Examining the Role of Hypothalamus-Derived Neuromedin-U (NMU) in Bone Remodeling of Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Rats
2.2. Hairpin Design
2.3. Cell Culture
2.4. In Vitro Hairpin Screening
2.5. Microinjection Surgery
2.6. In Vivo Hairpin Screening
2.7. Laser Capture Microdissection
2.8. Immunohistochemistry
2.9. Micro Computed Tomography (µCT)
2.10. Histomorphometry
2.11. Statistical Analyses
3. Results
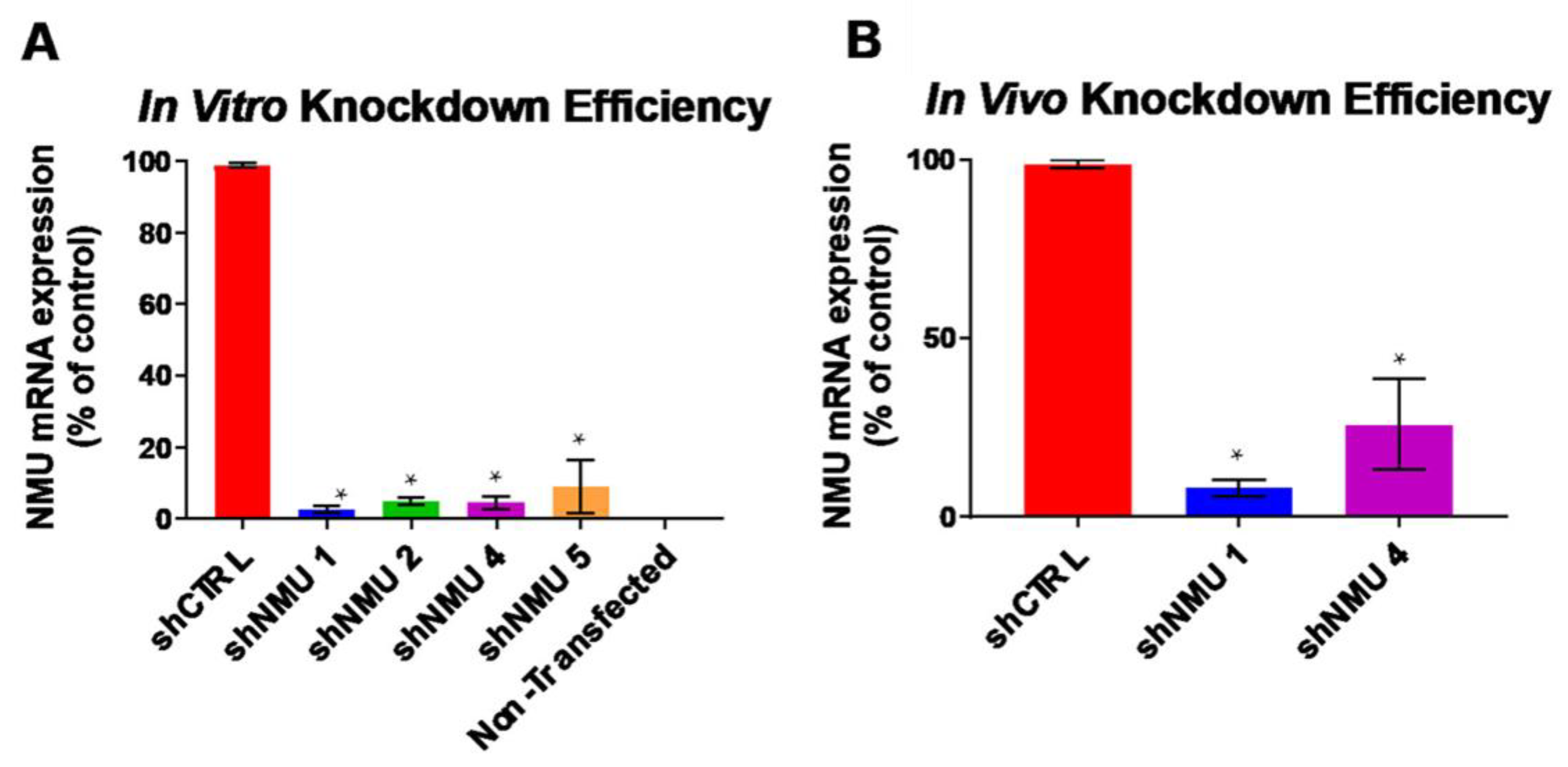
3.1. Validation of NMU Knockdown
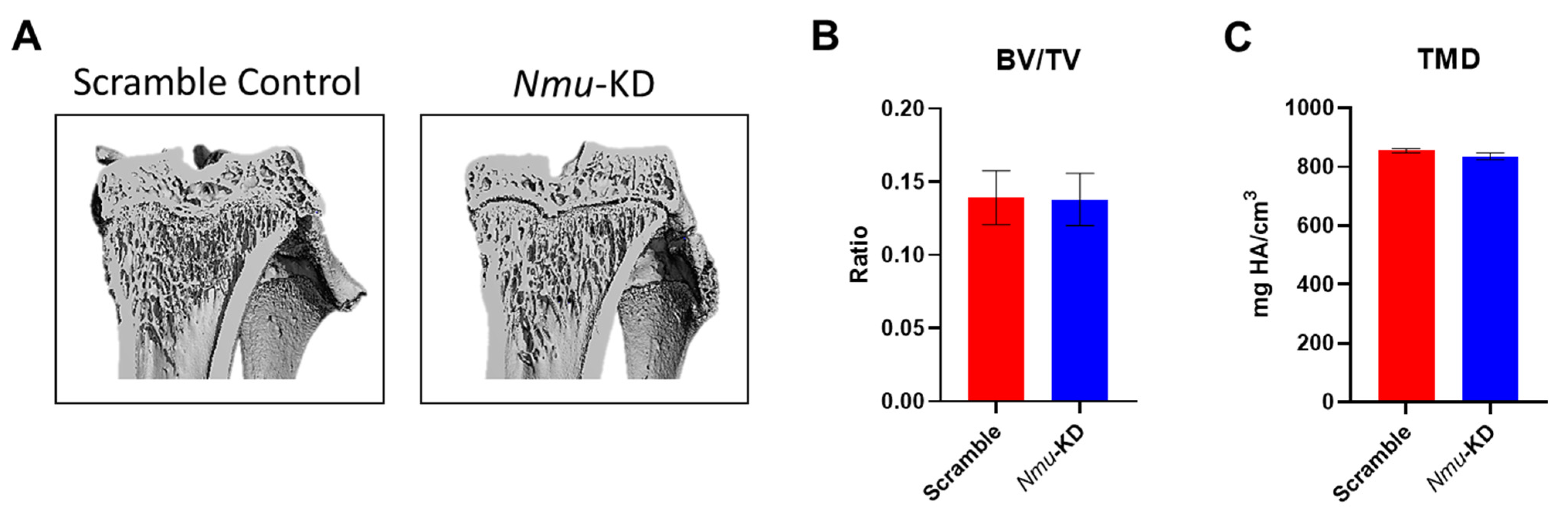
3.2. Bone Volume Is Unchanged in the Absence of Hypothalamic NMU
3.3. Histological Analyses of NMU-KD Bones
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Leboime, A.; Confavreux, C.B.; Mehsen, N.; Paccou, J.; David, C.; Roux, C. Osteoporosis and mortality. Jt. Bone Spine 2010, 77 (Suppl. S2), S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Brighton, P.J.; Szekeres, P.G.; Willars, G.B. Neuromedin U and its receptors: Structure, function, and physiological roles. Pharmacol. Rev. 2004, 56, 231–248. [Google Scholar] [CrossRef]
- Rao, S.M.; Auger, J.L.; Gaillard, P.; Weissleder, R.; Wada, E.; Torres, R.; Kojima, M.; Benoist, C.; Mathis, D.; Binstadt, B.A. The neuropeptide neuromedin U promotes autoantibody-mediated arthritis. Arthritis. Res. Ther. 2012, 14, R29. [Google Scholar] [CrossRef]
- Wallrapp, A.; Riesenfeld, S.J.; Burkett, P.R.; Abdulnour, R.E.; Nyman, J.; Dionne, D.; Hofree, M.; Cuoco, M.S.; Rodman, C.; Farouq, D.; et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 2017, 549, 351–356. [Google Scholar] [CrossRef]
