A.; Denadai, M.R. Protocolos Para o Monitoramento de Habitats Bentônicos Costeiros; Turra, A., Denadai, M.R., Eds.; Instituto Oceanográfico da Universidade de São Paulo: São Paulo, Brazil, 2015; ISBN 9788598729251. [Google Scholar]
- Rodolitos-Projeto. ReBentos Rodolitos: Um Oásis de Biodiversidade Marinha. Available online: https://rodolitos.wordpress.com/ (accessed on 20 January 2023).
- McKinley, E.; Fletcher, S. Improving marine environmental health through marine citizenship: A call for debate. Mar. Policy 2012, 36, 839–843. [Google Scholar] [CrossRef]
- Costa, D.A.; Lucena, R.F.P.; Christoffersen, M.L.; Piñeiro-Corbeira, C.; Dolbeth, M. Improving environmental awareness and ocean literacy through hands-on activities in the tropics. Appl. Environ. Educ. Commun. 2022, 21, 120–139. [Google Scholar] [CrossRef]
- Rodolitos. Rodolitos na Educação. Available online: https://rodolitos.wordpress.com/category/rodolitos-na-educacao/ (accessed on 19 January 2023).


| Drivers | Examples of Consequences/Impacts | References |
|---|---|---|
| Dredging/trawling | Production of a plume of fine sediment that amplifies the effect on the remaining organisms; physical perturbation in the fauna and associated algae; release of blue carbon stored in the beds | [129,130,131] |
| Coastal chemical pollution | Impacts the carbon cycle and algae’s ability to sequester carbonate | [117] |
| Aquaculture (including fish farming) | Due to deposition of fine sediments with high organic matter content/waste dispersion, the associated fauna changes, decreasing their complexity and biodiversity | [26,132,133] |
| Petroleum exploration/drilling activities | Disturbs photosynthesis due to sediment suspension; induces the burial of the algae | [134,135] |
| Overexploitation—rhodolith as raw material | Declines rhodoliths and associated fauna | [134] |
| Tourism activities | Alterations in the associated fauna due to increased and seasonal organic pollution; the trampling of the sediment beds containing rhodoliths | [136] |
| Indirect effects of invasive species/ecological competition | Excrement from invasive molluscs (e.g., Crepidula fornicuta) cover the spaces between the stalks of the rhodoliths or the ocean floor is covered by fleshy and/or invasive algae that make rhodoliths more vulnerable [95] | [12,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.d.A.; Dolbeth, M.; Christoffersen, M.L.; Zúñiga-Upegui, P.T.; Venâncio, M.; de Lucena, R.F.P. An Overview of Rhodoliths: Ecological Importance and Conservation Emergency. Life 2023, 13, 1556. https://doi.org/10.3390/life13071556
Costa DdA, Dolbeth M, Christoffersen ML, Zúñiga-Upegui PT, Venâncio M, de Lucena RFP. An Overview of Rhodoliths: Ecological Importance and Conservation Emergency. Life. 2023; 13(7):1556. https://doi.org/10.3390/life13071556
Chicago/Turabian StyleCosta, Dimítri de Araújo, Marina Dolbeth, Martin Lindsey Christoffersen, Pamela Tatiana Zúñiga-Upegui, Márcia Venâncio, and Reinaldo Farias Paiva de Lucena. 2023. "An Overview of Rhodoliths: Ecological Importance and Conservation Emergency" Life 13, no. 7: 1556. https://doi.org/10.3390/life13071556
APA StyleCosta, D. d. A., Dolbeth, M., Christoffersen, M. L., Zúñiga-Upegui, P. T., Venâncio, M., & de Lucena, R. F. P. (2023). An Overview of Rhodoliths: Ecological Importance and Conservation Emergency. Life, 13(7), 1556. https://doi.org/10.3390/life13071556






