Abstract
Few studies have focused on elderly (>80 years) amyotrophic lateral sclerosis (ALS) patients, who represent a fragile subgroup generally not included in clinical trials and often neglected because they are more difficult to diagnose and manage. We analyzed the clinical and genetic features of very late-onset ALS patients through a prospective, population-based study in the Emilia Romagna Region of Italy. From 2009 to 2019, 222 (13.76%) out of 1613 patients in incident cases were over 80 years old at diagnosis, with a female predominance (F:M = 1.18). Elderly ALS patients represented 12.02% of patients before 2015 and 15.91% from 2015 onwards (p = 0.024). This group presented with bulbar onset in 38.29% of cases and had worse clinical conditions at diagnosis compared to younger patients, with a lower average BMI (23.12 vs. 24.57 Kg/m2), a higher progression rate (1.43 vs. 0.95 points/month), and a shorter length of survival (a median of 20.77 vs. 36 months). For this subgroup, genetic analyses have seldom been carried out (25% vs. 39.11%) and are generally negative. Finally, elderly patients underwent less frequent nutritional- and respiratory-supporting procedures, and multidisciplinary teams were less involved at follow-up, except for specialist palliative care. The genotypic and phenotypic features of elderly ALS patients could help identify the different environmental and genetic risk factors that determine the age at which disease onset occurs. Since multidisciplinary management can improve a patient’s prognosis, it should be more extensively applied to this fragile group of patients.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a rare, fatal neurodegenerative disease in which the progressive degeneration of both upper and lower motor neurons leads to muscle weakness, bulbar palsy, and, finally, death within two to five years after the onset of the first symptoms [1]. Studies on the epidemiology of ALS are numerous, and increased incidence rates of motor neuron diseases (MNDs) with aging have been reported in different western countries despite the geographic variation among these countries [2]. Some population-based studies have reported that this age-specific incidence pattern is characterized by a low incidence of ALS before 40 years of age, a progressive increase that peaks at 70–74 years of age, and a sharp decrease between the ages of 80 and 100 [3,4]. This trend has not yet been fully explained, especially for elderly patients; it could be related to misdiagnosis due to prevalent comorbidities, the presence of a faster disease that can make diagnosis challenging, or a real decrease in incidence [5]. Recently, an increase in ALS incidence among elderly women has also been described [6]. Despite the great interest in ALS epidemiology and the many open questions regarding its risk factors, few studies have explored the epidemiological and clinical features of ALS patients diagnosed at a very old age, or over 80 years (defined as oldest-old ALS by Dandaba et al. [5]). Several papers have reported an increased frequency of bulbar cases and comorbidity with dementia among the elderly [7], but other clinical features are quite unexplored. However, the population-aging effect will probably increase the number of these patients [8,9]. Furthermore, these patients represent an even more fragile subgroup of people affected by ALS, as they are neglected because they are difficult to reach and diagnose, are not followed in tertiary centers, and are not included in clinical trials [10]. Since the Emilia Romagna Region (ERR) has a super-aged society [11], with 24.33% of its population aged 65 years or over [12], we aim to investigate the demographic, clinical, and genetic features of patients with late-onset ALS compared to those with early or adult-onset ALS.
2. Materials and Methods
This is a prospective, population-based, epidemiological study carried out in the Emilia Romagna Region (ERR) of Northern Italy. In this region, a prospective registry (Emilia Romagna Registry for ALS–ERRALS) has been active since 2009 [13], collecting all incident ALS cases among residents of the ERR, diagnosed according to the El Escorial Criteria-Revised (EEC-R) [14].
To identify eligible patients, neurologists from 17 ALS centers collected detailed descriptions of each ALS patient including their place of residence; sex; age, both at symptom onset and diagnosis; site of onset and progression; EEC-R classification; clinical ALS phenotype, categorized as bulbar ALS, classic ALS, upper motor neuron-predominant (UMNp) ALS, flail arm and flail leg ALS, or respiratory ALS in line with the definition proposed by Chiò et al. [15]; and drug use, including Riluzole. Other collected information includes their family history and genetic analysis results. Genetic analyses were performed as per clinical practice depending on the caring physician but included at least the four genes responsible for up to 70% of familial forms of ALS [1] (SOD1, FUS, TARDBP, and the C9ORF72 expansion), as described elsewhere [16]. Depending on their family histories and previous genetic results, some patients underwent an extended and customized panel using NGS probes (Illumina Nextera Rapid Capture Custom kit, Illumina), which include the causative and susceptibility genes of ALS/FTD, hereditary motor neuron disease, and hereditary spastic paraplegia [17]. Only pathogenic mutations are reported here.
Each patient’s respiratory function, measured as their forced vital capacity (FVC), was assessed with spirometry at both diagnosis and follow-up, and their ALS Functional Rating Scale-Revised (ALSFRS-R) score assessed at each visit. Data regarding their death was also assessed. Information on the patient’s use of nutritional support, gastrostomy (PEG), and non-invasive or invasive ventilation (NIV or IV) were noted, and missing data were retrieved and confirmed through administrative data [18,19,20]. In addition, comorbidities at diagnosis were recorded, including the presence of cognitive impairment; extrapyramidal signs; cardiovascular risk factors, such as hypertension and heart diseases; diabetes mellitus; thyroid dysfunction; metabolic disorders; chronic obstructive pulmonary disease (COPD) and other respiratory disorders; gastrointestinal, urological, hematological, autoimmune, and neoplastic diseases; and psychiatric disorders [21].
We also assessed the patients’ access to diagnostic facilities (neuroimaging and electromyography (EMG)) and multidisciplinary approaches using the number of specialist evaluations they had undergone adjusted by their disease duration (number of evaluations per year), prospectively collected in the ERRALS register. Every 3–4 months, patients were given multidisciplinary follow-ups, with data on their disease progression collected according to EFNS guidelines [22]. Home monitoring of patients was performed if it was no longer possible for them to reach an ALS center for the multidisciplinary visits.
