The Impact of Post-Stroke Depressive Symptoms on Cognitive Performance in Women and in Men: A 4 Month Prospective Study
Abstract
1. Introduction
2. Methods
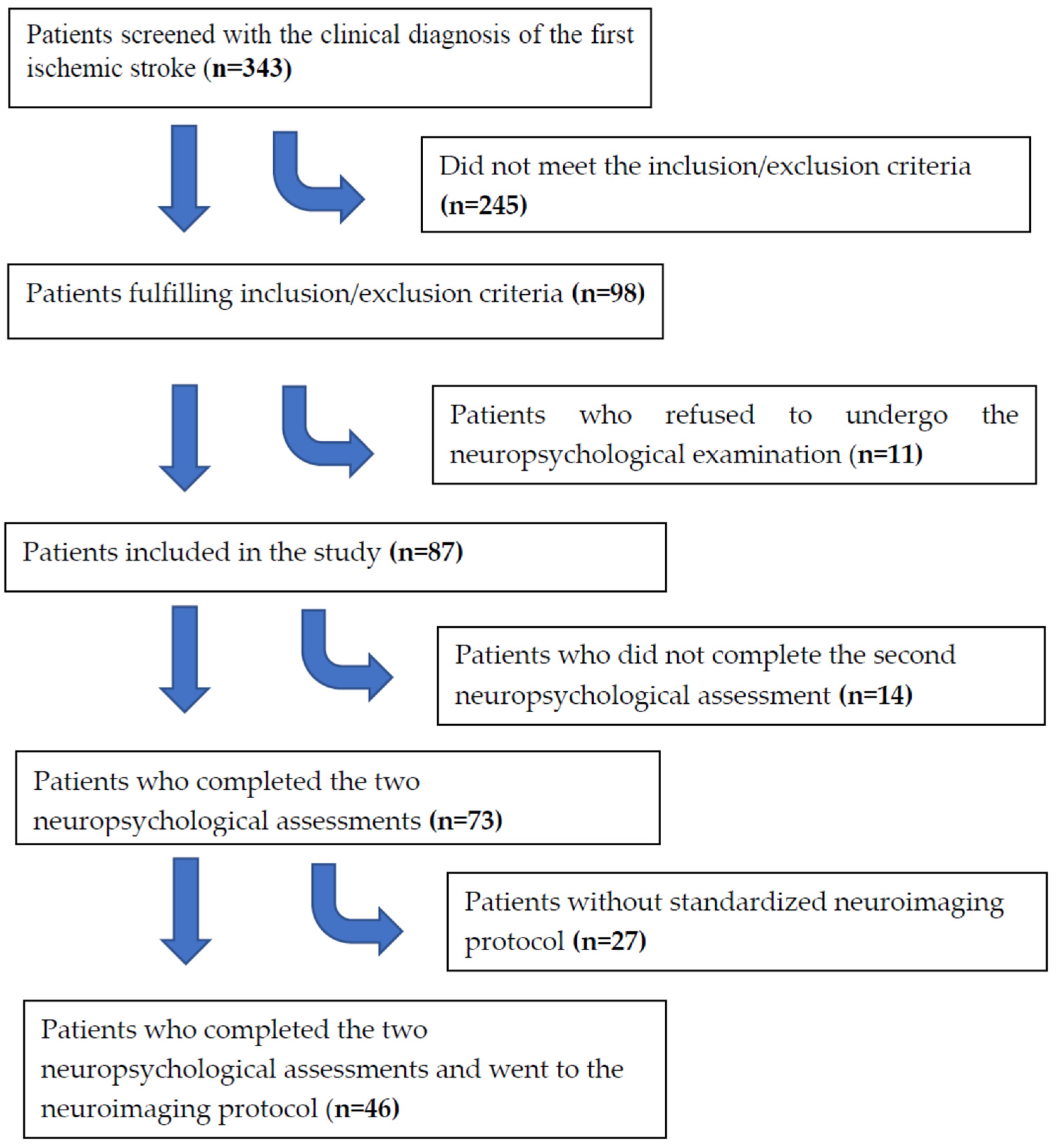
2.1. Sample
2.2. Procedures
2.3. Assessment of Depressive Symptoms
2.4. Neuropsychological Assessment
2.5. Assessment of Functional Status
2.6. MRI Methods and Assessment
2.7. Analysis
3. Results
3.1. Sample, and Men’s and Women’s Characteristics
3.2. Evolution from the First to the Fourth Month after Stroke
3.3. Specificities of Men and Women in the Evolution
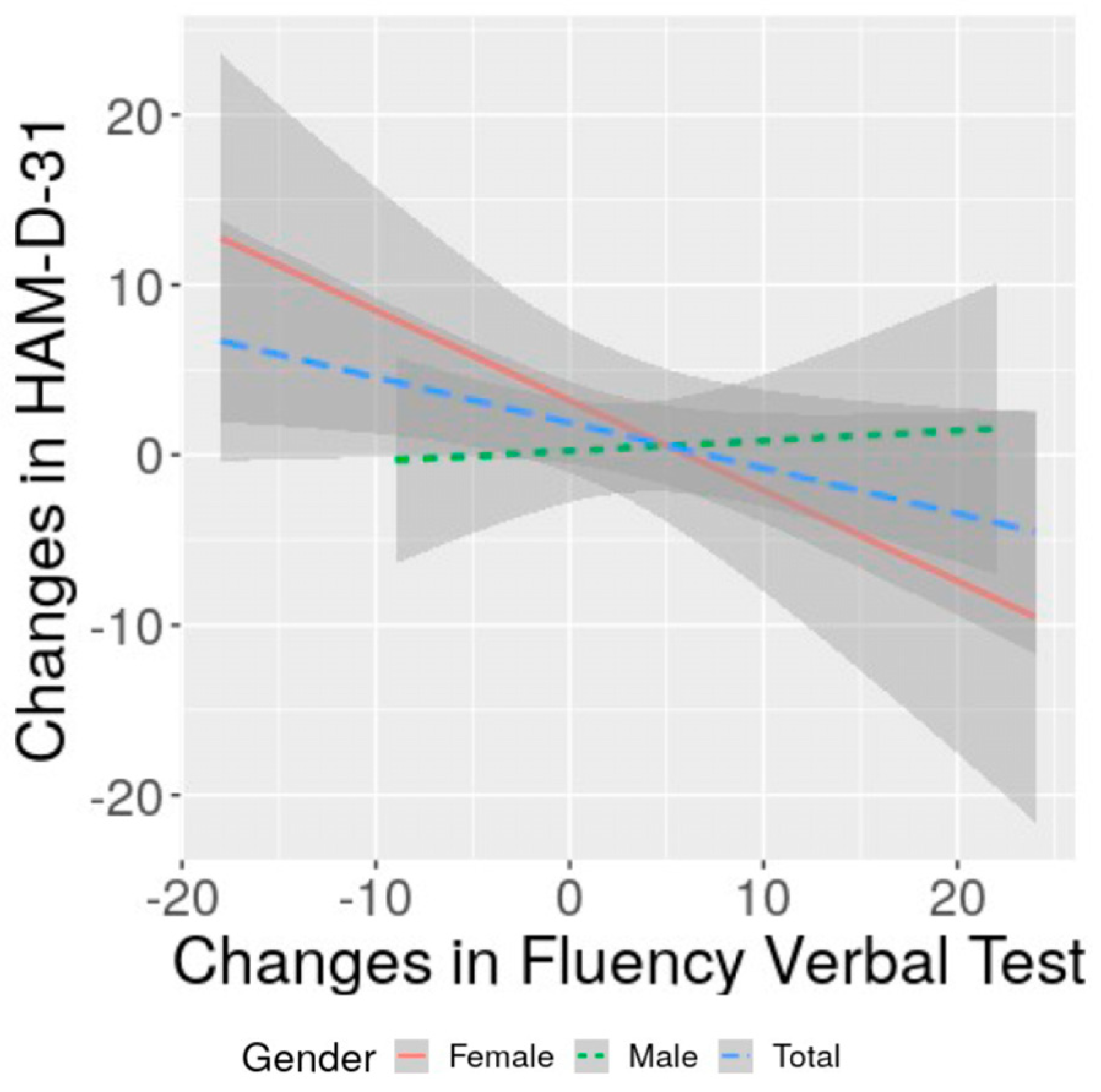
3.4. Correlation of Changes in Severity of Depression with Changes in Cognitive Performance and with Functional Status: Specificities of Women and Men
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordonnier, C.; Sprigg, N.; Sandset, E.C.; Pavlovic, A.; Sunnerhagen, K.S.; Caso, V.; Christensen, H.; Women Initiative for Stroke in Europe, g. Stroke in women—From evidence to inequalities. Nat. Rev. Neurol. 2017, 13, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Appelros, P.; Stegmayr, B.; Terent, A. Sex differences in stroke epidemiology: A systematic review. Stroke 2009, 40, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Leppert, M.H.; Burke, J.F.; Lisabeth, L.D.; Madsen, T.E.; Kleindorfer, D.O.; Sillau, S.; Schwamm, L.H.; Daugherty, S.L.; Bradley, C.J.; Ho, P.M.; et al. Systematic review of sex differences in ischemic strokes among young adults: Are young women disproportionately at risk? Stroke 2022, 53, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; van Os, H.J.A.; van der Weerd, N.; Schoones, J.W.; Heymans, M.W.; Kruyt, N.D.; Visser, M.C.; Wermer, M.J.H. Sex differences in presentation of stroke: A systematic review and meta-analysis. Stroke 2022, 53, 345–354. [Google Scholar] [CrossRef]
- Phan, H.T.; Reeves, M.J.; Blizzard, C.L.; Thrift, A.G.; Cadilhac, D.A.; Sturm, J.; Otahal, P.; Rothwell, P.; Bejot, Y.; Cabral, N.L.; et al. Sex differences in severity of stroke in the instruct study: A meta-analysis of individual participant data. J. Am. Heart Assoc. 2019, 8, e010235. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Amarilla Vallejo, A.; Cantalapiedra Calvete, C.; Rudd, A.; Wolfe, C.; O’Connell, M.D.L.; Douiri, A. Stroke outcomes in women: A population-based cohort study. Stroke 2022, 53, 3072–3081. [Google Scholar] [CrossRef]
- Blomgren, C.; Samuelsson, H.; Blomstrand, C.; Jern, C.; Jood, K.; Claesson, L. Long-term performance of instrumental activities of daily living in young and middle-aged stroke survivors-impact of cognitive dysfunction, emotional problems and fatigue. PLoS ONE 2019, 14, e0216822. [Google Scholar] [CrossRef]
- Kim, K.T.; Chang, W.K.; Jung, Y.S.; Jee, S.; Sohn, M.K.; Ko, S.H.; Shin, Y.I.; Leigh, J.H.; Kim, W.S.; Paik, N.J. Unmet needs for rehabilitative management in common health-related problems negatively impact the quality of life of community-dwelling stroke survivors. Front. Neurol. 2021, 12, 758536. [Google Scholar] [CrossRef]
