Epidemiology Pattern, Prevalent Genotype Distribution, Fighting Stigma and Control Options for Hepatitis D in Bulgaria and Other European Countries
Abstract
1. Introduction
2. The Contemporary Patterns of Chronic Hepatitis D: A Literature Review
2.1. Epidemiology of HDV in General and Hepatology Clinic Populations
2.2. Forms of HBV/HDV Infection
2.3. Prevalent HDV Genotypes and Their Pathogenicity
2.4. Management of Chronic Hepatitis D and Options for Viral Control
2.4.1. HDV Laboratory Tests
Anti-HDV Antibody (Ab) IgM and IgG
HDV Antigen (Ag)
HDV RNA
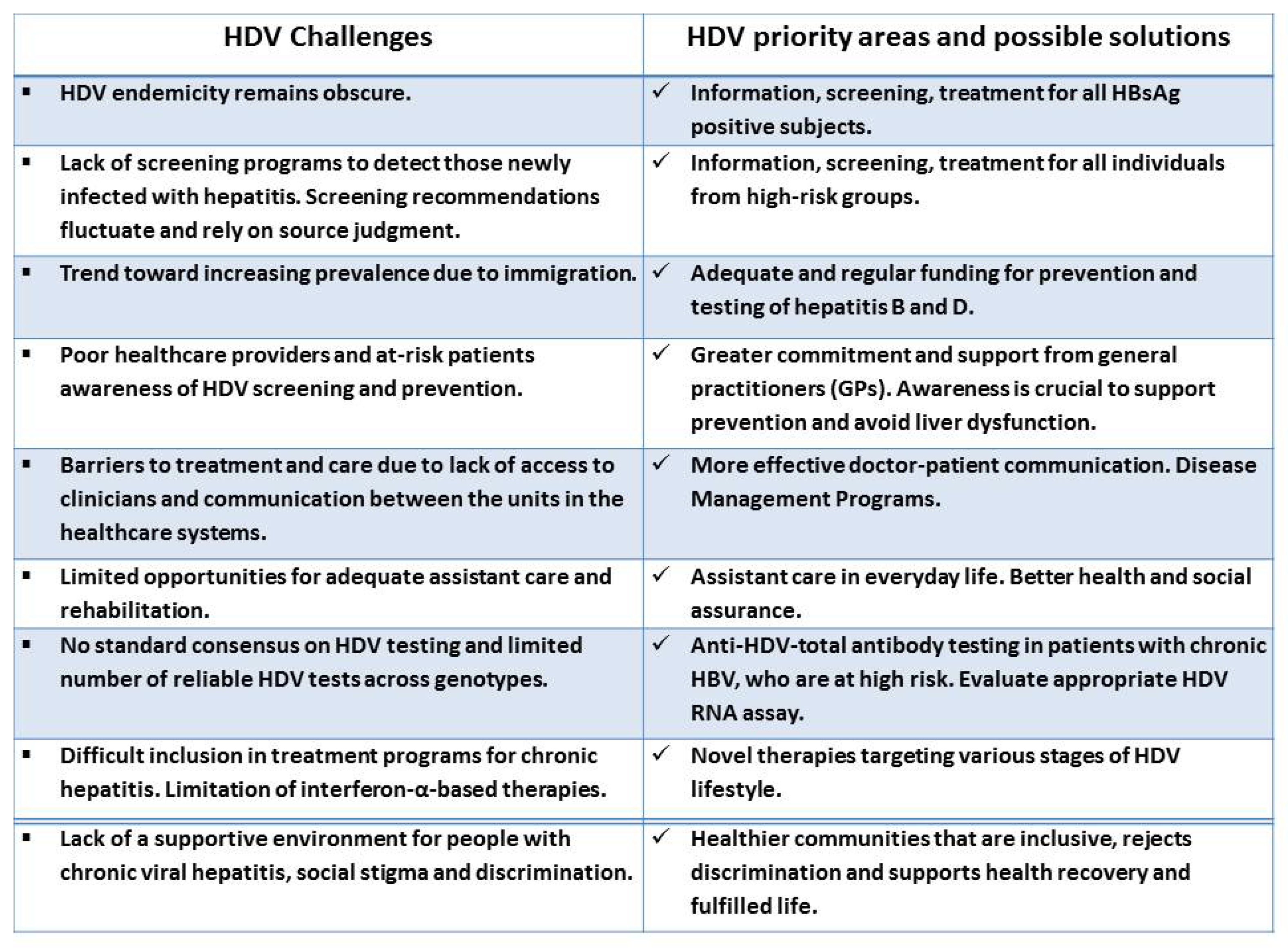
2.4.2. HDV Awareness and Screening Guidelines
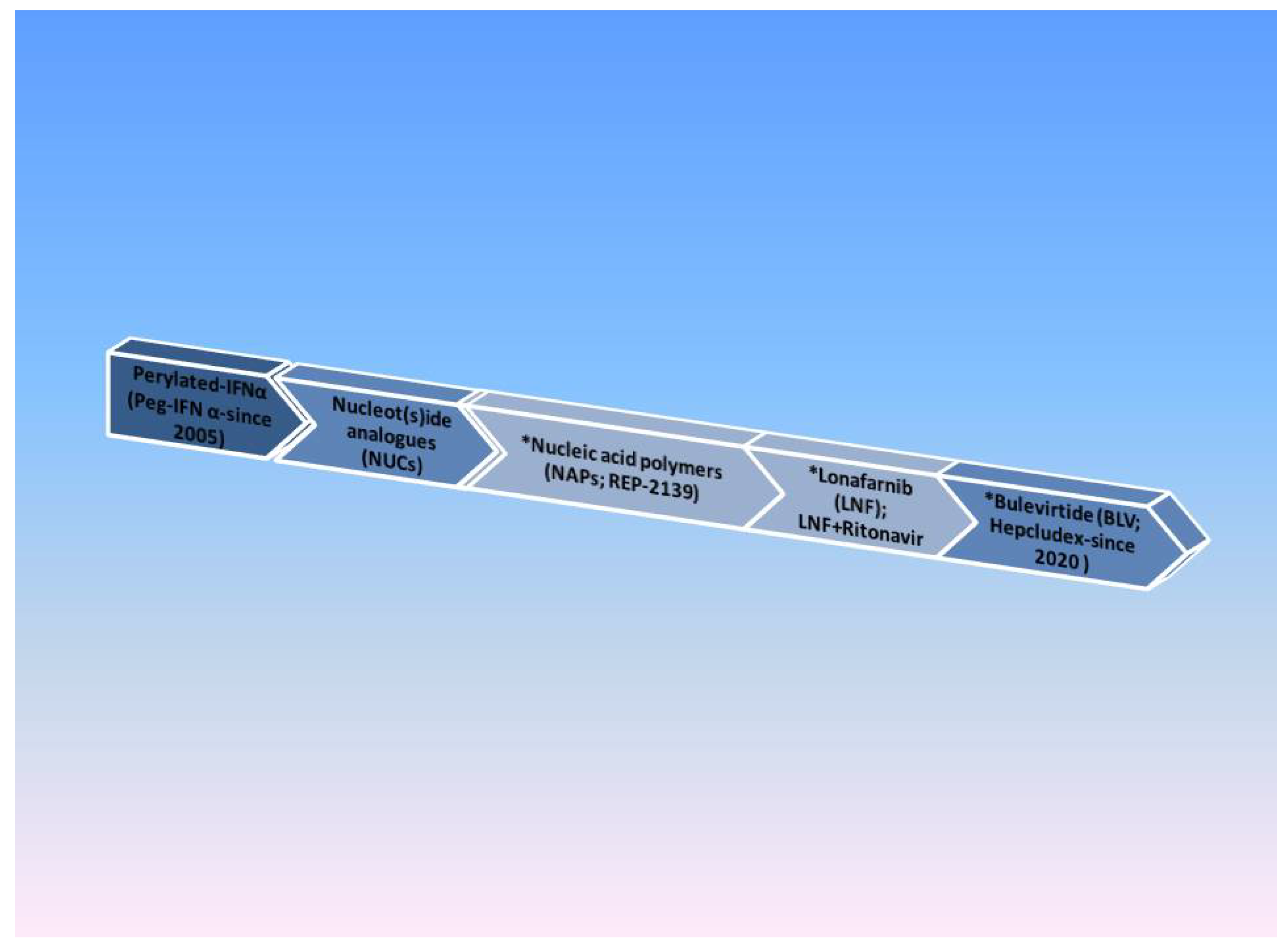
2.4.3. Management of Chronic HDV
Nucleic Acid Polymers (NAPs)
The Farnesyl-Transferase Inhibitor Lonafarnib (LNF)
Bulevirtide (BLV; Hepcludex)
2.4.4. Life with Chronic HDV—Fighting Stigma and Discrimination
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzetto, M. The Discovery of the Hepatitis D virus: Three Princes of serendip and the recognition of autoantibodies to liver-Kidney Microsomes. Clin. Liver Dis. 2020, 16, 2046–2484. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Ciancio, A.; Rizzetto, M. A Review of HDV Infection. Viruses 2022, 14, 1749. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, M.; Stroffolini, T. Forty-Five Years after the Discovery of the Hepatitis D Virus: Where Do We Stand? Viruses 2021, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Gerin, J.L. The Taxonomy of Hepatitis Delta Virus. In Viral Hepatitis and Liver Disease; Nishioka, K., Suzuki, H., Mishiro, S., Oda, T., Eds.; Springer: Tokyo, Japan, 1994; pp. 63–64. [Google Scholar]
- Bender, D.; Glitscher, M.; Hildt, E. Viral hepatitis A to E: Prevalence, pathogen characteristics, and pathogenesis. Bundesgesundheitsblatt Gesundh. Gesundh. 2022, 65, 139–148. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses (ICTV) 2023. Delta Virus. Available online: https://ictv.global/taxonomy (accessed on 1 April 2023).
- Urban, S.; Neumann-Haefelin, C.; Lampertico, P. Hepatitis D virus in 2021: Virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut 2021, 70, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Botelho-Souza, L.F.; Vasconcelos, M.P.A.; Dos Santos, A.O.; Salcedo, J.M.V.; Vieira, D.S. Hepatitis delta: Virological and clinical aspects. Virol. J. 2017, 14, 177. [Google Scholar] [CrossRef]
- Littlejohn, M.; Locarnini, S.; Yuen, L. Origins and Evolution of Hepatitis B Virus and Hepatitis D Virus. Cold Spring Harb. Perspect. Med. 2016, 6, a021360. [Google Scholar] [CrossRef]