As mentioned above, disease progression was measured using ALSFRS-R scores taken at each visit and progression rates at diagnosis, which are the monthly reductions in ALSFRS-R scores given the highest score of 48 points at disease onset [23].
Weight before symptom onset, at diagnosis, and during disease progression was collected. “Weight loss at diagnosis” was used as a categorical variable, dividing patients based on whether or not they had dropped at least 1 kg of weight between the time before onset and the time of diagnosis, and as a quantitative variable, with the difference in kilograms of body weight between the times already mentioned as datapoints.
The data of the population-based registry was supplemented by cases resulting from the regional hospitals as having a discharge code of 335.2 according to the International Classification of Diseases (ICD, 9th revision) or the corresponding G12.21 code from the ICD, 10th revision, and from death certificates of residents with the same codes.
This study was approved by the ethics committee of the coordinating center (Comitato Etico Provinciale di Modena, file number 124/08, on 2 September 2008) and participating centers. For this study, we considered the incident cases that were included in the registry and diagnosed between 1 January 2009 and 31 December 2019. Patients were grouped according to two age categories: oldest-old ALS (oALS) patients, or those having an age of over 80 years at diagnosis, and patients with an age of 80 years or less at diagnosis.
Differences between means were assessed with a 2-tailed t test, or ANOVA. A comparison of categorical variables was made with a chi-square test. Ninety-five percent confidence intervals (CIs) were calculated assuming a Poisson distribution. A logistic regression analysis was performed, considering bulbar or classic phenotypes as binary response variables and age at onset (according to the following classes: <50 years, from 50 to 65 years, from 65 to 80 years, over 80 years), sex, and the interaction between age and sex as independent variables in the adjusted analysis, which was previously described [24]. Adjusted analyses for each outcome included Cox proportional hazards models for time-to-event outcomes. A Cox regression analysis was used to estimate the hazard ratio (HR) and corresponding 95% confidence interval (95% CI) for each independent variable, including gender; site of onset; phenotype; age at onset; body mass index (BMI) at diagnosis; raw value of weight loss at diagnosis (considering the weight before onset of motor symptoms as the reference); frontotemporal dementia (FTD); genetic mutations (C9ORF72 versus other); ALSFRS-R score at diagnosis; FVC value at diagnosis; progression rate at diagnosis; diagnostic delay; use of Riluzole; family history of ALS/FTD; presence of hypertension, COPD, heart, and other respiratory diseases; autoimmune, thyroid, psychiatric, hematological, neoplastic, urologic, gastrointestinal, and metabolic disorders; and diabetes. Finally, a stepwise backward selection with a retention criterion of 0.1 was applied to the multivariate Cox regression analysis.
Statistical analyses were performed using the STATA statistical software (v15.1, StataCorp. LLC, 2017. College Station, TX, USA).
3. Results
Patients’ Clinical Features
From 1 January 2009 to 31 December 2019, 222 subjects older than 80 years of age and living in the ERR received a new diagnosis of ALS, corresponding to 13.76% of all patients in the ERR diagnosed with a disease in the same period. Table 1 shows the demographic and clinical features of oALS in comparison to people who were ≤80 years old at diagnosis. Among oALS patients, the mean age at diagnosis was 83.97 ± 3.66 years, and the majority had a bulbar onset and bulbar phenotype, differing from patients who were younger (p < 0.001). At diagnosis, oALS patients presented with a worse clinical profile in terms of BMI (23.12 ± 3.54 vs. 24.57 ± 4.01, p < 0.001), weight loss (70.17% vs. 55.11%, p = 0.002), total ALSFRS-R scores (34.60 ± 8.82 vs. 38.97 ± 7.32, p < 0.001), FVC percentage (72.33 ± 27.13 vs. 86.00 ± 25.37, p < 0.001), and monthly decline in ALSFRS-R scores (1.43 ± 1.31 vs. 0.95 ± 1.18, p < 0.001).

Table 1.
Demographic and clinical features of patients according to age group at diagnosis.
There were only 8 patients (7 women and 1 man) diagnosed after 90 years of age; 6 out of these 8 patients had a bulbar onset and phenotype, and the mean progression rate at diagnosis was 2.69 ± 2.20 points/month.
Genetic analyses were carried out less frequently in elderly patients (25.00% vs. 39.11%, p < 0.001) and were negative in almost all patients. All the reported mutations are classified as pathogenic according to the ACGM classification.
Table 2 shows the distribution of phenotypes according to sex and age, where a higher frequency of the bulbar phenotype in oALS patients was detected among women.

Table 2.
Distribution of phenotypes according to sex in oALS and younger ALS patients.
The bulbar phenotype was associated with older ages and the female sex without interaction between age and sex. The classic phenotype, on the contrary, was inversely associated with older ages and associated with the male sex without an interaction between age and sex. The UMNp phenotype was also inversely associated with age. There were no relevant associations among other phenotypes with sex and age, according to the binary logistic regression analysis (Table 3).

Table 3.
Correlation of motor phenotypes with age and sex: binary logistic regression analysis.
Figure 1 shows the number of ALS patients in the ERR from 2009 to 2019, categorized by sex and phenotype in five-year age groups.
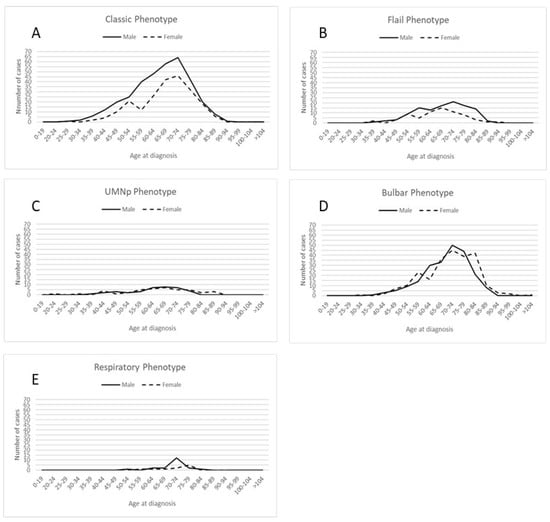
Figure 1.