- Brainin, M.; Tuomilehto, J.; Heiss, W.D.; Bornstein, N.M.; Bath, P.M.; Teuschl, Y.; Richard, E.; Guekht, A.; Quinn, T.; Post Stroke Cognition Study, G. Post-stroke cognitive decline: An update and perspectives for clinical research. Eur. J. Neurol. 2015, 22, 229–238, e213–e226. [Google Scholar] [CrossRef]
- Srikanth, V.K.; Thrift, A.G.; Saling, M.M.; Anderson, J.F.; Dewey, H.M.; Macdonell, R.A.; Donnan, G.A.; Community-Based Prospective Study of Nonaphasic English-Speaking, S. Increased risk of cognitive impairment 3 months after mild to moderate first-ever stroke: A community-based prospective study of nonaphasic english-speaking survivors. Stroke 2003, 34, 1136–1143. [Google Scholar] [CrossRef]
- Baccaro, A.; Wang, Y.P.; Brunoni, A.R.; Candido, M.; Conforto, A.B.; da Costa Leite, C.; Lotufo, P.A.; Bensenor, I.M.; Goulart, A.C. Does stroke laterality predict major depression and cognitive impairment after stroke? Two-year prospective evaluation in the emma study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109639. [Google Scholar] [CrossRef] [PubMed]
- Sagnier, S.; Munsch, F.; Bigourdan, A.; Debruxelles, S.; Poli, M.; Renou, P.; Olindo, S.; Rouanet, F.; Dousset, V.; Tourdias, T.; et al. The influence of stroke location on cognitive and mood impairment. A voxel-based lesion-symptom mapping study. J. Stroke Cerebrovasc. Dis. 2019, 28, 1236–1242. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, X. Post-stroke cognitive impairment: A review focusing on molecular biomarkers. J. Mol. Neurosci. 2020, 70, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Bako, A.T.; Potter, T.; Tannous, J.; Pan, A.P.; Johnson, C.; Baig, E.; Downer, B.; Vahidy, F.S. Sex differences in post-stroke cognitive decline: A population-based longitudinal study of nationally representative data. PLoS ONE 2022, 17, e0268249. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Briceno, E.; Morgenstern, L.B.; Lisabeth, L.D. Poststroke cognitive outcomes: Sex differences and contributing factors. J. Am. Heart Assoc. 2020, 9, e016683. [Google Scholar] [CrossRef]
- Mahadevan, S.; Chan, M.F.; Moghadas, M.; Shetty, M.; Burke, D.T.; Al-Rasadi, K.; Al-Adawi, S. Post-stroke psychiatric and cognitive symptoms in west asia, south asia and africa: A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 3655. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Sheth, B.; Gill, J.; Yadegarfar, M.; Stubbs, B.; Yadegarfar, M.; Meader, N. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatry 2017, 47, 48–60. [Google Scholar] [CrossRef]
- Hackett, M.L.; Pickles, K. Part i: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef]
- Liang, C.; Van Laar Veth, A.J.; Li, Q.; Zheng, D.; Hackett, M.L. Effect of mood on long-term disability in younger stroke survivors: Results from the psychosocial outcomes in stroke (poise) study. Top. Stroke Rehabil. 2022, 29, 286–294. [Google Scholar] [CrossRef]
- Poynter, B.; Shuman, M.; Diaz-Granados, N.; Kapral, M.; Grace, S.L.; Stewart, D.E. Sex differences in the prevalence of post-stroke depression: A systematic review. Psychosomatics 2009, 50, 563–569. [Google Scholar] [CrossRef]