- Modahl, L.E.; Macnaughton, T.B.; Zhu, N.; Johnson, D.L.; Lai, M.M. RNA-Dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol. Cell Biol. 2000, 20, 6030–6039. [Google Scholar] [CrossRef]
- Netter, H.J.; Barrios, M.H.; Littlejohn, M.; Yuen, L.K.W. Hepatitis Delta Virus (HDV) and Delta-Like Agents: Insights Into Their Origin. Front. Microbiol. 2021, 12, 652962. [Google Scholar] [CrossRef]
- Jelen, M.; Hošnjak, L.; Štunf, S.; Zagožen, A.; Komloš, K.; Markočič, P.; Poljak, M.; Seme, K. Hepatitis D virus infection in Slovenian patients with chronic hepatitis B virus infection: A national prevalence study and literature review. Acta Derm. APA 2016, 25, 49–54. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Heidrich, B.; Manns, M.P. Hepatitis D virus infection—Not a vanishing disease in Europe! Hepatology 2007, 45, 1331–1332. [Google Scholar] [CrossRef] [PubMed]
- Reinheimer, C.; Doerr, H.W.; Berger, A. Hepatitis delta: On soft paws across Germany. Infection 2012, 40, 621–625. [Google Scholar] [CrossRef]
- Servant-Delmas, A.; Le Gal, F.; Gallian, P.; Gordien, E.; Laperche, S. Increasing prevalence of HDV/HBV infection over 15 years in France. J. Clin. Virol. 2014, 59, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, A.J.; Kreuels, B.; Henrion, M.Y.R.; Giorgi, E.; Kyomuhangi, I.; de Martel, C.; Hutin, Y.; Geretti, A.M. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020, 73, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Roulot, D.; Brichler, S.; Layese, R.; BenAbdesselam, Z.; Zoulim, F.; Thibault, V.; Scholtes, C.; Roche, B.; Castelnau, C.; Poynard, T.; et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J. Hepatol. 2020, 73, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Giustina, G.; Christensen, E.; Pantalena, M.; Zagni, I.; Realdi, G.; Schalm, S.W. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European concerted action on viral hepatitis (Eurohep). Gut 2000, 46, 420–426. [Google Scholar] [CrossRef]
- Petrova, G. Strategic choice and model for integrated health care in the Republic of Bulgaria in the conditions of our membership in the European Union. In Dissertation Paper 2012; MU: Varna, Bulgaria, 2012. [Google Scholar]
- Miao, Z.; Xie, Z.; Ren, L.; Pan, Q. Hepatitis D: Advances and challenges. Chin. Med. J. 2022, 135, 767–773. [Google Scholar] [CrossRef]
- Chen, H.Y.; Shen, D.T.; Ji, D.Z.; Han, P.C.; Zhang, W.M.; Ma, J.F.; Chen, W.S.; Goyal, H.; Pan, S.; Xu, H.G. Prevalence and burden of hepatitis D virus infection in the global population: A systematic review and meta-analysis. Gut 2019, 68, 512–521. [Google Scholar] [CrossRef]
- WHO. Hepatitis D. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-d (accessed on 28 March 2023).