Distribution of cases by sex and 5–year age groups across different disease phenotypes: (A) classic phenotype; (B) flail phenotype, including both flail arm and flail leg phenotypes; (C) Upper Motor Neuron predominant (UMN-p) phenotype; (D) bulbar phenotype; (E) respiratory phenotype.
During the study decade, we observed an increase in the number of patients in the oldest group, with patients aged over 80 years representing 12.02% of patients before 2015 in comparison to 15.91% from 2015 onwards (p = 0.024).
Figure 2 displays the differences in comorbidities at the time of diagnosis between the two age groups. Comorbidities were more frequent in oALS patients than in younger patients, except for comorbidities with thyroid, psychiatric, metabolic, and respiratory diseases other than COPD.
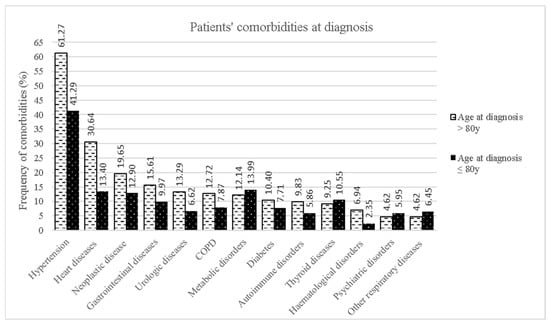
Figure 2.
Distribution of comorbidities at diagnosis for the two age groups.
As far as diagnostic ascertainment and the multidisciplinary approach are concerned, oALS patients underwent neuroimaging less frequently (brain MRI: 63.22% vs. 75.25%, p = 0–001; cervical MRI: 44.83% vs. 64.54%, p < 0.001), whereas access to EMG was similar between the two age groups (95.37% vs. 92.77%, p = 0.096) (Table 3).
The multidisciplinary team was less involved in oALS patients’ follow-ups as far as neurological, pneumological, and psychological evaluates are concerned. The oldest patients were treated less frequently with Riluzole than the others (68.79% vs. 84%, p < 0.001) (Table 4).

Table 4.
Diagnostic and clinical approaches to oALS patients compared to other ALS patients.
Elderly ALS patients underwent supportive procedures such as PEG, NIV, or IV earlier in the course of the disease but less frequently than other patients (Table 5).

Table 5.
Nutritional and respiratory support procedures and patient features at the time according to two age groups.
The elderly had a faster disease progression and a shorter survival length compared to younger ALS patients (Figure 3); the median tracheostomy-free survival length was 36 months (95% CI 33.57–37.43) in ALS patients who were ≤80 years old at diagnosis and 20.77 months (95% CI 18–23.93) in oALS patients (HR 1.87, 95% CI 1.61–2.17, p < 0.001)
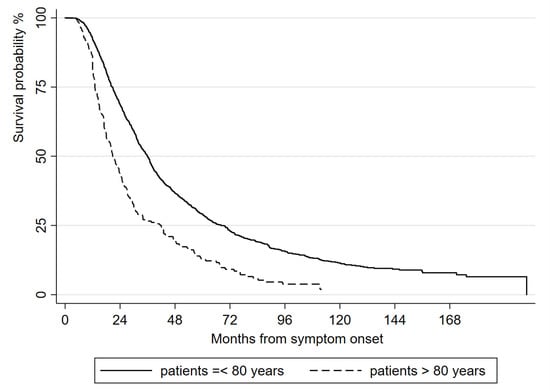
Figure 3.
Kaplan-Meier analysis of length of tracheostomy-free survival from symptom onset, comparing oALS and other ALS patients.
In oALS patients, the univariate analysis of the length of tracheostomy-free survival demonstrated that negative prognostic factors, included in the multivariable model, were weight loss at diagnosis (kg) (HR = 1.54, 95% CI: 0.98–2.42, p = 0.060), progression rate at diagnosis (HR = 1.53, 95% CI: 1.38–1.70, p < 0.001), age at onset (years) (HR = 1.04, 95% CI: 1.01–1.07, p = 0.007), and the presence of cardiovascular diseases (HR = 1.44, 95% CI: 1.02–2.03, p = 0.036), psychiatric diseases (HR = 1.84, 95% CI: 0.90–3.79, p = 0.093), or hematological diseases (HR = 0.53, 95% CI: 0.26–1.09, p = 0.083), whereas higher BMIs (HR = 0.92, 95% CI: 0.87–0.97, p = 0.005) and ALSFRS-R scores at diagnosis (1 point) (HR = 0.97, 95% CI: 0.96–0.99, p = 0.010) and longer diagnostic delays (months) (HR = 0.97, 95% CI: 0.94–0.97, p < 0.001) were associated with longer survivals (Table 6).

Table 6.
Univariate Cox regression analysis of survival in oldest-old ALS patients.
Independent prognostic factors for the length of tracheostomy-free survival in the multivariate analysis were ALSFRS-R score at diagnosis (1 point) (HR = 0.97, 95% CI: 0.95–0.98, p < 0.001), age at onset (years) (HR = 1.06, 95% CI: 1.00–1.11, p = 0.039), and diagnostic delay (months) (HR = 0.97, 95% CI: 0.95–0.98, p < 0.001) (Figure 4).