- Sienkiewicz-Jarosz, H.; Milewska, D.; Bochynska, A.; Chelmniak, A.; Dworek, N.; Kasprzyk, K.; Galecka, K.; Szczepanska-Szarej, A.; Chwojnicki, K.; Zyluk, B.; et al. Predictors of depressive symptoms in patients with stroke—A three-month follow-up. Neurol. Neurochir. Pol. 2010, 44, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Douven, E.; Kohler, S.; Rodriguez, M.M.F.; Staals, J.; Verhey, F.R.J.; Aalten, P. Imaging markers of post-stroke depression and apathy: A systematic review and meta-analysis. Neuropsychol. Rev. 2017, 27, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Terroni, L.; Amaro, E.; Iosifescu, D.V.; Tinone, G.; Sato, J.R.; Leite, C.C.; Sobreiro, M.F.; Lucia, M.C.; Scaff, M.; Fraguas, R. Stroke lesion in cortical neural circuits and post-stroke incidence of major depressive episode: A 4-month prospective study. World J. Biol. Psychiatry 2011, 12, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, J.; Xu, Q.; Song, Z.; Dai, J.; Wang, J. Altered functional connectivity in post-ischemic stroke depression: A resting-state functional magnetic resonance imaging study. Eur. J. Radiol. 2018, 100, 156–165. [Google Scholar] [CrossRef]
- Egorova, N.; Cumming, T.; Shirbin, C.; Veldsman, M.; Werden, E.; Brodtmann, A. Lower cognitive control network connectivity in stroke participants with depressive features. Transl. Psychiatry 2018, 7, 4. [Google Scholar] [CrossRef]
- Mayman, N.A.; Tuhrim, S.; Jette, N.; Dhamoon, M.S.; Stein, L.K. Sex differences in post-stroke depression in the elderly. J. Stroke Cerebrovasc. Dis. 2021, 30, 105948. [Google Scholar] [CrossRef]
- Volz, M.; Ladwig, S.; Werheid, K. Gender differences in post-stroke depression: A longitudinal analysis of prevalence, persistence and predictive value of known risk factors. Neuropsychol. Rehabil. 2021, 31, 1–17. [Google Scholar] [CrossRef]
- Lai, S.M.; Duncan, P.W.; Dew, P.; Keighley, J. Sex differences in stroke recovery. Prev. Chronic Dis. 2005, 2, A13. [Google Scholar]
- Williams, O.A.; Demeyere, N. Association of depression and anxiety with cognitive impairment 6 months after stroke. Neurology 2021, 96, e1966–e1974. [Google Scholar] [CrossRef]
- Kang, C. Predictors of post-stroke cognition among geriatric patients: The role of demographics, pre-stroke cognition, and trajectories of depression. Front. Psychol. 2021, 12, 717817. [Google Scholar] [CrossRef]
- Kanellopoulos, D.; Wilkins, V.; Avari, J.; Oberlin, L.; Arader, L.; Chaplin, M.; Banerjee, S.; Alexopoulos, G.S. Dimensions of poststroke depression and neuropsychological deficits in older adults. Am. J. Geriatr. Psychiatry 2020, 28, 764–771. [Google Scholar] [CrossRef] [PubMed]
- WHO. Stroke—1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke 1989, 20, 1407–1431. [Google Scholar] [CrossRef] [PubMed]
- Ducan, M.T. Assessement of normative data of stroop test performance in a group of elementary school students in niterói. J. Bras. Psiquiatr. 2006, 55, 42–48. [Google Scholar]
- Williams, J.B. A structured interview guide for the hamilton depression rating scale. Arch. Gen. Psychiatry 1988, 45, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Jamerson, B.D.; Krishnan, K.R.; Roberts, J.; Krishen, A.; Modell, J.G. Effect of bupropion sr on specific symptom clusters of depression: Analysis of the 31-item hamilton rating scale for depression. Psychopharmacol. Bull. 2003, 37, 67–78. [Google Scholar]