- Amini, N.; Alavian, S.M.; Kabir, A.; Aaalei-Andabili, S.H.; Hosseini, S.Y.S.; Rizzetto, M. Prevalence of Hepatitis D in the Eastern Mediterranean Region: Systematic Review and Meta Analysis. Hepat. Mon. 2013, 13, e8210. [Google Scholar] [CrossRef]
- Değertekin, H.; Yalçin, K.; Yakut, M.; Yurdaydin, C. Seropositivity for delta hepatitis in patients with chronic hepatitis B and liver cirrhosis in Turkey: A meta-analysis. Liver Int. 2008, 28, 494–498. [Google Scholar] [CrossRef]
- Coghill, S.; McNamara, J.; Woods, M.; Hajkowicz, K. Epidemiology and clinical outcomes of hepatitis delta (D) virus infection in Queensland, Australia. Int. J. Infect. Dis. 2018, 74, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.U.; Thio, C.L.; Boon, D.; Thomas, D.L.; Tobian, A.A.R. Prevalence of Hepatitis B and Hepatitis D Virus Infections in the United States, 2011–2016. Clin. Infect. Dis. 2019, 69, 709–712. [Google Scholar] [CrossRef]
- Hayashi, T.; Takeshita, Y.; Hutin, Y.J.F.; Harmanci, H.; Easterbrook, P.; Hess, S.; van Holten, J.; Oru, E.O.; Kaneko, S.; Yurdaydin, C.; et al. The global hepatitis delta virus (HDV) epidemic: What gaps to address in order to mount a public health response? Arch. Public Health 2021, 79, 180. [Google Scholar] [CrossRef]
- Gheorghe, L.; Csiki, I.E.; Iacob, S.; Gheorghe, C.; Trifan, A.; Grigorescu, M.; Motoc, A.; Suceveanu, A.; Curescu, M.; Caruntu, F.; et al. Hepatitis Delta Virus Infection in Romania: Prevalence and Risk Factors. J. Gastrointestin. Liver Dis. 2015, 24, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Dulger, A.C.; Suvak, B.; Gonullu, H.; Gonullu, E.; Gultepe, B.; Aydın, İ.; Batur, A.; Karadas, S.; Olmez, Ş. High prevalence of chronic hepatitis D virus infection in eastern Turkey: Urbanization of the disease. Arch. Med. Sci. 2016, 12, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ivaniushina, V.; Radjef, N.; Alexeeva, M.; Gault, E.; Semenov, S.; Salhi, M.; Kiselev, O.; Dény, P. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J. Gen. Virol. 2001, 82, 2709–2718. [Google Scholar] [CrossRef] [PubMed]
- Rex, K.F.; Krarup, H.B.; Laurberg, P.; Andersen, S. Population-based comparative epidemiological survey of hepatitis B, D, and C among Inuit migrated to Denmark and in high endemic Greenland. Scand. J. Gastroenterol. 2012, 47, 692–701. [Google Scholar] [CrossRef]
- Niro, G.; Fontana, R.; Ippolito, A.; Andriulli, A. Epidemiology and Diagnosis of Hepatitis D Virus. Future Virol. 2012, 7, 709–717. [Google Scholar] [CrossRef]
- Smedile, A.; Lavarini, C.; Farci, P.; Aricò, S.; Marinucci, G.; Dentico, P.; Giuliani, G.; Cargnel, A.; Del Vecchio Blanco, C.; Rizzetto, M. Epidemiologic patterns of infection with the hepatitis B virus-associated delta agent in Italy. Am. J. Epidemiol. 1983, 117, 223–229. [Google Scholar] [CrossRef]
- Sagnelli, E.; Stroffolini, T.; Ascione, A.; Bonino, F.; Chiaramonte, M.; Colombo, M.; Craxi, A.; Giusti, G.; Manghisi, O.G.; Pastore, G.; et al. The epidemiology of hepatitis delta infection in Italy. Promoting Group. J. Hepatol. 1992, 15, 211–215. [Google Scholar] [CrossRef]
- Sagnelli, E.; Stroffolini, T.; Ascione, A.; Chiaramonte, M.; Craxì, A.; Giusti, G.; Piccinino, F. Decrease in HDV endemicity in Italy. J. Hepatol. 1997, 26, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, G.B.; Stroffolini, T.; Chiaramonte, M.; Ascione, T.; Stornaiuolo, G.; Lobello, S.; Sagnelli, E.; Brunetto, M.R.; Rizzetto, M. Chronic hepatitis D: A vanishing disease? An Italian multicenter study. Hepatology 2000, 32, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Almasio, P.L.; Sagnelli, E.; Mele, A.; Gaeta, G.B. Evolving clinical landscape of chronic hepatitis B: A multicenter Italian study. J. Med. Virol. 2009, 81, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Badia, L.; Cultera, R.; Grilli, A.; De Togni, A. Epidemiological, clinical and laboratory features of chronic hepatitis B infection in a cohort of immigrant and Italian patients from Ferrara, Italy. Ann. Hepatol. 2012, 11, 862–869. [Google Scholar] [CrossRef]
- De Paschale, M.; Manco, M.T.; Belvisi, L.; Magnani, C.; Re, T.; Viganò, P.; Biagiotti, S.; Capelli, F.; Mazzone, A.; Baldacci, M.P.; et al. Epidemiology of hepatitis D virus (HDV) infection in an urban area of northern Italy. Infection 2012, 40, 485–491. [Google Scholar] [CrossRef]
- Brancaccio, G.; Giuberti, T.; Verucchi, G.; Levantesi, M.; Sacchini, D.; Fattovich, G.; Madona, S.; Fasano, M.; Gavrila, C.; Nardi, A.; et al. Epidemiological evolution of chronic hepatitis delta in Italy. An analysis of the Master-B cohort. Dig. Liver Dis. 2014, 46, 12–13. [Google Scholar] [CrossRef]
- Stroffolini, T.; Ciancio, A.; Furlan, C.; Vinci, R.; Fontana, M.R.; Russello, M.; Colloredo, G.; Morisco, F.; Coppola, N.; Babudieri, S.; et al. Migratory Flow and Hepatitis Delta Infection in Italy: A New Challenge at the Beginning of the Third Millennium. J. Viral. Hepat. 2020, 27, 941–947. [Google Scholar] [CrossRef]
- Naoumov, N.V.; Gueorgiev, A.; Ognyanov, M.; Maleev, A. Infection with hepatitis delta virus in patients with fulminant hepatitis B and chronic HBsAg carriers in Bulgaria. Hepatogastroenterology 1986, 33, 49–51. [Google Scholar]