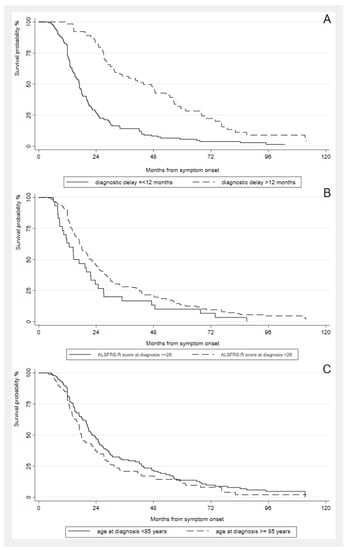
Figure 4.
Kaplan-Meier analysis of length of tracheostomy-free survival from symptom onset in oALS patients by (A) diagnostic delay (≤ or >12 months); (B) ALSFRS-R score at diagnosis (≤ or > 28/48); (C) age classes (< or ≥ 85 years).
4. Discussion
From 1 January 2009 to 31 December 2019, 14% of all new ALS cases diagnosed in the ERR were in subjects older than 80 years of age, a similar statistic to what was reported in the FRALim register [9]. These patients appear to have specific clinical features that differ from the remaining ALS population; elderly ALS patients are more likely to be female and show a higher prevalence of bulbar symptoms at onset and of the bulbar phenotype. We can confirm previous findings from the Scottish MND register [25] and the Piemonte and Valle d’Aosta register for ALS (PARALS) populations [24] with the evidence that age and sex also influence the phenotypes of our elderly study population. We could not find any evidence of an interaction among age and sex, probably as a result of the relatively small sample size, especially among the elderly. A relationship between very late-onset ALS and predominantly bulbar presentations was also already found in cohort studies [26].
This study found that, with increasing age, patients’ clinical statuses at diagnosis were worse in terms of BMI, ALSFRS-R, and FVC scores [5,27], and they had significantly higher progression rates and reduced lengths of survival compared to other ALS patients [27].
In our regional cohort, approximately 15% of patients reported a family history of ALS/dementia (fALS), among both the elderly and patients who were ≤80 years at diagnosis. Although there were no significant differences between age groups as far as family history is concerned, genetic testing was carried out less frequently among elderly patients, and only 5.45% of them showed a mutation in the genes related to ALS, namely SOD1 and FUS. These results are quite distinct from those in previous studies, which reported that, among patients carrying mutations in genes related to ALS, the elderly were the ones carrying the C9ORF72 expansion [28]. This discrepancy could be attributable in part to the low rate of genetic testing among the elderly in our population. The presence of FUS mutations in oALS patients is atypical because it represents the most common genetic defect in early onset ALS (<40 years) [28,29,30]. Some of the data in the literature, however, show that patients with an FUS mutation exhibit considerable variation in phenotypes, with some having an early onset and rapid disease progression and others having a later age of onset and slower progression [31]. This variability in the phenotypes of FUS-related ALS could result from the effects exerted by different missense and truncating mutations [32], or there could be influences from epigenetics or environmental factors on disease phenotype in patients carrying mutations. The lower frequency of pathogenic mutations detected among oALS patients could suggest that pathogenic mutations lead to an increased risk of early disease onset, according to the multistep hypothesis of ALS pathogenesis [33,34]. On the other hand, it cannot be ruled out that patients with a disease onset beyond 80 years of age carry mutations in unknown susceptibility genes.
Nevertheless, the fact that genetic analyses were performed based on clinical judgment may bias these results, and we acknowledge that this as a limitation of this study. The reason behind the low propensity for genetic testing could be the current clinical practice of performing genetic tests only on individuals with a positive family history or young people with sporadic diseases, although it is likely that genetic mutations are present in patients with apparently sporadic ALS at all ages [35]. With the use of precision treatments rising (e.g., antisense oligonucleotides), it is essential to identify all ALS patients carrying genetic mutations. Age at onset and family history should not be an obstacle to genetic testing. On the other hand, it must be considered that variants of unknown significance and uncertainties related to incomplete penetrance may require expert genetic and psychological counseling [36,37].
During this study, we observed an increase in the proportion of subjects over 80 years of age, probably reflecting the population aging [38], the accuracy of detection, and improved surveillance and reporting in the Emilia Romagna Region [6,13]. On the other hand, this increasing number of elderly patients might be due to not only better case ascertainment but also a combination of environmental, genetic, and individual factors that can contribute to the heterogeneity of disease presentation, including age at onset.
Our study highlights a generally poor prognosis for elderly ALS patients that could be related to the higher prevalence of comorbidities, such as cardiovascular diseases (e.g., hypertension, congestive heart failure, atrial fibrillation, and coronary disease) and hematological disorders, that were previously associated with a faster disease progression [21,39].
Comorbidities, an atypical clinical onset, and a faster disease progression can also complicate the evaluation and management of ALS by making the diagnosis more challenging [3,4,40,41,42]. Cognitive impairment, which is more prevalent with age, may limit an individual’s ability to provide an accurate history of symptoms. Moreover, access to neurological specialists may be limited for older adults who are institutionalized in nursing homes or long-term care facilities, or those with low health-care education. Elderly people come to the attention of neurological centers with greater difficulty or later in time [40,41], with a higher risk of being underdiagnosed or misdiagnosed. This may explain the underestimation of oALS patients in previous studies [3,4,43]. Accordingly, in our patients’ cohort, oALS patients underwent some diagnostic investigations, such as neuroimaging studies, less frequently. However, diagnostic delay in oALS patients, in the context of a faster disease, was not significantly different from other ALS patients [8,24].
