Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression
Abstract
1. Introduction
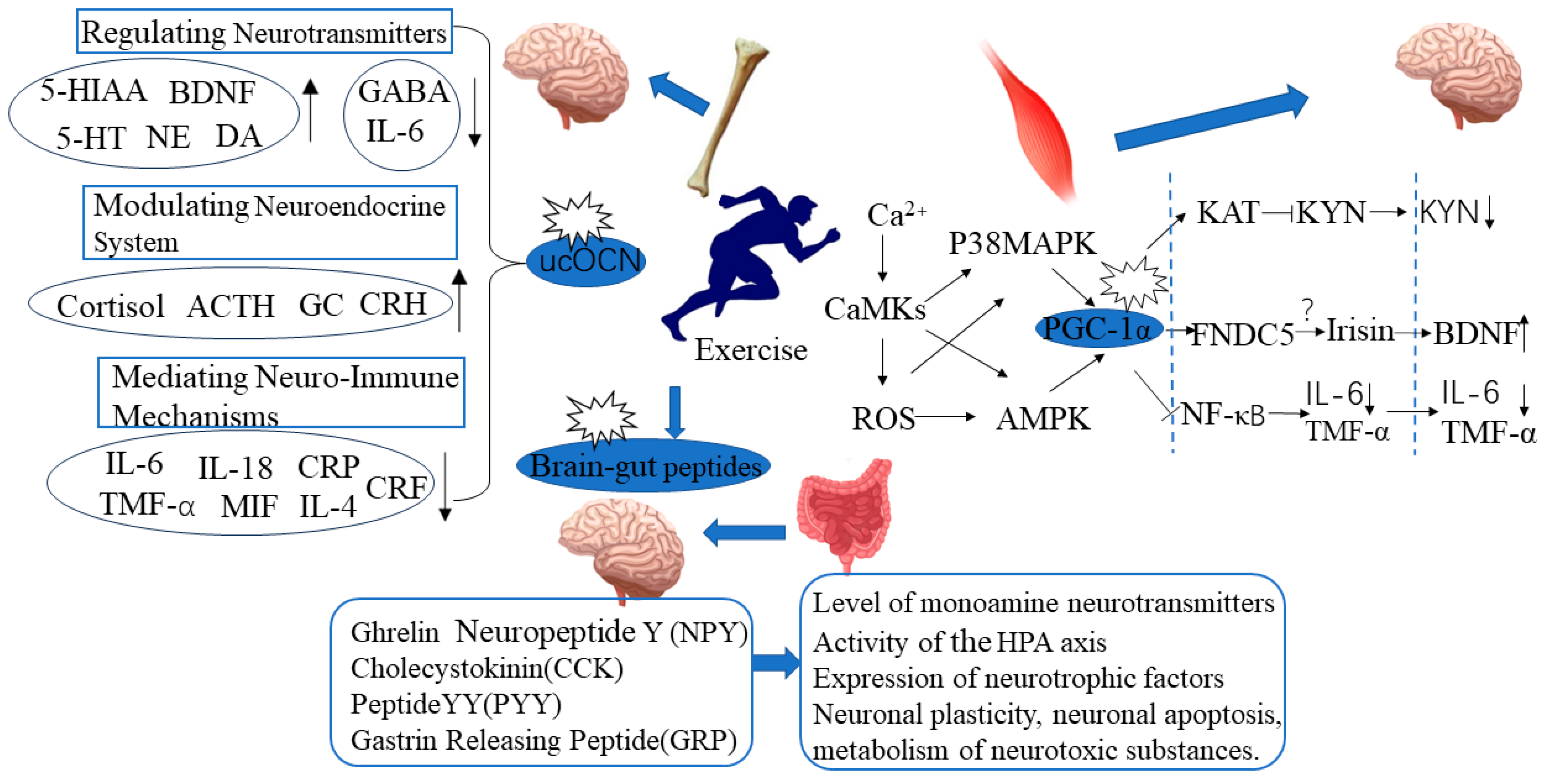
2. Exercise-Induced Skeletal Muscle PGC-1α Expression and Improvement in Depressive Behavior
- Exercise may balance skeletal muscle KYN metabolism through the PGC-1α/KAT pathway, alleviate the neurotoxic effects of KYN, and thereby exert an antidepressant effect. Aerobic exercise can increase the expression of skeletal muscle PGC-1α and KAT1-4 in healthy individuals [84]. Voluntary wheel running can promote the expression of skeletal muscle PGC-1α and KAT1, 3, 4, and increase the level of the KYN metabolite KYNA in the plasma of wild-type mice [84]. This suggests that the PGC-1α/KAT pathway activated by exercise regulates peripheral KYN metabolism.
- Exercise may reduce the risk of depression induced by peripheral inflammation activation by activating skeletal muscle PGC-1α. The regulatory effect of exercise on peripheral inflammatory response may be related to the activation of skeletal muscle PGC-1α. Exercise can increase the expression of skeletal muscle PGC-1α, reduce peripheral inflammation levels, and thus alleviate the systemic inflammatory response [85]. Kohut et al. [86] found that exercise can improve stress-induced depressive behavior by reducing levels of interleukin (IL-6, IL-18) and TNFα. After exercise intervention, the plasma levels of IL-1β, IL-6, and TNFα in patients with depression were significantly reduced, and depressive symptoms were improved [87].
- Exercise may exert an antidepressant effect by promoting the secretion of FNDC5/Irisin in skeletal muscle and increasing the expression of PGC-1α. Exercise-induced activation of PGC-1α can induce skeletal muscle to secrete FNDC5/Irisin [88]. Plasma Irisin levels in people who exercise regularly are significantly higher than those who lead sedentary lifestyles [89]. Wrann et al. [82] found that exercise can increase the expression of FNDC5 and BDNF in skeletal muscle and hippocampal tissue. When the FNDC5 gene in the liver of mice was activated, the level of BDNF in the hippocampal tissue also increased. This indicates that exercise can promote the secretion of FDNC5/Irisin in skeletal muscle through the activation of PGC-1α and that Irisin in the bloodstream can act as a remote secretion on brain tissue to exert a neuroprotective effect. However, there is still controversy surrounding Irisin research, as there are many sources of circulating Irisin in the blood, and it has not been proven yet whether the elevated circulating Irisin induced by exercise mainly originates from skeletal muscle, adipose tissue, or other tissues.
3. Exercise-Induced Expression of Bone-Derived Factor Ucocn Is Correlated with Improvement in Depressive Behavior
4. Exercise-Mediated Brain-Gut Peptide Expression and Improvement in Depressive Behavior
5. The Relationship between Exercise and Mental Health: The Role of Inflammatory Factors and Neurotransmitter Changes
6. Effects of Different Exercise Intervention Programs on Improving Depression
6.1. Effects of Different Types of Exercise on Improving Depression
6.1.1. Aerobic Exercise
6.1.2. Yoga
6.1.3. Resistance Training
6.1.4. Whole-Body Vibration
6.2. Effects of Different Intensity, Frequency, and Volume of Exercise on Depression
6.2.1. The Impact of Exercise Intensity on Treating Depression
6.2.2. The Influence of Exercise Frequency on Treating Depression
6.2.3. The Influence of Exercise Volume on Treating Depression
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- McCarron, R.M.; Shapiro, B.; Rawles, J.; Luo, J. Depression. Ann. Intern. Med. 2021, 174, ITC65–ITC80. [Google Scholar] [CrossRef] [PubMed]
- Hohls, J.K.; König, H.-H.; Quirke, E.; Hajek, A. Anxiety, Depression and Quality of Life—A Systematic Review of Evidence from Longitudinal Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 12022. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Storlino, G.; Oranger, A.; Sanesi, L.; Lovero, R.; Buccoliero, C.; Mori, G.; Colaianni, G.; et al. Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice. Int. J. Mol. Sci. 2022, 23, 7596. [Google Scholar] [CrossRef] [PubMed]
- Gładka, A.; Zatoński, T.; Rymaszewska, J. Association between the long-term exposure to air pollution and depression. Adv. Clin. Exp. Med. 2022, 31, 1139–1152. [Google Scholar] [CrossRef]
- Berton, O.; Nestler, E.J. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef]
- Carrera-González, M.D.P.; Cantón-Habas, V.; Rich-Ruiz, M. Aging, depression and dementia: The inflammatory process. Adv. Clin. Exp. Med. 2022, 31, 469–473. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Tanaka, M.; Spekker, E.; Szabo, A.; Polyák, H.; Vécsei, L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents—In celebration of 80th birthday of Professor Peter Riederer. J. Neural Transm. 2022, 129, 627–642. [Google Scholar] [CrossRef]
- Hunt, C.; Macedo, E.C.T.; Suchting, R.; de Dios, C.; Leal, V.A.C.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Maszlag-Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2,3-Dioxygenase 1 Influenced the Age Onset of Parkinson’s Disease. Front. Biosci. Landmrk. 2022, 27, 265. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, L.; Debnath, A.; Jamshed, S.; Wish, J.V.; Raine, J.C.; Tomy, G.T.; Thomas, P.J.; Holloway, A.C. An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway. Int. J. Mol. Sci. 2022, 23, 6300. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, A.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Integrating Armchair, Bench, and Bedside Research for Behavioral Neurology and Neuropsychiatry: Editorial. Biomedicines 2022, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT(1) receptor agonists effective anti-migraine drugs? Expert Opin. Pharmacother. 2021, 22, 1221–1225. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Tanaka, M.; Diano, M.; Battaglia, S. Editorial: Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry 2023, 14, 1225755. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the Role of vmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Peng, W.; Sweeney, J.A.; Jia, Z.-Y.; Gong, Q.-Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef]
- Battaglia, S.; Fabius, J.H.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Recent Advances in the Study of the Comorbidity of Depressive and Anxiety Disorders. Adv. Clin. Exp. Med. 2022, 31, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. It is time to investigate integrative approaches to enhance treatment outcomes for depression? Med. Hypotheses 2019, 126, 82–94. [Google Scholar] [CrossRef]
- Dunlop, B.W. Evidence-Based Applications of Combination Psychotherapy and Pharmacotherapy for Depression. Focus (Am. Psychiatr. Publ.) 2016, 14, 156–173. [Google Scholar] [CrossRef]
- Fuhr, K.; Meisner, C.; Batra, A. Long-Term Outcomes of Depression Treatment With Hypnotherapy or Cognitive Behavioral Therapy. J. Nerv. Ment. Dis. 2023, 211, 519–524. [Google Scholar] [CrossRef]
- Smith, V.A.D.; Maciejewski, M.L.; Berkowitz, T.S.M.; Mitchell, J.E.; Liu, C.-F.; Bradley, K.A.M.; Olsen, M.K.; Livingston, E.L.; Arterburn, D.E.M. The Effect of Bariatric Surgery on Long-term Depression Treatment in Patients With Obesity. Ann. Surg. 2022, 276, 318–323. [Google Scholar] [CrossRef]
- Serafini, G.; Adavastro, G.; Canepa, G.; De Berardis, D.; Valchera, A.; Pompili, M.; Nasrallah, H.; Amore, M. The Efficacy of Buprenorphine in Major Depression, Treatment-Resistant Depression and Suicidal Behavior: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 2410. [Google Scholar] [CrossRef]
- Gualano, M.; Bert, F.; Martorana, M.; Voglino, G.; Andriolo, V.; Thomas, R.; Gramaglia, C.; Zeppegno, P.; Siliquini, R. The long-term effects of bibliotherapy in depression treatment: Systematic review of randomized clinical trials. Clin. Psychol. Rev. 2017, 58, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Pavlova, B. Long-term effects of depression treatment. Lancet Psychiatry 2016, 3, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Rato, B.M.; Ley, C.; Schomoller, A.; Dumon, D. Mental Well-Being or Ill-Being through Coaching in Adult Grassroots Sport: A Systematic Mapping Review. Int. J. Environ. Res. Public Health 2021, 18, 6543. [Google Scholar] [CrossRef] [PubMed]
- Windle, G.; Hughes, D.; Linck, P.; Russell, I.; Woods, B. Is exercise effective in promoting mental well-being in older age? A systematic review. Aging Ment. Health 2010, 14, 652–669. [Google Scholar] [CrossRef]
- Belcher, B.R.; Zink, J.; Azad, A.; Campbell, C.E.; Chakravartti, S.P.; Herting, M.M. The Roles of Physical Activity, Exercise, and Fitness in Promoting Resilience During Adolescence: Effects on Mental Well-Being and Brain Development. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 225–237. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Physical Activity and Physical and Mental Well-Being in Church Settings. Am. J. Public Health 2017, 107, 1023–1024. [Google Scholar] [CrossRef]
- Black, S.V.; Cooper, R.; Martin, K.R.; Brage, S.; Kuh, D.; Stafford, M. Physical Activity and Mental Well-being in a Cohort Aged 60–64 Years. Am. J. Prev. Med. 2015, 49, 172–180. [Google Scholar] [CrossRef]
- Magdalena, K.; Karolina, Z.; Karolina, H.; Boberska, M.; Scholz, U.; Radtze, T.; Luszczynska, A. What comes first, negative emotions, positive emotions, or mod-erate-to-vigorous physical activity? Ment. Health Phys. Act. 2019, 16, 38–42. [Google Scholar]
- Li, J.; Theng, Y.-L.; Foo, S. Effect of Exergames on Depression: A Systematic Review and Meta-Analysis. Cyberpsychology Behav. Soc. Netw. 2016, 19, 34–42. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Hootman, J.M. Effects of exercise on depression in adults with arthritis: A systematic review with meta-analysis of randomized controlled trials. Arthritis Res. Ther. 2015, 17, 21. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, H.; Cho, H. The Effectiveness of Physical Activity Interventions on Depression in Korea: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Z.-D.; Jiang, W.-T.; Fang, Y.-Y.; Sun, W.-X. Systematic review and meta-analysis of the effects of exercise on depression in adolescents. Child Adolesc. Psychiatry Ment. Health 2022, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Mandy, X.H.; David, T.; Ellen, G.; Bos, D.; Ikram, M.K.; Ikram, M.A.; Cuijpers, P.; Penninx, B.W.J.H. Exercise interventions for the prevention of depression: A systematic review of meta-analyses. BMC Public Health 2020, 20, 1255. [Google Scholar]
- Hallgren, M.; Stubbs, B.; Vancampfort, D.; Lundin, A.; Jääkallio, P.; Forsell, Y. Treatment Guidelines for Depression: Greater Emphasis on Physical Activity is Needed. Eur. Psychiatry 2017, 40, 1–3. [Google Scholar] [CrossRef]
- Michalsen, A.; Grossman, P.; Acil, A.; Langhorst, J.; Lüdtke, R.; Esch, T.; Stefano, G.B.; Dobos, G.J. Rapid stress reduction and anxiolysis among distressed women as a consequence of a three-month intensive yoga program. Med. Sci. Monit. 2005, 11, CR555–CR561. [Google Scholar]
- Ross, R.E.; VanDerwerker, C.J.; Saladin, M.E.; Gregory, C.M. The role of exercise in the treatment of depression: Biological underpinnings and clinical outcomes. Mol. Psychiatry 2023, 28, 298–328. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, Z.; Sun, L.; Zhou, L.; Wang, G.; Xiao, L.; Wang, H. The Effects and Mechanisms of Exercise on the Treatment of Depression. Front. Psychiatry 2021, 12, 705559. [Google Scholar] [CrossRef]
- Lopez-Torres, H.J. Effectiveness of physical exercise in the treatment of depression in older adults as an alternative to antidepressant drugs in primary care. BMC Psychiatry 2019, 19, 21. [Google Scholar] [CrossRef]
- Martinsen, E.W. Benefits of Exercise for the Treatment of Depression. Sports Med. 1990, 9, 380–389. [Google Scholar] [CrossRef]
- Wegner, M.; Helmich, I.; Machado, S.; Nardi, A.E.; Arias-Carrion, O.; Budde, H. Effects of Exercise on Anxiety and Depression Disorders: Review of Meta-Analyses and Neurobiological Mechanisms. CNS Neurol. Disord. Drug Targets 2014, 13, 1002–1014. [Google Scholar] [CrossRef]
- Ströhle, A. Physical activity, exercise, depression and anxiety disorders. J. Neural Transm. 2009, 116, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Biddle, S.J.; Asare, M. Physical activity and mental health in children and adolescents: A review of reviews. Br. J. Sports Med. 2011, 45, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Bolívar, S.; Navarro, X.; Udina, E. New insights into peripheral nerve regeneration: The role of secretomes. Exp. Neurol. 2022, 354, 114069. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Madduri, S.; Gander, B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 2010, 15, 93–103. [Google Scholar] [CrossRef]
- Frostick, S.P.; Yin, Q.; Kemp, G.J. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 1998, 18, 397–405. [Google Scholar] [CrossRef]
- Meeusen, R. Exercise, Nutrition and the Brain. Sports Med. 2014, 44 (Suppl. S1), 47–56. [Google Scholar] [CrossRef]
- Schmidt, W.; Endres, M.; Dimeo, F.; Jungehulsing, G.J. Train the Vessel, Gain the Brain: Physical Activity and Vessel Function and the Impact on Stroke Prevention and Outcome in Cerebrovascular Disease. Cerebrovasc. Dis. 2013, 35, 303–312. [Google Scholar] [CrossRef]
- Perrey, S. Promoting Motor Function by Exercising the Brain. Brain Sci. 2013, 3, 101–122. [Google Scholar] [CrossRef]
- Qi, J.-Y.; Yang, L.-K.; Wang, X.-S.; Wang, M.; Li, X.-B.; Feng, B.; Wu, Y.-M.; Liu, S.-B.; Zhang, K. Mechanism of CNS regulation by irisin, a multifunctional protein. Brain Res. Bull. 2022, 188, 11–20. [Google Scholar] [CrossRef]
- Siteneski, A.; Olescowicz, G.; Pazini, F.L.; Camargo, A.; Fraga, D.B.; Brocardo, P.S.; Gil-Mohapel, J.; Cunha, M.P.; Rodrigues, A.L.S. Antidepressant-like and pro-neurogenic effects of physical exercise: The putative role of FNDC5/irisin pathway. J. Neural Transm. 2020, 127, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Smiles, W.J.; Kaiser, J.; McAloon, L.M.; Loh, K.; Galic, S.; Oakhill, J.S.; Gundlach, A.L.; Scott, J.W. Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4052. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, T.; Chu, J.M.-T.; Chen, Y.; Dunnett, S.; Ho, Y.-S.; Wong, G.T.-C.; Chang, R.C.-C. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig. 2019, 99, 943–957. [Google Scholar] [CrossRef]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.F.; de Mello, M.F.; Carpenter, L.L.; Price, L.H. Update on stress and depression: The role of the hypothalamic-pituitary-adrenal (HPA) axis. Braz. J. Psychiatry 2003, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Front. Horm. Res. 2016, 47, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Lim, E.Y.; Jung, W.R.; Shin, M.K.; Ann, E.S.; Kim, K.L. Effects of treadmill exercise on hypoactivity of the hypothalamo-pituitary-adrenal axis induced by chronic administration of corticosterone in rats. Neurosci. Lett. 2008, 434, 46–49. [Google Scholar] [CrossRef]
- Chennaoui, M.; Gomez, M.D.; Lesage, J.; Drogou, C.; Guezennec, C.Y. Effects of moderate and intensive training on the hypothalamo-pituitary-adrenal axis in rats. Acta Physiol. Scand. 2002, 175, 113–121. [Google Scholar] [CrossRef]
- Gerosa, L.; Lombardi, G. Bone-to-Brain: A Round Trip in the Adaptation to Mechanical Stimuli. Front. Physiol. 2021, 12, 623893. [Google Scholar] [CrossRef]
- Minoia, A.; Dalle, C.L.; Schwamborn, J.C.; Schwamborn, J.C.; Bolognin, S.; Valenti, M.T. Bone Tissue and the Nervous System: What Do They Have in Common? Cells 2022, 12, 51. [Google Scholar] [CrossRef]
- Köhler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.; Carvalho, A. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef] [PubMed]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020, 13, 520724184. [Google Scholar] [CrossRef] [PubMed]
- Codella, R.; Luzi, L.; Terruzzi, I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig. Liver Dis. 2018, 50, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Zarza-Rebollo, J.A.; Molina, E.; López-Isac, E.; Pérez-Gutiérrez, A.M.; Gutiérrez, B.; Cervilla, J.A.; Rivera, M. Interaction Effect between Physical Activity and the BDNF Val66Met Polymorphism on Depression in Women from the PISMA-ep Study. Int. J. Environ. Res. Public Health 2022, 19, 2068. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Otto, M.W. Assessing BDNF as a mediator of the effects of exercise on depression. J. Psychiatr. Res. 2020, 123, 114–118. [Google Scholar] [CrossRef]
- Archer, T.; Josefsson, T.; Lindwall, M. Effects of physical exercise on depressive symptoms and biomarkers in depression. CNS Neurol. Disord. Drug Targets 2014, 13, 1640–1653. [Google Scholar] [CrossRef]
- Eyre, H.; Baune, B.T. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology 2012, 37, 1397–1416. [Google Scholar] [CrossRef]
- Wallberg, A.E.; Yamamura, S.; Malik, S.; Spiegelman, B.M.; Roeder, R.G. Coordination of p300-Mediated Chromatin Remodeling and TRAP/Mediator Function through Coactivator PGC-1alpha. Mol. Cell 2003, 12, 1137–1149. [Google Scholar] [CrossRef]
- Pilegaard, H.; Saltin, B.; Neufer, P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J. Physiol. 2003, 546, 851–858. [Google Scholar] [CrossRef]
- Lucas, E.K.; Markwardt, S.J.; Gupta, S.; Meador-Woodruff, J.H.; Lin, J.D.; Overstreet-Wadiche, L.; Cowell, R.M. Parvalbumin Deficiency and GABAergic Dysfunction in Mice Lacking PGC-1alpha. J. Neurosci. 2010, 30, 7227–7235. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A.; Stouth, D.W.; Manta, A.; Ljubicic, V.; Hinkley, J.M.; Zou, K.; Park, S.; et al. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endoc. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal Muscle PGC-1α1 Modulates Kynurenine Metabolism and Mediates Resilience to Stress-Induced Depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1α in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kohut, M.L.; McCann, D.A.; Russell, D.; Konopka, D.; Cunnick, J.; Franke, W.; Castillo, M.; Reighard, A.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef]
- Rethorst, C.D.; Toups, M.S.; Greer, T.L.; Nakonezny, P.A.; Carmody, T.J.; Grannemann, B.D.; Huebinger, R.M.; Barber, R.C.; Trivedi, M.H. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol. Psychiatry 2013, 18, 1119–1124. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Sreekumaran Nair, K.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The two faces of serotonin in bone biology. J. Cell Biol. 2010, 191, 7–13. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kim, T.-W.; Park, S.-S.; Kim, C.-J.; Shin, M.-S.; Lee, S.-J.; Kim, S.-H.; Baek, S.-S. Wnt signaling pathway is implicated in the alleviating effect of treadmill exercise on maternal separation-induced depression. J. Exerc. Rehabil. 2019, 15, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Obri, A.; Khrimian, L.; Karsenty, G.; Oury, F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nat. Rev. Endocrinol. 2018, 14, 174–182. [Google Scholar] [CrossRef]
- Rentz, J.; Winberg, J.; Swardfager, W.; Mitchell, J. SAT-293 Osteocalcin and Exercise Improve Mood and Cognition in Female Mice with High-Fat Diet Induced Type 2 Diabetes. J. Endocr. Soc. 2020, 4, SAT-293. [Google Scholar] [CrossRef]
- Kang, Y.; Yao, J.; Gao, X.; Zhong, H.; Song, Y.; Di, X.; Feng, Z.; Xie, L.; Zhang, J. Exercise ameliorates anxious behavior and promotes neuroprotection through osteocalcin in VCD-induced menopausal mice. CNS Neurosci. Ther. 2023, 29, 3980–3994. [Google Scholar] [CrossRef]
- Nicola, N.; Rocky, S.; Angela, P.; Briganti, S.I.; Pozzili, P.; Epstein, S. The Alliance of Mesenchymal Stem Cells, Bone, and Diabetes. Int. J. Endocrinol. 2014, 2014, 690783. [Google Scholar]
- Millar, S.A.; Zala, I.; Anderson, S.I.; O’Sullivan, S.E. Osteocalcin does not influence acute or chronic inflammation in human vascular cells. J. Cell. Physiol. 2020, 235, 3414–3424. [Google Scholar] [CrossRef]
- Sen, E.I.; Esmaeilzadeh, S.; Eskiyurt, N. Effects of whole-body vibration and high impact exercises on the bone metabolism and functional mobility in postmenopausal women. J. Bone Miner Metab. 2020, 38, 392–404. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Ying, Z. Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience 2010, 168, 130–137. [Google Scholar] [CrossRef]
- Walker, A.K.; Rivera, P.D.; Wang, Q.; Chuang, J.-C.; Tran, S.; Osborne-Lawrence, S.; Estill, S.J.; Starwalt, R.; Huntington, P.; Morlock, L.; et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol. Psychiatry 2015, 20, 500–508. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Wang, Y.; Li, H.; Ji, L. Metabolic factors-triggered inflammatory response drives antidepressant effects of exercise in CUMS rats. Psychiatry Res. 2015, 228, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.P.; Perez, M.F.; Salde, E.; Schiöth, H.B.; A Ramirez, O.; de Barioglio, S.R. Ghrelin induced memory facilitation implicates nitric oxide synthase activation and decrease in the threshold to promote LTP in hippocampal dentate gyrus. Physiol. Behav. 2010, 101, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Li, H.; Ji, L. The Role of Nitric Oxide in the Antidepressant Actions of 5-Aminoimidazole-4-Carboxamide-1-beta-D-Ribofuranoside in Insulin-Resistant Mice. Psychosom. Med. 2016, 78, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bjørnebekk, A.; Mathé, A.A.; Brené, S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus 2010, 20, 820–828. [Google Scholar] [CrossRef]
- Jiang, P.; Dang, R.-L.; Li, H.-D.; Zhang, L.-H.; Zhu, W.-Y.; Xue, Y.; Tang, M.-M. The Impacts of Swimming Exercise on Hippocampal Expression of Neurotrophic Factors in Rats Exposed to Chronic Unpredictable Mild Stress. Evid.-Based Complement. Altern. Med. 2014, 2014, 729827. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Lennartsson, A.; Vakifahmetoglu-Norberg, H.; Wei, Y.; Aberg, E.; Werme, M.; Rogdaki, M.; Mannervik, M.; Wegener, G.; Brene, S.; et al. Allele-specific programming of Npy and epi-genetic effects of physical activity in a genetic model of depression. Transl. Psychiat. 2013, 3, e255. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B.; Castell, L.M.; Newsholme, E.A.; Calam, J. Physical exercise and normobaric hypoxia: Independent modulators of peripheral cholecystokinin metabolism in man. J. Appl. Physiol. 2001, 90, 105–113. [Google Scholar] [CrossRef]
- Sliwowski, Z.; Lorens, K.; Konturek, S.J.; Bielanski, W.; Zoladz, J.A. Leptin, gastrointestinal and stress hormones in response to exer-cise in fasted or fed subjects and before or after blood donation. J. Physiol. Pharmacol. 2001, 52, 53–70. [Google Scholar]
- Ströhle, A.; Feller, C.; Onken, M.; Godemann, F.; Heinz, A.; Dimeo, F. The Acute Antipanic Activity of Aerobic Exercise. Am. J. Psychiatry 2005, 162, 2376–2378. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’neil, A.; A Pasco, J.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Sigmundsson, H.; Dybendal, B.H.; Grassini, S. Motion, Relation, and Passion in Brain Physiological and Cognitive Aging. Brain Sci. 2022, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- DiLorenzo, T.M.; Bargman, E.P.; Stucky-Ropp, R.; Brassington, G.S.; Frensch, P.A.; LaFontaine, T. Long-Term Effects of Aerobic Exercise on Psychological Outcomes. Prev. Med. 1999, 28, 75–85. [Google Scholar] [CrossRef]
- Oertel-Knöchel, V.; Mehler, P.; Thiel, C.; Steinbrecher, K.; Malchow, B.; Tesky, V.; Ademmer, K.; Prvulovic, D.; Banzer, W.; Zopf, Y.; et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 589–604. [Google Scholar] [CrossRef]
- Olson, R.L.; Brush, C.J.; Ehmann, P.; Alderman, B.L. A randomized trial of aerobic exercise on cognitive control in major depression. Clin. Neurophysiol. 2017, 128, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Tsutsumimoto, K.; Doi, T.; Makino, K.; Nakakubo, S.; Liu-Ambrose, T.; Shimada, H. Exercise and Horticultural Programs for Older Adults with Depressive Symptoms and Memory Problems: A Randomized Controlled Trial. J. Clin. Med. 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Anheyer, D.; Lauche, R.; Dobos, G. A systematic review of yoga for major depressive disorder. J. Affect. Disord. 2017, 213, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bridges, L.; Sharma, M. The Efficacy of Yoga as a Form of Treatment for Depression. J. Evid.-Based Complement. Altern. Med. 2017, 22, 1017–1028. [Google Scholar] [CrossRef]
- James-Palmer, A.; Anderson, E.Z.; Zucker, L.; Kofman, Y.; Daneault, J.-F. Yoga as an Intervention for the Reduction of Symptoms of Anxiety and Depression in Children and Adolescents: A Systematic Review. Front. Pediatr. 2020, 8, 78. [Google Scholar] [CrossRef]
- Kwok, J.; Kwan, J.; Auyeung, M.; Mok, V.C.T.; Lau, C.K.Y.; Choi, K.C.; Chan, H.Y.L. Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef]
- Gordon, B.R.; McDowell, C.P.; Hallgren, M.; Meyer, J.; Lyons, M.; Herring, M.P. Association of Efficacy of Resistance Exercise Training With Depressive Symptoms: Meta-analysis and Meta-regression Analysis of Randomized Clinical Trials. JAMA Psychiatry 2018, 75, 566–576. [Google Scholar] [CrossRef]
- Aidar, F.J.; de Matos, D.G.; de Oliveira, R.J.; Carneiro, A.L.; Cabral, B.G.D.A.T.; Dantas, P.M.S.; Reis, V.M. Relationship Between Depression and Strength Training in Survivors of the Ischemic Stroke. J. Hum. Kinet. 2014, 43, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kekäläinen, T.; Kokko, K.; Sipilä, S.; Walker, S. Effects of a 9-month resistance training intervention on quality of life, sense of coherence, and depressive symptoms in older adults: Randomized controlled trial. Qual. Life Res. 2018, 27, 455–465. [Google Scholar] [CrossRef]
- Dziubek, W.; Kowalska, J.; Kusztal, M.; Rogowski, Ł.; Gołębiowski, T.; Nikifur, M.; Szczepańska-Gieracha, J.; Zembroń-Łacny, A.; Klinger, M.; Woźniewski, M. The Level of Anxiety and Depression in Dialysis Patients Undertaking Regular Physical Exercise Training—A Preliminary Study. Kidney Blood Press. Res. 2016, 41, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Moraes, H.S.; Silveira, H.S.; Oliveira, N.A.; Portugal, E.M.M.; Araújo, N.B.; Vasques, P.E.; Bergland, A.; Santos, T.M.; Engedal, K.; Coutinho, E.S.; et al. Is Strength Training as Effective as Aerobic Training for Depression in Older Adults? A Randomized Controlled Trial. Neuropsychobiology 2020, 79, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; O’Sullivan, D.M.; Shin, S.-K. Can 24 weeks strength training reduce feelings of depression and increase neurotransmitter in elderly females? Exp. Gerontol. 2019, 115, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, Y.; Wang, D. Endurance and resistance training mitigate the negative consequences of depression on synaptic plasticity through different molecular mechanisms. Int. J. Neurosci. 2020, 130, 541–550. [Google Scholar] [CrossRef]
- Peng, G.; Yang, L.; Wu, C.Y.; Zhang, L.L.; Wu, C.Y.; Li, F.; Shi, H.W.; Hou, J.; Zhang, L.M.; Ma, X.; et al. Whole body vibration training improves depression-like behaviors in a rat chronic restraint stress model. Neurochem. Int. 2021, 142, 104926. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Pallone, G.; Romagnoli, C.; Rinaldi, A.M.; Annino, G.; D’arcangelo, G.; Tancredi, V. Whole Body Vibration Improves Brain and Musculoskeletal Health by Modulating the Expression of Tissue-Specific Markers: FNDC5 as a Key Regulator of Vibration Adaptations. Int. J. Mol. Sci. 2022, 23, 10388. [Google Scholar] [CrossRef]
- Chawla, G.; Azharuddin, M.; Ahmad, I.; Hussain, M.E. Effect of Whole-body Vibration on Depression, Anxiety, Stress, and Quality of Life in College Students: A Randomized Controlled Trial. Oman Med. J. 2022, 37, e408. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Loukov, D.; Bowdish, D.M.; Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018, 133, 79–84. [Google Scholar] [CrossRef]
- Balchin, R.; Linde, J.; Blackhurst, D.; Rauch, H.L.; Schönbächler, G. Sweating away depression? The impact of intensive exercise on depression. J. Affect. Disord. 2016, 200, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.J.; Lewis, A.J.; Boyce, P.; Galbally, M. Exercise frequency and maternal mental health: Parallel process modelling across the perinatal period in an Australian pregnancy cohort. J. Psychosom. Res. 2018, 111, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Heuze, J.P. Antidepressant Effects Associated with Different Exercise Conditions in Participants with Depression: A Pilot Study. J. Sport Exerc. Psychol. 2007, 29, 348–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanamori, S.; Takamiya, T.; Inoue, S.; Kai, Y.; Tsuji, T.; Kondo, K. Frequency and pattern of exercise and depression after two years in older Japanese adults: The JAGES longitudinal study. Sci. Rep. 2018, 8, 11224. [Google Scholar] [CrossRef]
- Wang, S.; Ma, W.; Wang, S.-M.; Yi, X. A Cross Sectional Examination of the Relation Between Depression and Frequency of Leisure Time Physical Exercise among the Elderly in Jinan, China. Int. J. Environ. Res. Public Heal. 2018, 15, 2041. [Google Scholar] [CrossRef]
- Dunn, A.L.; Trivedi, M.H.; O’Neal, H.A. Physical activity dose-response effects on outcomes of depression and anxiety. Med. Sci. Sports Exerc. 2001, 33, S587–S597. [Google Scholar] [CrossRef]
- Dunn, A.L.; Trivedi, M.H.; Kampert, J.B.; Clark, C.G.; Chambliss, H.O. Exercise treatment for depression: Efficacy and dose response. Am. J. Prev. Med. 2005, 28, 1–8. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Park, J.-H.; Lee, M.Y.; Oh, K.-S.; Shin, D.-W.; Shin, Y.-C. Physical activity and the prevention of depression: A cohort study. Gen. Hosp. Psychiatry 2019, 60, 90–97. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lu, M.-C.; Hu, I.-H.; Wu, W.-C.I.; Hu, S.C. Effects of different amounts of exercise on preventing depressive symptoms in community-dwelling older adults: A prospective cohort study in Taiwan. BMJ Open 2017, 7, e014256. [Google Scholar] [CrossRef]
- Harvey, S.B.; Øverland, S.; Hatch, S.L.; Wessely, S.; Mykletun, A.; Hotopf, M. Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. Am. J. Psychiatry 2018, 175, 28–36. [Google Scholar] [CrossRef]
- Wang, R.; Bishwajit, G.; Zhou, Y.; Wu, X.; Feng, D.; Tang, S.; Chen, Z.; Shaw, I.; Wu, T.; Song, H.; et al. Intensity, frequency, duration, and volume of physical activity and its association with risk of depression in middle- and older-aged Chinese: Evidence from the China Health and Retirement Longitudinal Study, 2015. PLoS ONE 2019, 14, e0221430. [Google Scholar] [CrossRef] [PubMed]
- Rethorst, C.D.; Trivedi, M.H. Evidence-Based Recommendations for the Prescription of Exercise for Major Depressive Disorder. J. Psychiatr. Pract. 2013, 19, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.M.; Foright, R.M. Keystone Conference: New Insights into the Biology of Exercise. J. Appl. Physiol. 2020, 129, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Kyparos, A.; Spanou, C.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S. Redox biology of exercise: An integrative and comparative consideration of some overlooked issues. J. Exp. Biol. 2012, 215, 1615–1625. [Google Scholar] [CrossRef]
- Pierce, J.L.; Sharma, A.K.; Roberts, R.L.; Yu, K.; Irsik, D.L.; Choudhary, V.; Dorn, J.S.; Bensreti, H.; Benson, R.D.; Kaiser, H.; et al. The Glucocorticoid Receptor in Osterix-Expressing Cells Regulates Bone Mass, Bone Marrow Adipose Tissue, and Systemic Metabolism in Female Mice During Aging. J. Bone Miner. Res. 2021, 37, 285–302. [Google Scholar] [CrossRef]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a “Mediator” of Systemic Metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef]
- Becker, M.; Pinhasov, A.; Ornoy, A. Animal Models of Depression: What Can They Teach Us about the Human Disease? Diagnostics 2021, 11, 123. [Google Scholar] [CrossRef]
- Singh, V.P.; Pratap, K.; Sinha, J.; Desiraju, K.; Bahal, D.; Kukreti, R. Critical evaluation of challenges and future use of animals in experimentation for biomedical research. Int. J. Immunopathol. Pharmacol. 2016, 29, 551–561. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Wu, Y.; Yu, C. The efficacy and safety of St. John’s wort extract in depression therapy compared to SSRIs in adults: A meta-analysis of randomized clinical trials. Adv. Clin. Exp. Med. 2023, 32, 151–161. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, Y. A meta-analysis on the efficacy and safety of St John’s wort extract in depression therapy in comparison with selective serotonin reuptake inhibitors in adults. Neuropsychiatr. Dis. Treat. 2016, 12, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–391. [Google Scholar] [CrossRef]
- Tanaka, M.; Kádár, K.; Tóth, G.; Telegdy, G. Antidepressant-like effects of urocortin 3 fragments. Brain Res. Bull. 2011, 84, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Adamu, M.J.; Qiang, L.; Zakariyya, R.S.; Nyatega, C.O.; Kawuwa, H.B.; Younis, A. An Efficient Turbo Decoding and Frequency Domain Turbo Equalization for LTE Based Narrowband Internet of Things (NB-IoT) Systems. Sensors 2021, 21, 5351. [Google Scholar] [CrossRef] [PubMed]
- Liston, C.; Malter, C.M.; Teslovich, T.; Levenson, D.; Casey, B. Atypical Prefrontal Connectivity in Attention-Deficit/Hyperactivity Disorder: Pathway to Disease or Pathological End Point? Biol. Psychiatry 2011, 69, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, A.; Tanaka, M.; Vécsei, L. Exploring Novel Therapeutic Targets in the Common Pathogenic Factors in Migraine and Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 4114. [Google Scholar] [CrossRef]
| Author, Year | Exercise | Exercise Prescription | Effectiveness |
|---|---|---|---|
| Dilorenzo et al., 1999 [112] | Cycling | 70–80%HRR, 4 times a week, 12 weeks. | Beck Depression Inventory Scores show a significant reduction, intervention remains effective one year later. |
| Olson et al., 2017 [114] | Running | 40–65%HRR, 3 times a week, 8 weeks. | Improvement in cognitive control, depressive symptoms, and ruminative thinking patterns in patients with depression. |
| Dziubek et al., 2016 [123] | Endurance exercise | 3 times a week, 24 weeks. | Enhancing mood and reducing anxiety. |
| Kwok et al., 2019 [119] | Yoga | 90 min per session, 8 weeks. | Reducing anxiety and depression symptoms in Parkinson’s patients. |
| James-Paler et al., 2020 [118] | Yoga | 30 min per session, 2–3 times per week, 12 weeks. | Reducing anxiety and depression symptoms in teenagers. |
| Gordon et al., 2018 [120] | Resistance training | 3 times per week, 52 weeks. | Significantly reducing symptoms of depression in adults. |
| Kim et al., 2019 [125] | Resistance training | 30–60 min per session, 3 times a week, 24 weeks. | There is a significant decrease in neurotransmitters 5-HT, DA, NA, and NE in elderly female patients with depression. |
| Moraes et al., 2020 [124] | Resistance training | 2 times per week, 12 weeks. | Significantly reduces HAMD and BDI scores in elderly patients with depression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Xiao, H. Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression. Life 2023, 13, 1505. https://doi.org/10.3390/life13071505
Ren J, Xiao H. Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression. Life. 2023; 13(7):1505. https://doi.org/10.3390/life13071505
Chicago/Turabian StyleRen, Jianchang, and Haili Xiao. 2023. "Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression" Life 13, no. 7: 1505. https://doi.org/10.3390/life13071505
APA StyleRen, J., & Xiao, H. (2023). Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression. Life, 13(7), 1505. https://doi.org/10.3390/life13071505