- Iliev, B.; Mitov, G.; Radev, M.; Denchev, V.; Gancheva, T.; Baev, V.; Angelov, L.; Iliebva, P.; Miteva, R. Viral hepatitis B. In Infectology 2001; Prof. M.Drinov: Sofia, Bulgaria, 2001; pp. 654–666. (In Bulgarian) [Google Scholar]
- Krastev, Z.; Antonov, K.; Jelev, D.; Toieva, E.; Zheleva, N. HDV infection in Bulgaria. Rom. J. Hepatol. 2013, 9, 33–34. [Google Scholar]
- Tsaneva-Damyanova, D. Clinical and Laboratory Study on the Distribution of Virus Hepatitis B and D in General Population and for Patients with Chronic Liver Diseases in North-eastern Bulgaria. HBV Post-immune Response Persistence after HBV vaccination. In Dissertation Paper 2019; Medical University of Varna (Bulgaria): Varna, Bulgaria, PQDT-Global; 2019; Available online: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=UfWEl3MAAAAJ&cstart=20&pagesize=80&sortby=pubdate&citation_for_view=UfWEl3MAAAAJ:hC7cP41nSMkC (accessed on 28 March 2023). (In Bulgarian)
- Popov, G.; Andonova, R. Prevalence of hepatitis A, B, C and D infections among Bulgarian prison inmates. Malar. Control Elimin. 2020, 9, 3. [Google Scholar]
- Manesis, E.K.; Vourli, G.; Dalekos, G.; Vasiliadis, T.; Manolaki, N.; Hounta, A.; Koutsounas, S.; Vafiadis, I.; Nikolopoulou, G.; Giannoulis, G.; et al. Prevalence and clinical course of hepatitis delta infection in Greece: A 13-year prospective study. J. Hepatol. 2013, 59, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, B.; Deterding, K.; Tillmann, H.L.; Raupach, R.; Manns, M.P.; Wedemeyer, H. Virological and clinical characteristics of delta hepatitis in Central Europe. J. Viral. Hepat. 2009, 16, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Curran, R.A.; O’Neill, H.J.; Connolly, J.H. Hepatitis delta virus infection in Northern Ireland 1970–1989. Ulst. Med. J. 1991, 60, 159–163. [Google Scholar]
- Cross, T.J.S.; Rizzi, P.; Horner, M.; Jolly, A.; Hussain, M.J.; Smith, H.M.; Vergani, D.; Harrison, P. The increasingprevalence of hepatitis delta virus (HDV) infection in South London. J. Med. Virol. 2008, 80, 277–282. [Google Scholar] [CrossRef]
- William Tong, C.Y.; Asher, R.; Toby, M.; Ngui, S.L.; Tettmar, K.; Ijaz, S.; Tedder, R.; Kulasegaram, R.; Wilkinson, M.; Wong, T. A re-assessment of the epidemiology and patient characteristics of hepatitis D virus infection in inner city London. J. Infect. 2013, 66, 521–527. [Google Scholar] [CrossRef]
- El Bouzidi, K.; Elamin, W.; Kranzer, K.; Irish, D.N.; Ferns, B.; Kennedy, P.; Rosenberg, W.; Dusheiko, G.; Sabin, C.A.; Smith, B.C.; et al. Hepatitis delta virus testing, epidemiology and management: A multicentre cross-sectional study of patients in London. J. Clin. Virol. 2015, 66, 33–37. [Google Scholar] [CrossRef]
- Frisch-Niggemeyer, W.; Kunz, C. Delta virus: Now also detected in Austria. A defective virus as a pathogenic agent. Wien. Klin. Wochenschr. 1985, 97, 460–463. (In German) [Google Scholar]
- Jachs, M.; Binter, T.; Schmidbauer, C.; Hartl, L.; Strasser, M.; Laferl, H.; Hametner-Schreil, S.; Lindorfer, A.; Dax, K.; Stauber, R.E.; et al. Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis. United Eur. Gastroenterol. J. 2021, 9, 1119–1127. [Google Scholar] [CrossRef]
- Kondili, L.A.; Cuko, L.; Chionne, P.; Candido, A.; Madonna, E.; Dentico, P.; Resuli, B.; Taliani, G.; Brunetto, M.R.; Rapicetta, M. Hepatitis B, C and delta virus infections in Albanian patients with chronic liver disease: Evaluation of possible changes during the last 10 years. Eur. J. Gastroenterol. Hepatol. 2010, 22, 167–171. [Google Scholar] [CrossRef]
- Ho, E.; Deltenre, P.; Nkuize, M.; Delwaide, J.; Colle, I.; Michielsen, P. Belgian Association for the Study of the Liver. Coinfection of hepatitis B and hepatitis delta virus in Belgium: A multicenter BASL study. Prospective epidemiology and comparison with HBV mono-infection. J. Med. Virol. 2013, 85, 1513–1517. [Google Scholar] [CrossRef]
- Krogsgaard, K.; Mathiesen, L.R.; Aldershvile, J.; Kryger, P.; Andersson, P.; Hansson, B.G.; Resuli, B.; Taliani, G.; Brunetto, M.R.; Rapicetta, M. Delta infection and hepatitis B virus replication in Danish patients with fulminant hepatitis B. Scand. J. Infect. Dis. 1988, 20, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Jelić, D.; Jelić, O. Epidemiological characteristics of HBV and HDV chronic liver diseases. Acta Med. Croat. 1994, 48, 7–13. [Google Scholar]
- Horváth, G.; Tolvaj, G.; Stotz, G.; Dávid, K. The incidence of hepatitis delta virus infection in chronic liver diseases in Hungary. Acta Med. Hung. 1993, 49, 109–117. [Google Scholar]
- Hříbek, P.; Klasová, J.; Tůma, T.; Kupsa, T.; Urbánek, P. Etiopathogenetic Factors of Hepatocellular Carcinoma, Overall Survival, and Their Evolution over Time—Czech Tertiary Center Overview. Medicina 2022, 58, 1099. [Google Scholar] [CrossRef] [PubMed]
- Turcanu, A.; Pitel, E.; Dumbrava, V.T.; Tcaciuc, E.; Donscaia, A.; Peltec, A.; Pineau, P. Profile of hepatocellular carcinoma in the Republic of Moldova: First-hand information on the presentation, distribution and etiologies. Rom. J. Intern. Med. 2019, 57, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, G.; Ramadani, N.; Pattaro, C.; Cami, A.; Dentico, P.; Volpe, A.; Pellizzer, G.; Berisha, A.; Smacchia, C.; Figliomeni, M.; et al. Prevalence and risk factors for viral hepatitis in the Kosovarian population: Implications for health policy. J. Med. Virol. 2008, 80, 833–840. [Google Scholar] [CrossRef]