This study points out that only 69% of the oldest-old patients had been prescribed Riluzole, the only disease-modifying therapy currently approved in Italy. This could be related to the patients’ worse clinical statuses at the time of diagnosis and the higher frequency of FTD or cognitive impairment among older ALS patients (10,98% vs. 7.87%), although this difference is not statistically significant. This observation could represent a limitation to healthcare access, given the aging of the Western population, that needs to be accounted for using population-based studies [9]. Multidisciplinary teams were also less involved in the management of oALS patients (especially as far as neurological, pneumological, and psychological evaluations are concerned), partly as a result of the shorter length of ALS survival in these patients. This is probably also due to the more frequent denial of support procedures by oALS patients, which is more likely to be a patient’s choice than ageism. Although the involvement of specialist palliative care is of undoubtable value for every patient [44], multidisciplinary management in close cooperation with general practitioners, home carers, and a dedicated healthcare network should also be recommended for the proper assessment of all patients to minimize morbidity and maximize their quality of life [44,45].
Together with differences in healthcare management, more severe frontotemporal cortical thinning [46], and worse clinical statuses at disease onset and diagnosis (such as lower BMI, lower ALSFRS-R score, and higher progression rate) [47,48,49,50] in elderly patients, the harmful effects of aging on known prognostic factors [8] may contribute to the worse prognoses of these patients.
On the contrary, the bulbar-onset form, which is a well-known negative predictor of survival in the general ALS population [15,47], was not significantly associated with poor prognoses in oALS patients. This could be explained by the fact that other disease factors may have a stronger impact on survival in this age group. Consistently, the multivariate analysis of length of survival showed that an older age at onset, ALSFRS-R scores at diagnosis, and shorter diagnostic delays are independently associated with poor survival outcomes among the elderly [51,52].
5. Conclusions
Our study shows that elderly ALS patients are represented more frequently by women and tend to have a bulbar onset, a more rapid disease course, and shorter length of survival. Furthermore, we documented the limited application of treatments and procedures in this group. The main strengths of our study are the use of a population-based registry for data collection, involving an extensive network of ALS neurologists in the ERR, the length of follow-up, and the high number of cases described, reflecting the high accuracy of case ascertainment. Some limitations should be recognized in the presence of missing data on clinical phenotypes, cognitive/behavioral impairment details, and disease progression rates.
To improve the diagnosis, prognosis, and quality of life in patients in this extremely fragile group, diagnostic tests and multidisciplinary management should be more extensively applied and aimed at minimizing diagnostic latency.
Author Contributions
Conceptualization, J.M.; data curation, G.G., I.M., C.S., E.Z., N.F., M.C. (Maria Caputo), A.G. (Andrea Ghezzi) and J.M.; formal analysis, G.G., I.M., E.Z., N.F., T.F., M.V. and J.M.; funding acquisition, J.M.; investigation, G.G., I.M., C.S., E.Z., N.F., M.C. (Maria Caputo), A.G. (Andrea Ghezzi), A.G. (Annalisa Gessani), E.C., M.C. (Mario Casmiro), P.D.M., M.C.D., S.D.P., R.L., M.L., D.M., S.M., A.P., M.P., M.S., E.S., F.S., E.T., V.V., L.Z., M.V. and J.M.; methodology, G.G., I.M., C.S., E.Z., N.F., A.A. and J.M.; project administration, J.M.; resources, S.F. and J.M.; software, I.M., E.Z. and J.M.; supervision, A.A., M.V. and J.M.; validation, E.Z., S.F., M.V. and J.M.; visualization, G.G., M.V. and J.M.; writing—original draft, G.G. and I.M.; writing—review and editing, G.G., I.M., C.S., E.Z., N.F., M.C. (Maria Caputo), A.G. (Andrea Ghezzi), A.G. (Annalisa Gessani), E.C., M.C. (Mario Casmiro), P.D.M., M.C.D., S.D.P., R.L., M.L., D.M., S.M., A.P., M.P., M.S., E.S., F.S., E.T., V.V., L.Z., A.A., T.F., M.V. and J.M. All authors will be informed about each step of manuscript processing including submission, revision, revision reminders, etc., via emails from our system or assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
The Emilia Romagna Registry for ALS (ERRALS) is supported by a grant from the Emilia Romagna Regional Health Authority (GPG/2022/1343).
Institutional Review Board Statement
This study was approved by the ethics committee of the coordinating center (Comitato Etico Provinciale di Modena, file number 124/08, on 2 September 2008) and participating centers.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
The authors thank all the participants in the ERRALS group (see Appendix A).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
ERRALS group
Project coordinator: Dr J. Mandrioli.
Collaborating centers:
Department of Neurosciences, Azienda Ospedaliero Universitaria di Modena and Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy (Jessica Mandrioli, Nicola Fini, Ilaria Martinelli, Elisabetta Zucchi, Giulia Gianferrari, Cecilia Simonini, Annalisa Gessani, Maria Caputo, Andrea Ghezzi, and Marco Vinceti);
Dipartimento di Scienze Biomediche e Neuromotorie, University of Bologna, and IRCCS Istituto delle Scienze Neurologiche di Bologna, Bellaria Hospital, Bologna, Italy (Veria Vacchiano and Rocco Liguori);
IRCCS Istituto delle Scienze Neurologiche di Bologna, Bellaria Hospital, Bologna, Italy (Fabrizio Salvi, Ilaria Bartolomei, and Roberto Michelucci);
Dipartimento di Scienze Biomediche e Neuromotorie, University of Bologna, Bologna, Italy (Pietro Cortelli);
IRCCS Istituto delle Scienze Neurologiche di Bologna, Department of Neurology and Stroke Center, Maggiore Hospital, Bologna, Italy (Anna Maria Borghi and Andrea Zini);
IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Interaziendale Clinica Neurologica Metropolitana (NeuroMet), Bologna, Italy (Rita Rinaldi and Pietro Cortelli);
Department of Neurosciences and Rehabilitation, St Anna Hospital, Ferrara, Italy (Elisabetta Sette and Valeria Tugnoli);
Department of Neuroscience and Rehabilitation, University of Ferrara, Ferrara, Italy (Maura Pugliatti);
Department of Neurology, IRCCS Arcispedale Santa Maria Nuova, Reggio Emilia, Italy (Elena Canali, Luca Codeluppi, and Franco Valzania);
Department of General and Specialized Medicine, University Hospital of Parma, Parma, Italy (Lucia Zinno, Filippo Stragliati, Pietro Anceschi, Andi Nuredini, Sonia Romano, Alessandro D’Orsi, and Giovanni Pavesi);
Department of Neurology, Fidenza Hospital, Parma, Italy (Doriana Medici and Giovanna Pilurzi);
Department of Neurology, G. Da Saliceto Hospital, Piacenza, Italy (Emilio Terlizzi and Donata Guidetti);
Department of Neurology, Carpi Hospital, Modena, Italy (Silvia De Pasqua and Mario Santangelo);
Department of Neurology, Imola Hospital, Bologna, Italy (Martina Bracaglia and Patrizia De Massis);
Department of Neurology, Faenza and Ravenna Hospital, Ravenna, Italy (Mario Casmiro and Pietro Querzani);
Department of Neurology, Bufalini Hospital, Cesena, Italy (Simonetta Morresi, Maria Vitiello, and Marco Longoni);
Department of Neurology, Forlì Hospital, Forlì, Italy (Alberto Patuelli, Susanna Malagù, Francesca Bianchi, and Marco Longoni);
Department of Neurology, Infermi Hospital, Rimini, Italy (Marco Currò Dossi, Cristiana Ganino, and Marco Longoni);
Department of Hospital Services, Emilia Romagna Regional Health Authority, Bologna, Italy (Salvatore Ferro).
References
- Masrori, P.; van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.L.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; Desai, R.; et al. Burden of Neurological Disorders across the US from 1990-2017: A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Marin, B.; Fontana, A.; Arcuti, S.; Copetti, M.; Boumédiene, F.; Couratier, P.; Beghi, E.; Preux, P.M.; Logroscino, G. Age-Specific ALS Incidence: A Dose–Response Meta-Analysis. Eur. J. Epidemiol. 2018, 33, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global Variation in Prevalence and Incidence of Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. J. Neurol. 2020, 267, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Dandaba, M.; Couratier, P.; Labrunie, A.; Nicol, M.; Hamidou, B.; Raymondeau, M.; Logroscino, G.; Preux, P.M.; Marin, B. Characteristics and Prognosis of Oldest Old Subjects with Amyotrophic Lateral Sclerosis. Neuroepidemiology 2017, 49, 64–73. [Google Scholar] [CrossRef]
- Gianferrari, G.; Martinelli, I.; Zucchi, E.; Simonini, C.; Fini, N.; Vinceti, M.; Ferro, S.; Gessani, A.; Canali, E.; Valzania, F.; et al. Epidemiological, Clinical and Genetic Features of ALS in the Last Decade: A Prospective Population-Based Study in the Emilia Romagna Region of Italy. Biomedicines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Fang, F. Epidemiology of Amyotrophic Lateral Sclerosis: An Update of Recent Literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Aragones, J.M.; Altimiras, J.; Roura-Poch, P.; Homs, E.; Bajo, L.; Povedano, M.; Cortés-Vicente, E.; Illa, I.; Al-Chalabi, A.; Rojas-García, R. Amyotrophic Lateral Sclerosis: A Higher than Expected Incidence in People over 80 Years of Age. Amyotroph. Lateral. Scler. Front. Degener. 2016, 17, 522–527. [Google Scholar] [CrossRef]
- Marin, B.; Hamidou, B.; Couratier, P.; Nicol, M.; Delzor, A.; Raymondeau, M.; Druet-Cabanac, M.; Lautrette, G.; Boumediene, F.; Preux, P.M. Population-Based Epidemiology of Amyotrophic Lateral Sclerosis (ALS) in an Ageing Europe—The French Register of ALS in Limousin (FRALim Register). Eur. J. Neurol. 2014, 21, 1292-e79. [Google Scholar] [CrossRef]
- Chio, A.; Canosa, A.; Gallo, S.; Cammarosano, S.; Moglia, C.; Fuda, G.; Calvo, A.; Gabriele, M. For the PARALS group ALS clinical trials: Do enrolled patients accurately represent the ALS population? Neurology 2011, 77, 1432–1437. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP/248; UN: New York, NY, USA, 2017. [Google Scholar]
- Regione Emilia Romagna-Statistica. Available online: https://Statistica.Regione.Emilia-Romagna.It/Servizi-Online/Statistica-SelfService/Popolazione/Popolazione-per-Eta-e-Sesso/Pop_eta_ammontare (accessed on 10 July 2022).
- Mandrioli, J.; Biguzzi, S.; Guidi, C.; Venturini, E.; Sette, E.; Terlizzi, E.; Ravasio, A.; Casmiro, M.; Salvi, F.; Liguori, R.; et al. Epidemiology of Amyotrophic Lateral Sclerosis in Emilia Romagna Region (Italy): A Population Based Study. Amyotroph. Lateral. Scler. Front. Degener. 2014, 15, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial Revisited: Revised Criteria for the Diagnosis of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Calvo, A.; Moglia, C.; Mazzini, L.; Mora, G.; Mutani, R.; Balma, M.; Cammarosano, S.; Canosa, A.; Gallo, S.; et al. Phenotypic Heterogeneity of Amyotrophic Lateral Sclerosis: A Population Based Study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, J.; Zucchi, E.; Martinelli, I.; Van der Most, L.; Gianferrari, G.; Moglia, C.; Manera, U.; Solero, L.; Vasta, R.; Canosa, A.; et al. Factors predicting disease progression in C9ORF72 ALS patients. J. Neurol. 2023, 270, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Pensato, V.; Magri, S.; Bella, E.D.; Tannorella, P.; Bersano, E.; Sorarù, G.; Gatti, M.; Ticozzi, N.; Taroni, F.; Lauria, G.; et al. Sorting Rare ALS Genetic Variants by Targeted Re-Sequencing Panel in Italian Patients: OPTN, VCP, and SQSTM1 Variants Account for 3% of Rare Genetic Forms. J. Clin. Med. 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Fini, N.; Georgoulopoulou, E.; Vinceti, M.; Monelli, M.; Pinelli, G.; Vacondio, P.; Giovannini, M.; Dallari, R.; Marudi, A.; Mandrioli, J. Noninvasive and Invasive Ventilation and Enteral Nutrition for ALS in Italy. Muscle Nerve 2014, 50, 508–516. [Google Scholar] [CrossRef]
- Georgoulopoulou, E.; Fini, N.; Vinceti, M.; Monelli, M.; Vacondio, P.; Bianconi, G.; Sola, P.; Nichelli, P.; Mandrioli, J. The Impact of Clinical Factors, Riluzole and Therapeutic Interventions on ALS Survival: A Population Based Study in Modena, Italy. Amyotroph. Lateral. Scler. Front. Degener. 2013, 14, 338–345. [Google Scholar] [CrossRef]
- Fasano, A.; Fini, N.; Ferraro, D.; Ferri, L.; Vinceti, M.; Mandrioli, J.; Nichelli, P.; Biguzzi, S.; Handouk, Y.; Venturini, E.; et al. Percutaneous Endoscopic Gastrostomy, Body Weight Loss and Survival in Amyotrophic Lateral Sclerosis: A Population-Based Registry Study. Amyotroph. Lateral. Scler. Front. Degener. 2017, 18, 233–242. [Google Scholar] [CrossRef]
- Mandrioli, J.; Ferri, L.; Fasano, A.; Zucchi, E.; Fini, N.; Moglia, C.; Lunetta, C.; Marinou, K.; Ticozzi, N.; Drago Ferrante, G.; et al. Cardiovascular Diseases May Play a Negative Role in the Prognosis of Amyotrophic Lateral Sclerosis. Eur. J. Neurol. 2018, 25, 861–868. [Google Scholar] [CrossRef]
- Andersen, P.M.; Abrahams, S.; Borasio, G.D.; de Carvalho, M.; Chio, A.; van Damme, P.; Hardiman, O.; Kollewe, K.; Morrison, K.E.; Petri, S.; et al. EFNS Guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS)—Revised Report of an EFNS Task Force. Eur. J. Neurol. 2012, 19, 360–375. [Google Scholar] [CrossRef]
- Kimura, F.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; D’Ovidio, F.; Vasta, R.; Grassano, M.; Brunetti, M.; Barberis, M.; Corrado, L.; et al. ALS Phenotype Is Influenced by Age, Sex, and Genetics: A Population-Based Study. Neurology 2020, 94, e802–e810. [Google Scholar] [CrossRef] [PubMed]
- forbes, r.b.; colville, s.; swingler, r.j. The Epidemiology of Amyotrophic Lateral Sclerosis (ALS/MND) in People Aged 80 or over. Age Ageing 2004, 33, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.O.; Gromicho, M.; Pinto, S.; de Carvalho, M. Very Late-Onset Amyotrophic Lateral Sclerosis in a Portuguese Cohort. Amyotroph. Lateral. Scler. Front. Degener. 2018, 19, 619–622. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshikura, N.; Harada, N.; Yamada, M.; Koumura, A.; Sakurai, T.; Hayashi, Y.; Kimura, A.; Hozumi, I.; Inuzuka, T. Late-Onset Patients with Sporadic Amyotrophic Lateral Sclerosis in Japan Have a Higher Progression Rate of ALSFRS-R at the Time of Diagnosis. Intern. Med. 2012, 51, 579–584. [Google Scholar] [CrossRef]
- Millecamps, S.; Boillée, S.; le Ber, I.; Seilhean, D.; Teyssou, E.; Giraudeau, M.; Moigneu, C.; Vandenberghe, N.; Danel-Brunaud, V.; Corcia, P.; et al. Phenotype Difference between ALS Patients with Expanded Repeats in C9ORF72 and Patients with Mutations in Other ALS-Related Genes. J. Med. Genet. 2012, 49, 258–263. [Google Scholar] [CrossRef]
- Connolly, O.; Le Gall, L.; McCluskey, G.; Donaghy, C.G.; Duddy, W.J.; Duguez, S. A Systematic Review of Genotype-Phenotype Correlation across Cohorts Having Causal Mutations of Different Genes in ALS. J. Pers. Med. 2020, 10, 58. [Google Scholar] [CrossRef]
- Assoni, A.F.; Foijer, F.; Zatz, M. Amyotrophic Lateral Sclerosis, FUS and Protein Synthesis Defects. Stem Cell Rev. Rep. 2022. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Ansorge, O.; Strong, M.; Bilbao, J.; Zinman, L.; Ang, L.C.; Baker, M.; Stewart, H.; Eisen, A.; Rademakers, R.; et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: Two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011, 122, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Waibel, S.; Neumann, M.; Rosenbohm, A.; Birve, A.; Volk, A.E.; Weishaupt, J.H.; Meyer, T.; Müller, U.; Andersen, P.M.; Ludolph, A.C. Truncating mutations in FUS/TLS give rise to a more aggressive ALS-phenotype than missense mutations: A clinico-genetic study in Germany. Eur. J. Neurol. 2013, 20, 540–546. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Calvo, A.; Chio, A.; Colville, S.; Ellis, C.M.; Hardiman, O.; Heverin, M.; Howard, R.S.; Huisman, M.H.B.; Keren, N.; et al. Analysis of amyotrophic lateral sclerosis as a multistep process: A population-based modelling study. Lancet Neurol. 2014, 13, 1108–1113. [Google Scholar] [CrossRef]
- Chiò, A.; Mazzini, L.; D’Alfonso, S.; Corrado, L.; Canosa, A.; Moglia, C.; Manera, U.; Bersano, E.; Brunetti, M.; Barberis, M.; et al. The multistep hypothesis of ALS revisited: The role of genetic mutations. Neurology 2018, 91, e635–e642. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.R.; Iacoangeli, A.; Opie-Martin, S.; van Vugt, A.J.J.F.; Al Khleifat, A.; Bredin, A.; Ossher, L.; Andersen, P.M.; Hardiman, O.; Mehta, A.R.; et al. The impact of age on genetic testing decisions in amyotrophic lateral sclerosis. Brain 2022, 145, 4440–4447. [Google Scholar] [CrossRef]
- Dharmadasa, T.; Scaber, J.; Edmond, E.; Marsden, R.; Thompson, A.; Talbot, K.; Turner, M.R. Genetic testing in motor neurone disease. Pract. Neurol. 2022, 22, 107–116. [Google Scholar] [CrossRef]
- Salmon, K.; Kiernan, M.C.; Kim, S.H.; Andersen, P.M.; Chio, A.; Berg, L.H.V.D.; Van Damme, P.; Al-Chalabi, A.; Lillo, P.; Andrews, J.A.; et al. The importance of offering early genetic testing in everyone with amyotrophic lateral sclerosis. Brain 2022, 145, 1207–1210. [Google Scholar] [CrossRef]
- Arthur, K.C.; Calvo, A.; Price, T.R.; Geiger, J.T.; Chiò, A.; Traynor, B.J. Projected Increase in Amyotrophic Lateral Sclerosis from 2015 to 2040. Nat. Commun. 2016, 7, 12408. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, K.; Kuzma-Kozakiewicz, M.; Piotrkiewicz, M.; Gromicho, M.; Grosskreutz, J.; Andersen, P.M.; de Carvalho, M.; Uysal, H.; Osmanovic, A.; Schreiber-Katz, O.; et al. Impact of Comorbidities and Co-Medication on Disease Onset and Progression in a Large German ALS Patient Group. J. Neurol. 2020, 267, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Paganoni, S.; Macklin, E.A.; Lee, A.; Murphy, A.; Chang, J.; Zipf, A.; Cudkowicz, M.; Atassi, N. Diagnostic Timelines and Delays in Diagnosing Amyotrophic Lateral Sclerosis (ALS). Amyotroph. Lateral. Scler. Front. Degener. 2014, 15, 453–456. [Google Scholar] [CrossRef]
- Richards, D.; Morren, J.A.; Pioro, E.P. Time to Diagnosis and Factors Affecting Diagnostic Delay in Amyotrophic Lateral Sclerosis. J. Neurol. Sci. 2020, 417, 117054. [Google Scholar] [CrossRef]
- Samaras, N.; Chevalley, T.; Samaras, D.; Gold, G. Older Patients in the Emergency Department: A Review. Ann. Emerg. Med. 2010, 56, 261–269. [Google Scholar] [CrossRef]
- Alonso, A.; Logroscino, G.; Jick, S.S.; Hernán, M.A. Incidence and Lifetime Risk of Motor Neuron Disease in the United Kingdom: A Population-Based Study. Eur. J. Neurol. 2009, 16, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Hobson, E.V.; McDermott, C.J. Supportive and Symptomatic Management of Amyotrophic Lateral Sclerosis. Nat. Rev. Neurol. 2016, 12, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Soriani, M.H.; Desnuelle, C. Care Management in Amyotrophic Lateral Sclerosis. Rev. Neurol. 2017, 173, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Cabona, C.; Meo, G.; Rolla-Bigliani, C.; Castellan, L.; Pardini, M.; Inglese, M.; Caponnetto, C.; Roccatagliata, L. Age at symptom onset influences cortical thinning distribution and survival in amyotrophic lateral sclerosis. Neuroradiology 2021, 63, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Little, J.; Gomes, J.; Cashman, N.R.; Krewski, D. Identification of Risk Factors Associated with Onset and Progression of Amyotrophic Lateral Sclerosis Using Systematic Review and Meta-Analysis. Neurotoxicology 2017, 61, 101–130. [Google Scholar] [CrossRef] [PubMed]
- Broussalis, E.; Grinzinger, S.; Kunz, A.B.; Killer-Oberpfalzer, M.; Haschke-Becher, E.; Hartung, H.P.; Kraus, J. Late Age Onset of Amyotrophic Lateral Sclerosis Is Often Not Considered in Elderly People. Acta Neurol. Scand. 2018, 137, 329–334. [Google Scholar] [CrossRef]
- Kaufmann, P.; Levy, G.; Thompson, J.L.; DelBene, M.L.; Battista, V.; Gordon, P.H.; Rowland, L.P.; Levin, B.; Mitsumoto, H. The ALSFRSr predicts survival time in an ALS clinic population. Neurology 2005, 64, 38–43. [Google Scholar] [CrossRef]
- Kollewe, K.; Mauss, U.; Krampfl, K.; Petri, S.; Dengler, R.; Mohammadi, B. ALSFRS-R Score and Its Ratio: A Useful Predictor for ALS-Progression. J. Neurol. Sci. 2008, 275, 69–73. [Google Scholar] [CrossRef]
- Yates, E.; Rafiq, M.K. Prognostic Factors for Survival in Patients with Amyotrophic Lateral Sclerosis: Analysis of a Multi-Centre Clinical Trial. J. Clin. Neurosci. 2016, 32, 51–56. [Google Scholar] [CrossRef]
- Pupillo, E.; Messina, P.; Logroscino, G.; Beghi, E.; Micheli, A.; Rosettani, P.; Baldini, D.; Bianchi, G.; Rigamonti, A.; Bonito, V.; et al. Long-Term Survival in Amyotrophic Lateral Sclerosis: A Population-Based Study. Ann. Neurol. 2014, 75, 287–297. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).