- Wechsler, D. Adult Intelligence Scale-Revised; Psychological Corporation: San Antonio, TX, USA, 1981; Volume 1. [Google Scholar]
- Brandão, E. Adaptação da Esccala Wechsler de Inteligencia Para Adultos iii-Revisada (Wais iii-r). Unpublished. Ph.D. Thesis, Pontificia Universidade Católica-SP, São Paulo, Brazil, 1997. [Google Scholar]
- Benton, A.L.; Hamsher, K.S. Multilingual Aphasia Examination; AJA Associates: Iowa City, IA, USA, 1989. [Google Scholar]
- Spreen, O.S.E. A compendium Neuropsychological Tests; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- Sobreiro, M.F.; Miotto, E.C.; Terroni, L.; Tinone, G.; Iosifescu, D.V.; de Lucia, M.C.; Scaff, M.; Leite Cda, C.; Amaro, E., Jr.; Fraguas, R. Executive function and depressive symptoms of retardation in nonelderly stroke patients. J. Clin. Exp. Neuropsychol. 2014, 36, 636–647. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.C.; Collins, D.L.; Mills, S.R.; Brown, E.D.; Kelly, R.L.; Peters, T.M. 3D statistical neuroanatomical models from 305 mri volumes. In Proceedings of the IEEE-Nuclear Science Symposium and Medical Imaging Conference, San Francisco, CA, USA, 31 October–6 November 1993; pp. 1813–1817. [Google Scholar]
- Friston, K.J.; Frith, C.D.; Fletcher, P.; Liddle, P.F.; Frackowiak, R.S. Functional topography: Multidimensional scaling and functional connectivity in the brain. Cereb. Cortex 1996, 6, 156–164. [Google Scholar] [CrossRef]
- Talairach, P.T.J. Co-Planar Stereotaxic Atlas of the Human Brain; Thieme Medical Publishers Inc: New York, NY, USA, 1988. [Google Scholar]
- Rorden, C.; Brett, M. Stereotaxic display of brain lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Kaiser, U.B.; Chen, G.L.; Manson, J.E.; Goldstein, J.M. Sex and gender differences research design for basic, clinical, and population studies: Essentials for investigators. Endocr. Rev. 2018, 39, 424–439. [Google Scholar] [CrossRef]
- Mohd Zulkifly, M.F.; Ghazali, S.E.; Che Din, N.; Singh, D.K.; Subramaniam, P. A review of risk factors for cognitive impairment in stroke survivors. ScientificWorldJournal 2016, 2016, 3456943. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.M.; Gerraty, R.T. A meta-analytic investigation of neurocognitive deficits in bipolar illness: Profile and effects of clinical state. Neuropsychology 2009, 23, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Helmreich, I.; Wollschlager, D.; Meyer, K.; Kaaden, S.; Reiff, J.; Roll, S.C.; Braus, D.; Tuscher, O.; Muller-Dahlhaus, F.; et al. Early improvement of executive test performance during antidepressant treatment predicts treatment outcome in patients with major depressive disorder. PLoS ONE 2018, 13, e0194574. [Google Scholar] [CrossRef]
- Wroolie, T.E.; Williams, K.E.; Keller, J.; Zappert, L.N.; Shelton, S.D.; Kenna, H.A.; Reynolds, M.F.; Rasgon, N.L. Mood and neuropsychological changes in women with midlife depression treated with escitalopram. J. Clin. Psychopharmacol. 2006, 26, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, J.S.; Holtzer, R. Depressive symptoms are associated with decline over time in verbal fluency performance in female but not male community-dwelling older adults. Exp. Aging Res. 2023, 1–16, ahead of print. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, S.; Kim, S. Gender differences in the impact of depression on cognitive decline among korean older adults. Asia Pac. J. Public Health 2021, 33, 67–75. [Google Scholar] [CrossRef]
- Henry, J.; Crawford, J.R. A meta-analytic review of verbal fluency deficits in depression. J. Clin. Exp. Neuropsychol. 2005, 27, 78–101. [Google Scholar] [CrossRef]
- Degl’Innocenti, A.; Agren, H.; Backman, L. Executive deficits in major depression. Acta Psychiatr. Scand. 1998, 97, 182–188. [Google Scholar] [CrossRef]
- Klumpp, H.; Deldin, P. Review of brain functioning in depression for semantic processing and verbal fluency. Int. J. Psychophysiol. 2010, 75, 77–85. [Google Scholar] [CrossRef]
- Morsund, A.H.; Ellekjaer, H.; Gramstad, A.; Reiestad, M.T.; Midgard, R.; Sando, S.B.; Jonsbu, E.; Naess, H. Cognitive and emotional impairment after minor stroke and non-st-elevation myocardial infarction (nstemi): A prevalence study. Stroke Res. Treat. 2019, 2019, 2527384. [Google Scholar] [CrossRef]
- Kauhanen, M.; Korpelainen, J.T.; Hiltunen, P.; Brusin, E.; Mononen, H.; Maatta, R.; Nieminen, P.; Sotaniemi, K.A.; Myllyla, V.V. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke 1999, 30, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Barker-Collo, S.L. Depression and anxiety 3 months post stroke: Prevalence and correlates. Arch. Clin. Neuropsychol. 2007, 22, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, A.; Pekmezovic, T.; Mijajlovic, M.; Tomic, G.; Zidverc Trajkovic, J. Is the female sex associated with an increased risk for long-term cognitive decline after the first-ever lacunar stroke? Prospective study on small vessel disease cohort. Front. Neurol. 2022, 13, 1052401. [Google Scholar] [CrossRef]
- Kalbouneh, H.M.; Toubasi, A.A.; Albustanji, F.H.; Obaid, Y.Y.; Al-Harasis, L.M. Safety and efficacy of ssris in improving poststroke recovery: A systematic review and meta-analysis. J. Am. Heart Assoc. 2022, 11, e025868. [Google Scholar] [CrossRef]
- Kimura, M.; Robinson, R.G.; Kosier, J.T. Treatment of cognitive impairment after poststroke depression: A double-blind treatment trial. Stroke 2000, 31, 1482–1486. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, F.C.; Guan, B.; Zhang, N.; Wang, A.; Yu, P.; Zhou, L.; Wang, C.Y.; Wang, C. Predictors of remission of early-onset poststroke depression and the interaction between depression and cognition during follow-up. Front. Psychiatry 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.S.; Chang, D.I.; Park, J.H.; Ahn, S.H.; Cha, J.K.; Heo, J.H.; Sohn, S.I.; Lee, B.C.; Kim, D.E.; et al. Depressive symptoms in stroke patients: Are there sex differences? Cerebrovasc. Dis. 2020, 49, 19–25. [Google Scholar] [CrossRef]
- Pinter, D.; Fandler-Hofler, S.; Fruhwirth, V.; Berger, L.; Bachmaier, G.; Horner, S.; Eppinger, S.; Kneihsl, M.; Enzinger, C.; Gattringer, T. Relevance of cognition and emotion for patient-reported quality of life after stroke in working age: An observational cohort study. Front. Neurol. 2022, 13, 869550. [Google Scholar] [CrossRef]
- De Ryck, A.; Brouns, R.; Fransen, E.; Geurden, M.; Van Gestel, G.; Wilssens, I.; De Ceulaer, L.; Marien, P.; De Deyn, P.P.; Engelborghs, S. A prospective study on the prevalence and risk factors of poststroke depression. Cerebrovasc. Dis. Extra 2013, 3, 1–13. [Google Scholar] [CrossRef]
- Rabi-Zikic, T.; Zivanovic, Z.; Dajic, V.; Simic, S.; Ruzicka-Kaloci, S.; Slankamenac, S.; Zikic, M. Predictors of early-onset depression after first-ever stroke. Acta Clin. Croat. 2020, 59, 81–90. [Google Scholar] [CrossRef]
- Rohde, D.; Gaynor, E.; Large, M.; Mellon, L.; Hall, P.; Brewer, L.; Bennett, K.; Williams, D.; Dolan, E.; Callaly, E.; et al. The impact of cognitive impairment on poststroke outcomes: A 5-year follow-up. J. Geriatr. Psychiatry Neurol. 2019, 32, 275–281. [Google Scholar] [CrossRef]
- Wheeler, M.; Williams, O.A.; Johns, L.; Chiu, E.G.; Slavkova, E.D.; Demeyere, N. Unravelling the complex interactions between self-awareness, cognitive change, and mood at 6-months post-stroke using the y-shaped model. Neuropsychol. Rehabil. 2023, 33, 680–702. [Google Scholar] [CrossRef] [PubMed]
- Siotto, M.; Germanotta, M.; Santoro, M.; Cipollini, V.; Guardati, G.; Papadopoulou, D.; Bray, E.; Mastrorosa, A.; Aprile, I. Serotonin levels and cognitive recovery in patients with subacute stroke after rehabilitation treatment. Brain Sci. 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Ding, S.; Ding, S. Astroglia abnormalities in post-stroke mood disorders. Adv. Neurobiol. 2021, 26, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, R.; Zhang, L.; Cao, Z. Altered topology of the structural brain network in patients with post-stroke depression. Front. Neurosci. 2019, 13, 776. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Lan, Y.; Miao, J.; Pan, C.; Sun, W.; Li, G.; Wang, Y.; Zhao, X.; Zhu, Z.; et al. Blood biomarkers of post-stroke depression after minor stroke at three months in males and females. BMC Psychiatry 2022, 22, 162. [Google Scholar] [CrossRef]
- Bonkhoff, A.K.; Bretzner, M.; Hong, S.; Schirmer, M.D.; Cohen, A.; Regenhardt, R.W.; Donahue, K.L.; Nardin, M.J.; Dalca, A.V.; Giese, A.K.; et al. Sex-specific lesion pattern of functional outcomes after stroke. Brain Commun. 2022, 4, fcac020. [Google Scholar] [CrossRef]
- Collie, A.; Maruff, P.; Darby, D.G.; McStephen, M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J. Int. Neuropsychol. Soc. 2003, 9, 419–428. [Google Scholar] [CrossRef]
- Scharfen, J.; Jansen, K.; Holling, H. Retest effects in working memory capacity tests: A meta-analysis. Psychon. Bull. Rev. 2018, 25, 2175–2199. [Google Scholar] [CrossRef]
- Morsund, A.H.; Ellekjaer, H.; Gramstad, A.; Reiestad, M.T.; Midgard, R.; Sando, S.B.; Jonsbu, E.; Naess, H. The development of cognitive and emotional impairment after a minor stroke: A longitudinal study. Acta Neurol. Scand. 2019, 140, 281–289. [Google Scholar] [CrossRef]