- Delić, D.; Gotić, M.; Ostrić, V.; Fridman, V.; Nikolić, P.; Jemuović, L.; Nikolov, V.; Zerjav, S.; Groza, S. Epidemiology of hepatitis D virus (delta) infection in Yugoslavia. Liver 1993, 13, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Chlabicz, S.; Grzeszczuk, A.; Lapiński, T.W.; Prokopowicz, D.; Panasiuk, A. Search for hepatitis delta virus (HDV) infection in hepatitis C patients in north-eastern Poland. Comparison with anti-HDV prevalence in chronic hepatitis B. Eur. J. Epide Miol. 2003, 18, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, K.P.; Zietkowski, D.; Charmuszko, U.; Sikorska, K.; Stalke, P. Hepatitis delta virus infection in chronically HBV-infected patients from northern Poland. Arch. Virol. 2006, 151, 1207–1215. [Google Scholar] [CrossRef]
- Popescu, G.A.; Otelea, D.; Gavriliu, L.C.; Neaga, E.; Popescu, C.; Paraschiv, S.; Fratila, M. Epidemiology of hepatitis D in patients infected with hepatitis B virus in Bucharest: A cross-sectional study. J. Med. Virol. 2013, 85, 769–774. [Google Scholar] [CrossRef]
- Ramalho, F.; Carvalho, G.; Bonino, F.; Baptista, A.; de Moura, M.C. Clinical and epidemiological significance of hepatitis delta virus (HDV) infection in chronic HBV carriers in Portugal. Prog. Clin. Biol. Res. 1987, 234, 409–417. [Google Scholar] [PubMed]
- Rivas, P.; Herrero, M.D.; Poveda, E.; Madejón, A.; Treviño, A.; Gutiérrez, M.; Ladrón de Guevara, C.; Lago, M.; de Mendoza, C.; Soriano, V.; et al. Hepatitis B, C, and D and HIV infections among immigrants from Equatorial Guinea living in Spain. Am. J. Trop. Med. Hyg. 2013, 88, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Montero, J.V.; Vispo, E.; Barreiro, P.; Sierra-Enguita, R.; de Mendoza, C.; Soriano, V. Hepatitis delta is a major determinant of liver decompensation events and death in HIV-infected patients. Clin. Infect. Dis. 2014, 58, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Cuenza-Gómez, J.A.; Salas-Coronas, J.; Soriano-Pérez, M.J.; Vázquez-Villegas, J.; Lozano-Serrano, A.B.; Cabezas-Fernández, M.T. Viral hepatitis and immigration: A challenge for the healthcare system. Rev. Clin. Esp. 2016, 216, 248–252. [Google Scholar]
- Ordieres, C.; Navascués, C.A.; González-Diéguez, M.L. Prevalence and epidemiology of hepatitis D among patients with chronic hepatitis B virus infection: A report from Northern Spain. Eur. J. Gastroenterol. Hepatol. 2017, 29, 277–283. [Google Scholar]
- Aguilera, A.; Trastoy, R.; Barreiro, P.; Costa, J.J.; de Mendoza, C.; Peña, J.M.; Soriano, V. Decline and changing profile of hepatitis delta among injection drug users in Spain. Antivial Ther. 2018, 23, 87–90. [Google Scholar] [CrossRef]
- Ji, J.; Sundquist, K.; Sundquist, J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J. Natl. Cancer Inst. 2012, 104, 790–792. [Google Scholar] [CrossRef]
- Genné, D.; Rossi, I. Hepatitis delta in Switzerland: A silent epidemic. Swiss Med. Wkly. 2011, 141, w13176. [Google Scholar] [CrossRef]
- Hirzel, C.; Wandeler, G.; Owczarek, M.; Gorgievski-Hrisoho, M.; Dufour, J.F.; Semmo, N.; Zucher, S. Molecular epidemiology of hepatitis B virus infection in Switzerland: A retrospective cohort study. BMC Infect. Dis. 2015, 15, 483. [Google Scholar] [CrossRef]
- Wedemeyer, H.L.; Manns, M.P. Epidemiology, pathogenesis and management of hepatitis D: Update and challenges ahead. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 31–40. [Google Scholar] [CrossRef]
- Tsaneva-Damyanova, D. Disease Management and Survival in Patients with Chronic Hepatitis D (HDV). Master’s Thesis, Medical University of Varna (Bulgaria), Varna, Bulgaria, 2021. (In Bulgarian). [Google Scholar]
- EASL. Clinical Practive Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar]
- Mentha, N.; Clément, S.; Negro, F.; Alfaiate, D. A review on hepatitis D: From virology to new therapies. J. Adv. Res. 2019, 17, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M. Infection by Hepatitis Delta Virus. Viruses 2020, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Sausen, D.G.; Shechter, O.; Bietsch, W.; Shi, Z.; Miller, S.M.; Gallo, E.S.; Dahari, H.; Borenstein, R. Hepatitis B and Hepatitis D Viruses:A Comprehensive Update with an Immunological Focus. Int. J. Mol. Sci. 2022, 23, 15973. [Google Scholar] [CrossRef]
- Lee, A.U.; Lee, C. Hepatitis D Review: Challenges for the Resource-Poor Setting. Viruses 2021, 13, 1912. [Google Scholar] [CrossRef]
- Negro, F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb. Perspect. Med. 2014, 3, a021550. [Google Scholar] [CrossRef]
- Jang, T.-Y.; Wei, Y.-J.; Yeh, M.-L.; Liu, S.-F.; Hsu, C.-T.; Hsu, P.-Y.; Liu, T.W.; Lin, Y.H.; Liang, P.C.; Hsieh, M.H.; et al. Role of hepatitis D virus in persistent alanine aminotransferase abnormality among chronic hepatitis B patients treated with nucleotide/nucleoside analogues. J. Formos. Med. Assoc. 2021, 120, 303–331. [Google Scholar] [CrossRef]
- Farci, P.; Niro, G.A. Clinical features of hepatitis D. Semin. Liver Dis. 2012, 32, 228–236. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, S.; Ou, X.; Li, S.; Ma, Z.; Wang, W.; Peppelenbosch, M.P.; Liu, J.; Pan, Q. Estimating the Global Prevalence, Disease Progression, and Clinical Outcome of Hepatitis Delta Virus Infection. J. Infect. Dis. 2019, 221, 1677–1687. [Google Scholar] [CrossRef]
- Romeo, R.; Perbellini, R. Hepatitis delta virus: Making the point from virus isolation up to 2014. World J. Hepatol. 2015, 7, 2389–2395. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar] [PubMed]
- Benegiamo, G.; Vinciguerra, M.; Guarnieri, V.; Niro, G.A.; Andriulli, A.; Pazienza, V. Hepatitis delta virus induces specific DNA methylation processes in Huh-7 liver cancer cells. FEBS Lett. 2013, 587, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Yacoubi, L.; Brichler, S.; Mansour, W.; Le Gal, F.; Hammami, W.; Sadraoui, A.; Ben Mami, N.; Msaddek, A.; Cheikh, I.; Triki, H.; et al. Molecular epidemiology of hepatitis B and Delta virus strains that spread in the Mediterranean North East Coast of Tunisia. J. Clin. Virol. 2015, 72, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Su, C.W.; Huang, Y.H.; Huo, T.I.; Shih, H.H.; Sheen, I.J.; Chen, S.W.; Lee, P.C.; Lee, S.D.; Wu, J.C. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology 2006, 130, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Borzacov, L.M.; de Figueiredo Nicolete, L.D.; Souza, L.F.; Dos Santos, A.O.; Vieira, D.S.; Salcedo, J.M. Treatment of hepatitis delta virus genotype 3 infection with peg-interferon and entecavir. Int. J. Infect. Dis. 2016, 46, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Nagayama, K.; Enomoto, N.; Chinzei, R.; Yamashiro, T.; Izumi, N.; Yatsuhashi, H.; Nakano, T.; Robertson, B.; Nakasone, H.; et al. Chronic hepatitis delta virus infection with genotype IIb variant is correlated with progressive liver disease. J. Gen. Virol. 2003, 84, 3275–3289. [Google Scholar] [CrossRef] [PubMed]
- Radjef, N.; Gordien, E.; Ivaniushina, V.; Gault, E.; Anaïs, P.; Drugan, T.; Trinchet, J.C.; Roulot, D.; Tamby, M.; Milinkovitch, M.C.; et al. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J. Virol. 2004, 78, 2537–2544. [Google Scholar] [CrossRef]
- Tsaneva-Damyanova, D.; Stoykova, Z.; Ivanova, I.; Kostadinova, T.; Ivanova, L. Hepatitis D virus in Bulgaria: Virology, epidemiology and pathogenesis in chronic HBV carriers with liver dysfunction. Scr. Sci. Med. 2020, 52, 12–18. [Google Scholar] [CrossRef]
- Tsaneva-Damyanova, D. Clinical significance of Hepatitis D virus genotype I infection. Suppl. J. IMAB 2021, 27, 40–42. [Google Scholar]
- Heidrich, B.; Serrano, B.; Idilman, R.; Kabaçam, G.; Bremer, B.; Raupach, R.; Önder, F.O.; Deterding, K.; Zacher, B.J.; Taranta, A.; et al. HBeAg—Positive hepatitis delta. Liver Int. 2012, 32, 1415–1425. [Google Scholar] [CrossRef]
- Da, B.L.; Heller, T.; Koh, C. Hepatitis D infection: From initial discovery to current investigational therapies. Gastroenterol. Rep. 2019, 7, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Khan, M.A.; Salih, M.; Jafri, W. Interferon alpha for chronic hepatitis D. Cochrane Database Syst. Rev. 2011, 12, CD006002. [Google Scholar] [CrossRef] [PubMed]
- Alfaiate, D.; Dény, P.; Durantel, D. Hepatitis delta virus: From biological and medical aspects to current and investigational therapeutic options. Antiviral Res. 2015, 122, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Gish, R. Diagnosing and Screening for Hepatitis D Viral Infection. Hepatitis B Foundation. Available online: https://www.hepb.org/assets/Uploads/Gish-HDVDiagnositic-Analysis-Whitepaper-1.pdf (accessed on 30 March 2023).
- Robinson, A.; Wong, R.; Gish, R.G. Chronic Hepatitis B Virus and Hepatitis D Virus: New Developments. Clin. Liver Dis. 2023, 27, 17–25. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06eng.pdf?sequence=1 (accessed on 31 March 2023).
- Sagnelli, C.; Pisaturo, M.; Curatolo, C.; Codella, A.V.; Coppola, N.; Sagnelli, E. Hepatitis B virus/hepatitis D virus epidemiology: Changes over time and possible future influence of the SARS-CoV-2 pandemic. World J. Gastroenterol. 2021, 27, 7271–7284. [Google Scholar] [CrossRef]
- National Program for the Prevention and Control of Viral Hepatitis in the Republic of Bulgaria 2021–2025. Available online: https://www.mh.government.bg/media/filer_public/2021/04/01/np_hepatitis__2021-2025.pdf (accessed on 1 April 2023). (In Bulgarian)
- Hepactive. Available online: https://www.hepactive.org/ (accessed on 31 March 2023).
- Bazinet, M.; Pântea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Iarovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology 2020, 158, 2180–2194. [Google Scholar] [CrossRef]
- Yurdaydin, C.; Idilman, R.; Kalkan, C.; Karakaya, F.; Kartal, A.C.; Keskin, O.; Karatayli, E.; Karatayli, S.C.; Bozdayi, A.M.; Koh, C.; et al. Exploring optimal dosing of Lonafarnib with ritonavir for the treatment of chronic delta hepatitis-interim results from the lowr HDV-2 study. Hepatology 2016, 64, 910A. [Google Scholar]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Yonghe, Q.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012, 1, e00049. [Google Scholar] [CrossRef]
- European Medicines Agency Hepcludex: European Medicines Agency 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/hepcludex (accessed on 23 April 2023).
- Smith-Palmer, J.; Cerri, K.; Sbarigia, U.; Chan, E.K.H.; Pollock, R.F.; Valentine, W.J.; Bonroy, K. Impact of Stigma on People Living with Chronic Hepatitis, B. Patient Relat. Outcome Meas. 2020, 11, 95–107. [Google Scholar] [CrossRef]
- Rice, W.S.; Logie, C.H.; Napoles, T.M.; Walcott, M.; Batchelder, A.W.; Kempf, M.C.; Wingood, G.M.; Konkle-Parker, D.J.; Turan, B.; Wilson, T.E.; et al. Perceptions of intersectional stigma among diverse women living with HIV in the United States. Soc. Sci. Med. 2018, 208, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tsaneva-Damyanova, D. Hepatitis D virus (HDV)-satellite in the orbit of chronic hepatitis B virus. In Monograph; Steno: Varna, Bulgaria, 2022. (In Bulgarian) [Google Scholar]
- World Hepatitis Alliance. Available online: https://www.worldhepatitisalliance.org/ (accessed on 1 April 2023).
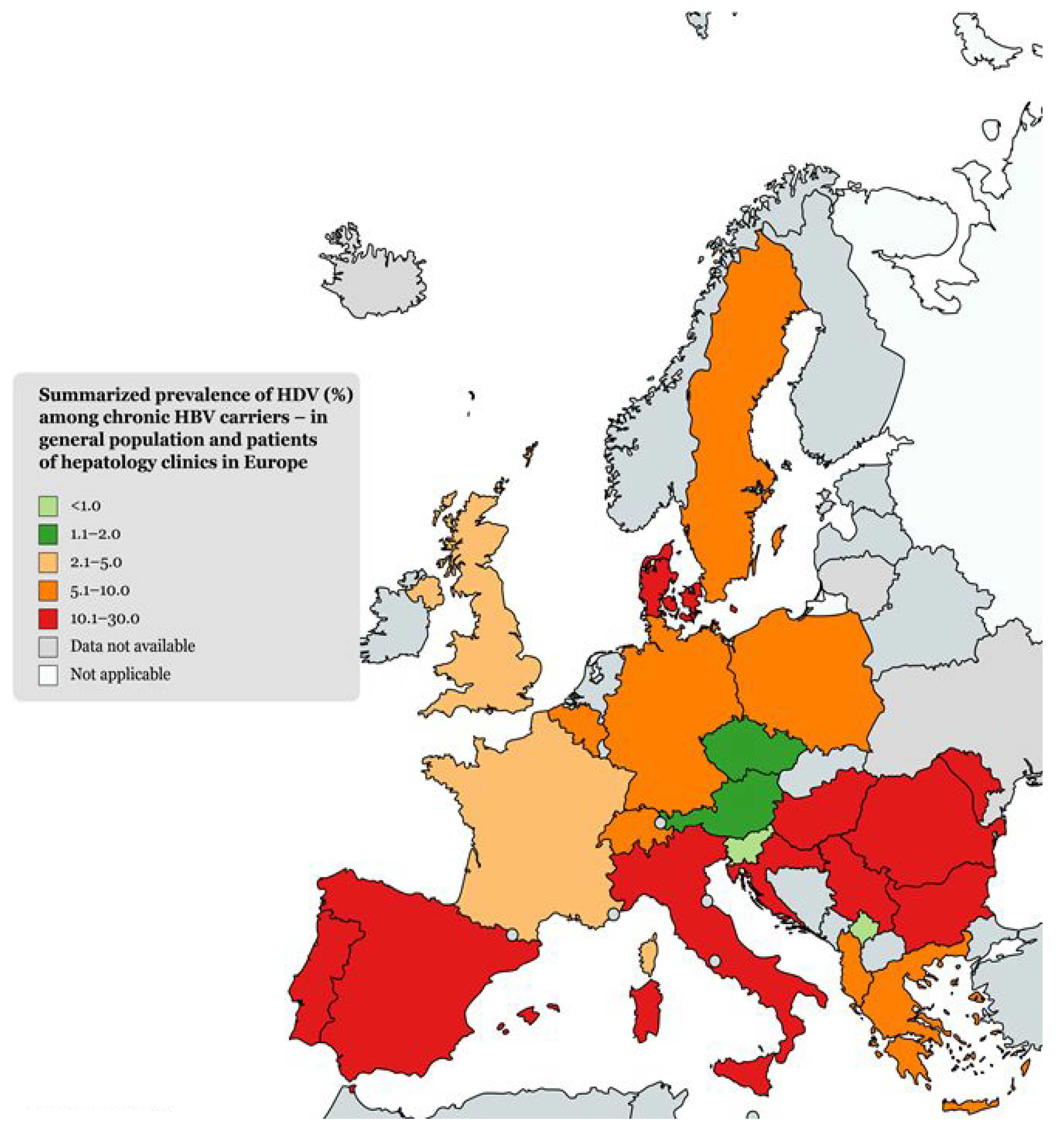
| Country | HDV Prevalence among HBsAg-Positive Carriers (%) | Tested Population (Years of Research) | Reference |
|---|---|---|---|
| Italy | 494/2001 (24.7%) 364/1556 (23.4%) 143/996 (14.4%) 69/834 (8.3%) 112/1386 (8.1%) Native: 1/78 (1.3%); Immigrants: 6/76 (7.9%) Native: 19/381 (5.0%); Immigrants: 5/107 (4.7%) Native: 53/716 (7.4%); Immigrants: 34/295 (11.5%) 78/786 (9.9%) Native: (6.4%); Immigrants: (26.4%) | HBV-infected patients (1978–1981) Chronic HBsAg carriers (1987) Chronic HBsAg carriers (1992) HBsAg carriers (1997) Chronic HBsAg carriers (2006–2007) Chronic HBsAg carriers in Ferrara (1997–2009) Chronic HBsAg carriers in Milan (2007–2008) Chronic HBsAg carriers Chronic HBsAg carriers (2019) | Smedile et al., 1983 [33] Sagnelli et al., 1992 [34] Sagnelli et al., 1997 [35] Gaeta et al., 2000 [36] Stroffolini et al., 2009 [37] Contini et al., 2012 [38] De Paschale et al., 2012 [39] Brancaccio et al., 2014 [40] Stroffolini et al., 2020 [41] |
| Bulgaria | 9/105 (8.6%) 151/1465 (10.3%, of which 47.06% were haemophiliacs) 28/173 (16.1%) (49/1280) (3.8%) 65/391 (16.6%) 84/788 (10.6%) | Chronic HBsAg carriers (HDAg) (1985–1986) Chronic HBsAg carriers (1997–1998) Patients with chronic HBsAg (1986–1997) Chronic HBsAg carriers on antiviral therapy (2008–2013) Chronic HBsAg patients with liver dysfunction (2013–2018) Inmates in 5 prisons (2018–2019) | Naoumov et al., 1986 [42] Iliev et al., 2001 [43] Krastev et al., 2013 [44] Tsaneva-Damyanova, 2019 [45] Popov, et al., 2020 [46] |
| Greece | 1997–2010: 90/2137 (4.2%):
| Chronic HBsAg carriers (1997–2010) | Manesis et al., 2013 [47] |
| Germany | 258/2083 (8.0%) (1992); (10.9%) (2006) 266/2354 (11.3%) 210/2844 (7.4%) | HDV infected patients and chronic HBsAg carriers (1992–2006) Chronic HBsAg carriers in Hannover (1992–2006) Chronic HBsAg carriers in Frankfurt (2000–2011) | Heidrich B et al., 2009 [48] Wedemeyer et al., 2007 [13] Rehnheimer et al., 2012 [14] |
| France | 89/4492 (2.0%); (1997–2011) 1997–2005: 33/2831 (1.2%) 2010: 13/200 (6.5%) 2011: 2/234 (0.9%) | HBsAg-positive blood donors (1997–2011) HDV-Ab; HDV RNA | Servant-Delmas et al., 2014 [15] |
| United Kingdom | 9/401 (2.2%) 82/962 (8.5%) (2.6%) (2000) 22/1048 (2.1%) 162/3610 (4.5%) | Chronic HBsAg carriers in Northern Ireland (1970–1989) Chronic HBV patients (mostly immigrants) (2000–2006) in London Chronic HBsAg carriers in London HBsAg carriers (2008–2012) in London (anti-HDV Ab, anti-HDV IgM, HDV RNA) HBsAg carriers (mostly immigrants) (2005–2012) in London | Curran et al., 1991 [49] Cross et al., 2008 [50] Stockdale, et al., 2020 [16] William Tong et al., 2013 [51] El Bouzidi et al., 2015 [52] |
| Austria | 4/138 (2.9%) (N/A) (0.8%) | HBsAg carriers (N/A) HBV patients (N/A) | Frisch-Niggemeyer and Kunz, 1985 [53] Jachs M et al., 2021 [54] |
| Albania | 1995: 10/106 (9.4%); 2005: 7/99 (7.1%) | Patients with chronic viral and/or alcohol-induced liver disease (1995 and 2005) | Kondili et al., 2010 [55] |
| Slovenia | 3/1305 (0.23%) | Patients with chronic HBV infection (1998–2015) | Jelen et al., 2016 [12] |
| Belgium | 44/800 (5.5%) | Chronic HBsAg carriers (2008–2009) | Ho et al., 2013 [56] |
| Denmark | 29/100 (29.0%) | Chronic HBV patients (1970–1985) | Krogsgaard et al., 1988 [57] |
| Croatia | 19/100 (19.0%) | Chronic HBsAg carriers (N/A) | Jelić and Jelić, 1994 [58] |
| Hungary | 16/118 (13.6%) | Chronic HBsAg carriers (N/A) | Horváth et al., 1992–1993 [59] |
| Chech Republic | 3/170 (2.0%) | Patients with coinfection HBV + chronic hepatitis D (2011–2020) | Hříbek, et al., 2022 [60] |
| Moldova | 27/148 (18.5%) | Patients with primary liver malignancies | Turcanu et al., 2019 [61] |
| Kosovo | 1/1287 (0.08%) | General population included in routine blood testing | Quaglio et al., 2008 [62] |
| Serbia and Montenegro | 69/614 (11.2%) | Chronic HBsAg carriers (N/A) | Delić et al., 1993 [63] |
| Poland | 4/102 (3.9%) 3/63 (4.8%) 5/63 (7.9%) | Chronic HBV patients (N/A) Chronic HBsAg carriers (2002–2004) (anti-HDV Ab, HDV RNA) | Chlabicz et al., 2003 [64] Bielawski et al., 2006 [65] |
| Romania | 223/1094 (20.4%) 617/2761 (23.1%) | Chronic HBsAg carriers (2005) Chronic HBsAg carriers (N/A) | Popescu et al., 2013 [66] Gheorghe et al., 2015 [28] |
| Portugal | N/A (17.3%) | Chronic HBsAg carriers (N/A) | Ramalho et al., 1987 [67] |
| Spain | 249/1220 (20.4%) 17/1147 (1.5%) 1984/2518 (78.8%) 100/1215 (8.2%) N/A (30%—1990s to 4.2%—2018) | Immigrants (HBsAg carriers) from Equatorial Guinea (2002–2008) HIV-positive patients (2004) African immigrants (HBsAg carriers) Chronic hepatitis B virus (HBV) patients (1983–2012) Anti-HDV Ab among active HBsAg-positive IVDUs (1990–2018) | Rivas et al., 2013 [68] Fernández-Montero et al., 2014 [69] Cuenza-Gómez et al., 2016 [70] Ordieres et al., 2017 [71] Aguilera et al., 2018 [72] |
| Sweden | N/A 650/9160 (7.1%) | Chronic HBsAg carriers (1997–2008) | Ji et al., 2012 [73] |
| Switzerland | 101/1699 (5.9%) 15/338 (4.4%) | Chronic HBV patients (mostly immigrants) (N/A)-HDV Ab, HDV Ag, HDV RNA HBsAg carriers (2002–2013) | Genné and Rossi, 2011 [74] Hirzel et al., 2015 [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaneva-Damyanova, D.T.; Georgieva, L.H. Epidemiology Pattern, Prevalent Genotype Distribution, Fighting Stigma and Control Options for Hepatitis D in Bulgaria and Other European Countries. Life 2023, 13, 1115. https://doi.org/10.3390/life13051115
Tsaneva-Damyanova DT, Georgieva LH. Epidemiology Pattern, Prevalent Genotype Distribution, Fighting Stigma and Control Options for Hepatitis D in Bulgaria and Other European Countries. Life. 2023; 13(5):1115. https://doi.org/10.3390/life13051115
Chicago/Turabian StyleTsaneva-Damyanova, Denitsa Todorova, and Lora Hristova Georgieva. 2023. "Epidemiology Pattern, Prevalent Genotype Distribution, Fighting Stigma and Control Options for Hepatitis D in Bulgaria and Other European Countries" Life 13, no. 5: 1115. https://doi.org/10.3390/life13051115
APA StyleTsaneva-Damyanova, D. T., & Georgieva, L. H. (2023). Epidemiology Pattern, Prevalent Genotype Distribution, Fighting Stigma and Control Options for Hepatitis D in Bulgaria and Other European Countries. Life, 13(5), 1115. https://doi.org/10.3390/life13051115