- Sato, S.; Hanada, R.; Kimura, A.; Abe, T.; Matsumoto, T.; Iwasaki, M.; Inose, H.; Ida, T.; Mieda, M.; Takeuchi, Y.; et al. Central control of bone remodeling by neuromedin U. Nat. Med. 2007, 13, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.T.; Jestes, K.J.; Jackson, K.L.; Zukosky, T.; Squire, M.E.; Hum, J.M.; Lowery, J.W. Neuromedin U (NMU) regulates osteoblast differentiation and activity. Biochem. Biophys. Res. Commun. 2020, 524, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.T.; Manikowski, K.J.; Snyder, S.; Griffin, N.; Orr, A.L.; Hulsey, E.Q.; Born-Evers, G.; Zukosky, T.; Squire, M.E.; Hum, J.M.; et al. NMUR1 in the NMU-Mediated Regulation of Bone Remodeling. Life 2021, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Rucinski, M.; Ziolkowska, A.; Tyczewska, M.; Szyszka, M.; Malendowicz, L.K. Neuromedin U directly stimulates growth of cultured rat calvarial osteoblast-like cells acting via the NMU receptor 2 isoform. Int. J. Mol. Med. 2008, 22, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Benzon, C.R.; Johnson, S.B.; McCue, D.L.; Li, D.; Green, T.A.; Hommel, J.D. Neuromedin U receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high-fat food and leads to increased body weight. Neuroscience 2014, 258, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hommel, J.D.; Sears, R.M.; Georgescu, D.; Simmons, D.L.; DiLeone, R.J. Local gene knockdown in the brain using viral-mediated RNA interference. Nat. Med. 2003, 9, 1539–1544. [Google Scholar] [CrossRef]
- Kasper, J.M.; McCue, D.L.; Milton, A.J.; Szwed, A.; Sampson, C.M.; Huang, M.; Carlton, S.; Meltzer, H.Y.; Cunningham, K.A.; Hommel, J.D. Gamma-Aminobutyric Acidergic Projections From the Dorsal Raphe to the Nucleus Accumbens Are Regulated by Neuromedin U. Biol. Psychiatry 2016, 80, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Scramble Control | Nmu-KD | p Value | |
|---|---|---|---|---|
| