Retromode Scanning Laser Ophthalmoscopy for Choroidal Nevi: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Acquisition and Grading
2.2. Statistical Analysis
3. Results
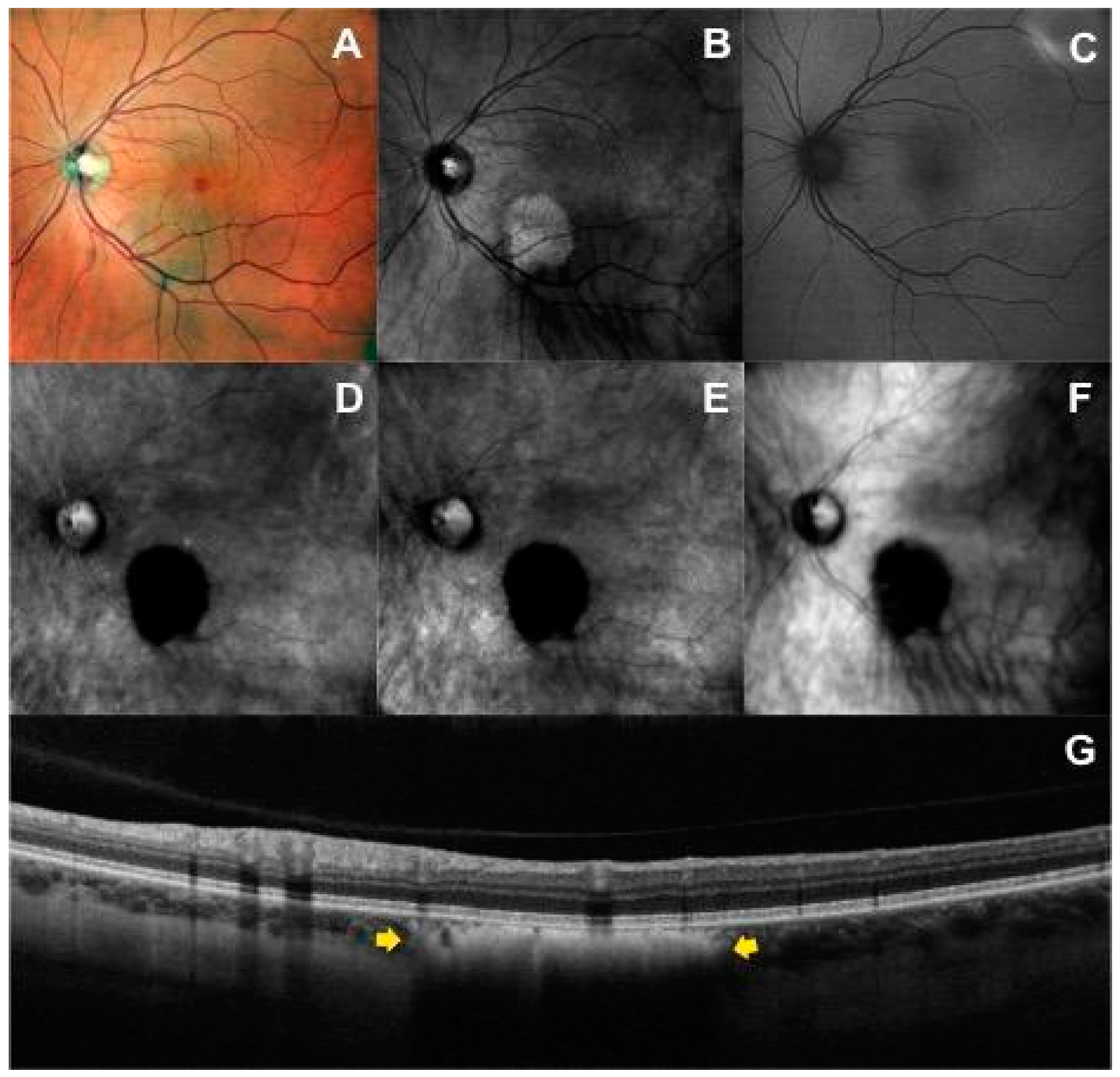
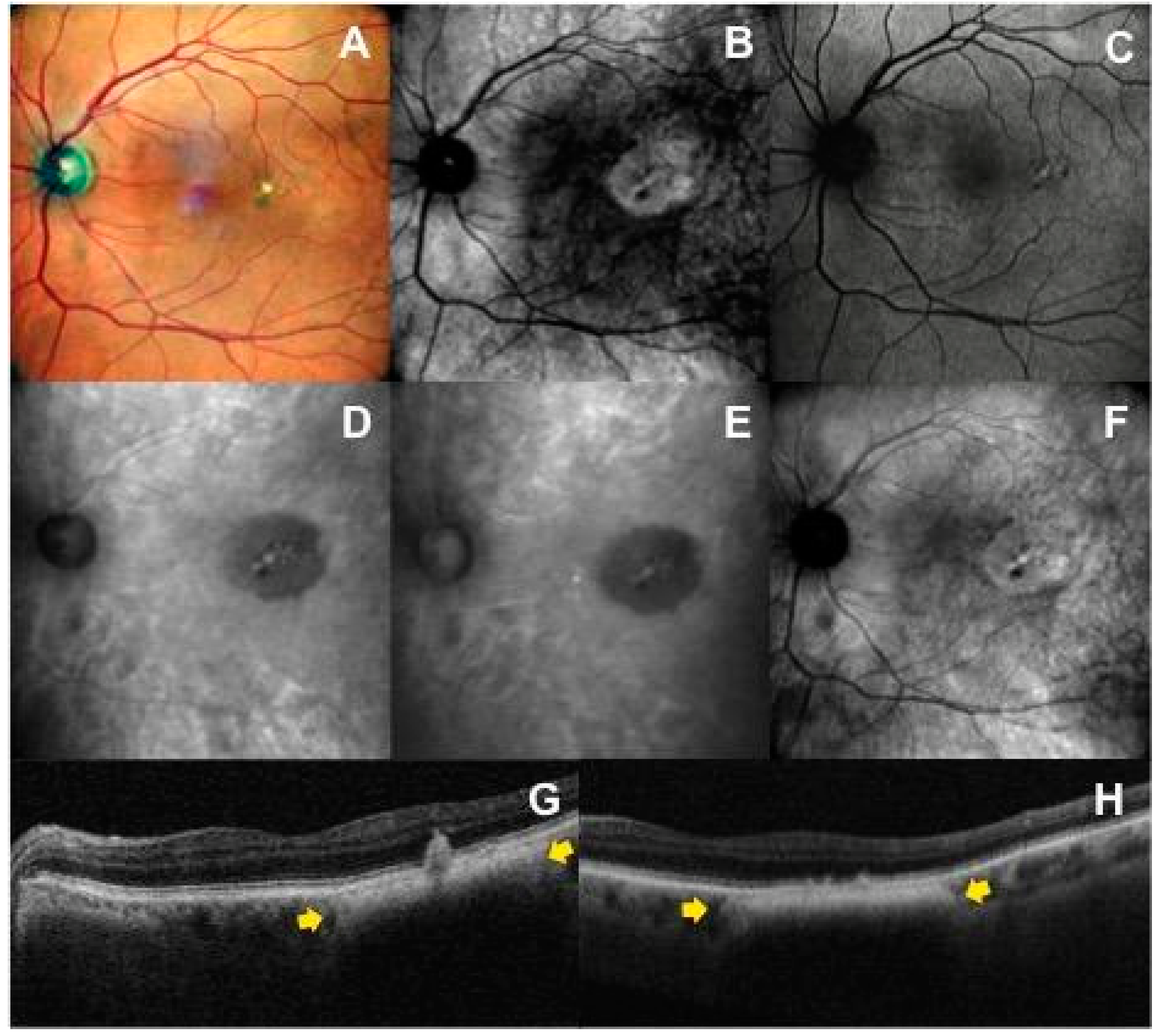
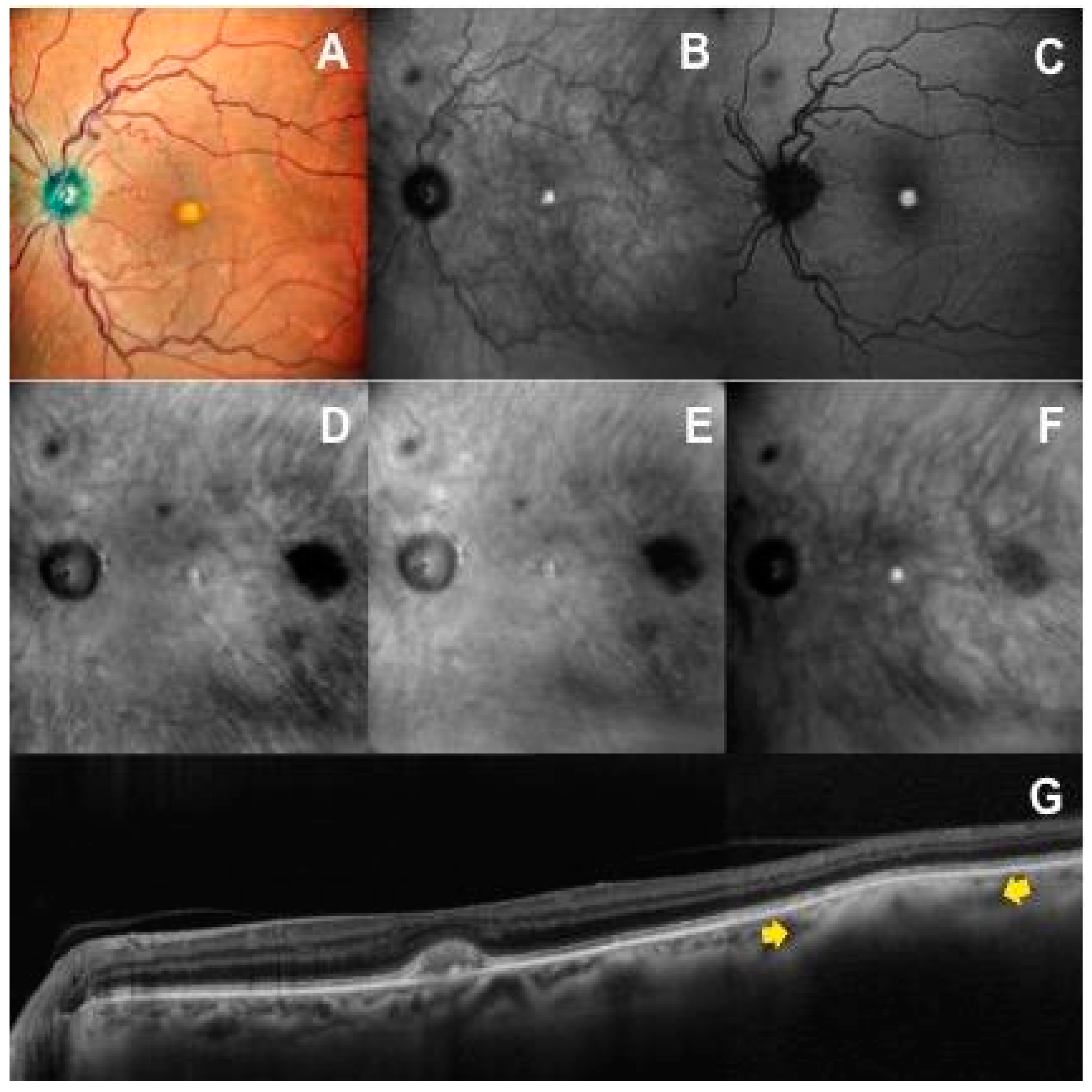
3.1. Retromode and Dark-Field Imaging
3.2. Correlation between Imaging Modalities
3.2.1. Spectral-Domain Optical Coherence Tomography Imaging
3.2.2. Multicolor Fundus Imaging
3.2.3. Infrared Reflectance Imaging
3.2.4. Fundus Autofluorescence Imaging
3.2.5. Visualization of Lesion Border
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sumich, P.; Mitchell, P. Choroidal nevi in a white population: The Blue Mountains Eye Study. Arch. Ophthalmol. 1998, 116, 645–650. [Google Scholar] [CrossRef]
- Chien, J.L.; Sioufi, K. Choroidal nevus: A review of prevalence, features, genetics, risks, and outcomes. Curr. Opin. Ophthalmol. 2017, 28, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Kalyani, P. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005, 112, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Dalvin, L.A. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina 2019, 39, 1840–1851. [Google Scholar] [CrossRef]
- Geiger, F.; Said, S. Assessing Choroidal Nevi, Melanomas and Indeterminate Melanocytic Lesions Using Multimodal Imaging-A Retrospective Chart Review. Curr. Oncol. 2022, 29, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L. Application of Multimodal and Molecular Imaging Techniques in the Detection of Choroidal Melanomas. Front. Oncol. 2021, 10, 617868. [Google Scholar] [CrossRef]
- Elsner, A.E.; Burns, S.A. Infrared imaging of sub-retinal structures in the human ocular fundus. Vis. Res. 1996, 36, 191–205. [Google Scholar] [CrossRef]
- Geeraets, W.J.; Williams, R.C. The loss of light energy in retina and choroid. Arch. Ophthalmol. 1960, 64, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lee, B.R. Retromode imaging: Review and perspectives. Saudi J. Ophthalmol. 2014, 28, 88–94. [Google Scholar] [CrossRef]
- Mainster, M.A.; Desmettre, T. Scanning laser ophthalmoscopy retroillumination: Applications and illusions. Int. J. Retin. Vitr. 2022, 8, 71. [Google Scholar] [CrossRef]
- Elsner, A.E.; Burns, S.A. Reflectometry with a scanning laser ophthalmoscope. Appl. Opt. 1992, 31, 3697–3710. [Google Scholar] [CrossRef] [PubMed]
- Woon, W.H.; Fitzke, F.W. Confocal imaging of the fundus using a scanning laser ophthalmoscope. Br. J. Ophthalmol. 1992, 76, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Vallabh, N.A.; Sahni, J.N. Near-infrared reflectance and autofluorescence imaging characteristics of choroidal nevi. Eye 2016, 30, 1593–1597. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Pucci, P. Extent of diabetic macular edema by scanning laser ophthalmoscope in the retromode and its functional correlations. Retina 2014, 34, 2416–2422. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, X. The noninvasive retro-mode imaging of confocal scanning laser ophthalmoscopy in myopic maculopathy: A prospective observational study. Eye 2014, 28, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, K.; Tsuiki, E. Visualization of subthreshold micropulse diode laser photocoagulation by scanning laser ophthalmoscopy in the retro mode. Am. J. Ophthalmol. 2010, 150, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Mercuri, S. Scanning Laser Ophthalmoscopy Retromode Imaging Compared to Fundus Autofluorescence in Detecting Outer Retinal Features in Central Serous Chorioretinopathy. Diagnostics 2022, 12, 2638. [Google Scholar] [CrossRef]
- Pilotto, E.; Sportiello, P. Confocal scanning laser ophthalmoscope in the retromode imaging modality in exudative age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 27–34. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, X. The noninvasive retro-mode imaging modality of confocal scanning laser ophthalmoscopy in polypoidal choroidal vasculopathy: A preliminary application. PLoS ONE 2013, 8, e75711. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, S.U. Evaluation of Retromode Imaging for Use in Hydroxychloroquine Retinopathy. Am. J. Ophthalmol. 2018, 196, 44–52. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology 2008, 115, 546–552. [Google Scholar] [CrossRef]
- Song, W.; Zhang, L. Wavelength-dependent optical properties of melanosomes in retinal pigmented epithelium and their changes with melanin bleaching: A numerical study. Biomed. Opt. Express 2017, 8, 3966–3980. [Google Scholar] [CrossRef]
- Acton, J.H.; Cubbidge, R.P. Drusen detection in retro-mode imaging by a scanning laser ophthalmoscope. Acta Ophthalmol. 2011, 89, e404–e411. [Google Scholar] [CrossRef]
- Corradetti, G.; Corvi, F. Subretinal Drusenoid Deposits Revealed by Color SLO and Retro-Mode Imaging. Ophthalmology 2021, 128, 409. [Google Scholar] [CrossRef] [PubMed]
- Monteduro, D.; Cozzi, M. Topographic detection and characterization of drusen and subretinal drusenoid deposits: Contribution of Retro mode modality in a multimodal imaging approach. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3471. [Google Scholar]
- Kulikov, A.N.; Maltsev, D.S. Characterization of choroidal nevi with dark-field infrared scanning laser ophthalmoscopy. Ophthalmol. Retin. 2019, 3, 703–708. [Google Scholar] [CrossRef]
- Bindewald-Wittich, A.; Holz, F.G. Fundus Autofluorescence Imaging in Patients with Choroidal Melanoma. Cancers 2022, 14, 1809. [Google Scholar] [CrossRef] [PubMed]
- Lavinsky, D.; Belfort, R.N. Fundus autofluorescence of choroidal nevus and melanoma. Br. J. Ophthalmol. 2007, 91, 1299–1302. [Google Scholar] [CrossRef]
- Muftuoglu, I.K.; Gaber, R. Comparison of conventional color fundus photography and multicolor imaging in choroidal or retinal lesions. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, R.; Pereira, A. Variability in Imaging Findings in Choroidal Nevus Using Multicolor Imaging Vis-à-vis Color Fundus Photography. J. Curr. Ophthalmol. 2020, 32, 285–289. [Google Scholar] [CrossRef] [PubMed]

| Retromode-DR (DR Aperture) | Retromode-DL (DL Aperture) | Dark-Field (RA Aperture) | |
|---|---|---|---|
| Level of retro-reflectivity | |||
| Hypo-retro-reflective | 40/41 (97.6%) | 41/41 (100%) | 36/41 (87.8%) |
| - Dark shadow | 37/41 (90.2%) | 37/41 (90.2%) | 27/41 (65.9%) |
| - Gray shadow | 3/41 (7.3%) | 4/41 (9.8%) | 9/41 (22.0%) |
| Iso-retro-reflective | 0 | 0 | 3/41 (7.3%) |
| Hyper-retro-reflective | 0 | 0 | 2/41 (4.9%) |
| Not available | 1/41 (2.4%) | 0 | 0 |
| Multicolor | Infrared | Retromode-DR (DR Aperture) | Retromode-DL (DL Aperture) | Dark-Field (RA Aperture) | |
|---|---|---|---|---|---|
| Visualization of nevus | |||||
| - Not available | 0 | 0 | 1/41 (2.4%) | 0 | 0 |
| - Not visible | 13/41 (31.7%) | 4/41 (9.8%) | 0 | 0 | 0 |
| - Visible | 28/41 (68.3%) | 37/41 (90.2%) | 40/41 (97.6%) | 41/41 (100%) | 41/41 (100%) |
| Visualization of border | |||||
| - Barely visible | 13/41 (31.7%) | 10/41 (24.4%) | 6/41 (14.6%) | 7/41 (17.1%) | 12/41 (29.3%) |
| - Clearly visible | 15/41 (36.6%) | 27/41 (65.9%) | 34/41 (82.9%) | 34/41 (82.9%) | 29/41 (70.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzolini, C.; Di Nicola, M.; Pozzo Giuffrida, F.; Cappelli, F.; Bellina, C.; Viola, F.; Chelazzi, P. Retromode Scanning Laser Ophthalmoscopy for Choroidal Nevi: A Preliminary Study. Life 2023, 13, 1253. https://doi.org/10.3390/life13061253
Azzolini C, Di Nicola M, Pozzo Giuffrida F, Cappelli F, Bellina C, Viola F, Chelazzi P. Retromode Scanning Laser Ophthalmoscopy for Choroidal Nevi: A Preliminary Study. Life. 2023; 13(6):1253. https://doi.org/10.3390/life13061253
Chicago/Turabian StyleAzzolini, Claudia, Maura Di Nicola, Francesco Pozzo Giuffrida, Francesca Cappelli, Claudia Bellina, Francesco Viola, and Paolo Chelazzi. 2023. "Retromode Scanning Laser Ophthalmoscopy for Choroidal Nevi: A Preliminary Study" Life 13, no. 6: 1253. https://doi.org/10.3390/life13061253
APA StyleAzzolini, C., Di Nicola, M., Pozzo Giuffrida, F., Cappelli, F., Bellina, C., Viola, F., & Chelazzi, P. (2023). Retromode Scanning Laser Ophthalmoscopy for Choroidal Nevi: A Preliminary Study. Life, 13(6), 1253. https://doi.org/10.3390/life13061253