- Rauwenhoff, J.C.C.; Bronswijk, S.C.; Peeters, F.; Bol, Y.; Geurts, A.C.H.; van Heugten, C.M. Personalized predictions of treatment outcome in patients with post-stroke depressive symptoms. J. Rehabil. Med. 2020, 52, jrm00120. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Lee, S.Y.; Song, M.K.; et al. Post-stroke depression and cognitive aging: A multicenter, prospective cohort study. J. Pers. Med. 2022, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Hillis, A.E. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol 2010, 9, 895–905. [Google Scholar] [CrossRef] [PubMed]
| T1 | T2 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total (N = 73) | Female (N = 27) | Male (N = 46) | Total (N = 73) | Female (N = 27) | Male (N = 46) | ||||||||||||
| N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD | |
| Age (years) | 73 | 52.1 | 15.5 | 27 | 46.8 | 14.8 | 46 | 55.2 | 15.2 | |||||||||
| Education (years) | 73 | 7.0 | 4.0 | 27 | 7.7 | 4.2 | 46 | 6.5 | 3.8 | |||||||||
| Neuropsychological tests | ||||||||||||||||||
| Digit Span Forward | 73 | 5.2 | 1.9 | 27 | 5.4 | 1.5 | 46 | 5.1 | 2.1 | 73 | 5.9 | 1.9 | 27 | 6.2 | 1.8 | 46 | 5.7 | 2.0 |
| Digit Span Backward | 73 | 3.4 | 1.8 | 27 | 3.2 | 1.7 | 46 | 3.5 | 1.8 | 73 | 3.8 | 1.7 | 27 | 3.7 | 1.8 | 46 | 3.9 | 1.7 |
| Verbal Fluency Test | 72 | 21.3 | 11.5 | 27 | 24.4 | 13.4 | 45 | 19.4 | 9.9 | 72 | 23.9 | 10.8 | 27 | 26.1 | 12.0 | 45 | 22.6 | 10.0 |
| Stroop Dots | 39 | 26.6 | 14.1 | 13 | 24.0 | 14.6 | 26 | 27.8 | 13.9 | 43 | 23.9 | 11.6 | 14 | 18.1 | 5.7 | 29 | 26.7 | 12.7 |
| Stroop Color | 38 | 50.9 | 29.8 | 13 | 48.8 | 37.9 | 25 | 52.0 | 25.3 | 43 | 56.4 | 35.7 | 14 | 43.3 | 29.4 | 29 | 62.8 | 37.3 |
| Stroop Interference | 38 | 0.5 | 0.4 | 13 | 0.7 | 0.4 | 25 | 0.4 | 0.3 | 43 | 0.6 | 0.4 | 14 | 0.8 | 0.4 | 29 | 0.6 | 0.3 |
| Clinical characteristics | ||||||||||||||||||
| HAM-D-31 | 71 | 8.2 | 6.5 | 25 | 9.7 | 6.5 | 46 | 7.5 | 6.5 | 71 | 9.6 | 8.6 | 25 | 12.2 | 9.1 | 46 | 8.2 | 8.1 |
| NIHSS | 69 | 3.4 | 3.2 | 24 | 3.0 | 2.7 | 45 | 3.6 | 3.5 | 69 | 2.1 | 2.1 | 24 | 2.0 | 2.0 | 45 | 2.1 | 2.2 |
| Volume of stroke lesion | ||||||||||||||||||
| Total cortex | 51 | 2685.9 | 4058.2 | 23 | 2471.7 | 3751.4 | 28 | 2861.9 | 4354.4 | |||||||||
| Dorsolateral prefrontal cortex | 50 | 34.3 | 120.2 | 23 | 44.1 | 154.0 | 27 | 25.9 | 83.8 | |||||||||
| Medial prefrontal cortex | 50 | 102.8 | 235.4 | 23 | 69.1 | 168.5 | 27 | 131.5 | 280.3 | |||||||||
| Orbital prefrontal cortex | 50 | 49.0 | 167.7 | 23 | 18.0 | 56.5 | 27 | 75.4 | 220.8 | |||||||||
| Time | Time × Gender | Gender | ||||
|---|---|---|---|---|---|---|
| Variables | p | ES | p | ES | p | ES |
| Digit Span Forward | 0.001 | 0.031 | 0.723 | 0.000 | 0.347 | 0.010 |
| Digit Span Backward | 0.050 | 0.017 | 0.822 | 0.000 | 0.423 | 0.006 |
| Verbal Fluency Test | 0.005 | 0.011 | 0.415 | 0.001 | 0.108 | 0.033 |
| Stroop Dots | <0.001 | 0.069 | 0.930 | 0.000 | 0.149 | 0.044 |
| Stroop Color | 0.487 | 0.002 | 0.412 | 0.002 | 0.509 | 0.011 |
| Stroop Interference | 0.011 | 0.042 | 0.637 | 0.001 | 0.061 | 0.070 |
| HAM-D-31 | 0.194 | 0.010 | 0.481 | 0.003 | 0.031 | 0.038 |
| NIHSS | <0.001 | 0.044 | 0.320 | 0.002 | 0.628 | 0.003 |
| T1 | T2 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total (N = 73) | Female (N = 27) | Male (N = 46) | Total (N = 73) | Female(N = 27) | Male (N = 46) | ||||||||||||
| N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD | |
| Age (years) | 73 | 52.1 | 15.5 | 27 | 46.8 | 14.8 | 46 | 55.2 | 15.2 | |||||||||
| Education (years) | 73 | 7.0 | 4.0 | 27 | 7.7 | 4.2 | 46 | 6.5 | 3.8 | |||||||||
| Neuropsychological tests | ||||||||||||||||||
| Digit Span Forward | 73 | 5.2 | 1.9 | 27 | 5.4 | 1.5 | 46 | 5.1 | 2.1 | 73 | 5.9 | 1.9 | 27 | 6.2 | 1.8 | 46 | 5.7 | 2.0 |
| Digit Span Backward | 73 | 3.4 | 1.8 | 27 | 3.2 | 1.7 | 46 | 3.5 | 1.8 | 73 | 3.8 | 1.7 | 27 | 3.7 | 1.8 | 46 | 3.9 | 1.7 |
| Verbal Fluency Test | 72 | 21.3 | 11.5 | 27 | 24.4 | 13.4 | 45 | 19.4 | 9.9 | 72 | 23.9 | 10.8 | 27 | 26.1 | 12.0 | 45 | 22.6 | 10.0 |
| Stroop Dots | 39 | 26.6 | 14.1 | 13 | 24.0 | 14.6 | 26 | 27.8 | 13.9 | 43 | 23.9 | 11.6 | 14 | 18.1 | 5.7 | 29 | 26.7 | 12.7 |
| Stroop Color | 38 | 50.9 | 29.8 | 13 | 48.8 | 37.9 | 25 | 52.0 | 25.3 | 43 | 56.4 | 35.7 | 14 | 43.3 | 29.4 | 29 | 62.8 | 37.3 |
| Stroop Interference | 38 | 0.5 | 0.4 | 13 | 0.7 | 0.4 | 25 | 0.4 | 0.3 | 43 | 0.6 | 0.4 | 14 | 0.8 | 0.4 | 29 | 0.6 | 0.3 |
| Clinical characteristics | ||||||||||||||||||
| HAM-D-31 | 71 | 8.2 | 6.5 | 25 | 9.7 | 6.5 | 46 | 7.5 | 6.5 | 71 | 9.6 | 8.6 | 25 | 12.2 | 9.1 | 46 | 8.2 | 8.1 |
| NIHSS | 69 | 3.4 | 3.2 | 24 | 3.0 | 2.7 | 45 | 3.6 | 3.5 | 69 | 2.1 | 2.1 | 24 | 2.0 | 2.0 | 45 | 2.1 | 2.2 |
| Volume of stroke lesion | ||||||||||||||||||
| Total cortex | 51 | 2685.9 | 4058.2 | 23 | 2471.7 | 3751.4 | 28 | 2861.9 | 4354.4 | |||||||||
| Dorsolateral prefrontal cortex | 50 | 34.3 | 120.2 | 23 | 44.1 | 154.0 | 27 | 25.9 | 83.8 | |||||||||
| Medial prefrontal cortex | 50 | 102.8 | 235.4 | 23 | 69.1 | 168.5 | 27 | 131.5 | 280.3 | |||||||||
| Orbital prefrontal cortex | 50 | 49.0 | 167.7 | 23 | 18.0 | 56.5 | 27 | 75.4 | 220.8 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobreiro, M.F.M.; Terroni, L.; Guajardo, V.D.; Mattos, P.F.; Leite, C.d.C.; Amaro, E., Jr.; Tinone, G.; Iosifescu, D.V.; Fraguas, R. The Impact of Post-Stroke Depressive Symptoms on Cognitive Performance in Women and in Men: A 4 Month Prospective Study. Life 2023, 13, 1554. https://doi.org/10.3390/life13071554
Sobreiro MFM, Terroni L, Guajardo VD, Mattos PF, Leite CdC, Amaro E Jr., Tinone G, Iosifescu DV, Fraguas R. The Impact of Post-Stroke Depressive Symptoms on Cognitive Performance in Women and in Men: A 4 Month Prospective Study. Life. 2023; 13(7):1554. https://doi.org/10.3390/life13071554
Chicago/Turabian StyleSobreiro, Matildes F. M., Luisa Terroni, Valeri Delgado Guajardo, Patricia Ferreira Mattos, Claudia da Costa Leite, Edson Amaro, Jr., Gisela Tinone, Dan V. Iosifescu, and Renerio Fraguas. 2023. "The Impact of Post-Stroke Depressive Symptoms on Cognitive Performance in Women and in Men: A 4 Month Prospective Study" Life 13, no. 7: 1554. https://doi.org/10.3390/life13071554
APA StyleSobreiro, M. F. M., Terroni, L., Guajardo, V. D., Mattos, P. F., Leite, C. d. C., Amaro, E., Jr., Tinone, G., Iosifescu, D. V., & Fraguas, R. (2023). The Impact of Post-Stroke Depressive Symptoms on Cognitive Performance in Women and in Men: A 4 Month Prospective Study. Life, 13(7), 1554. https://doi.org/10.3390/life13071554






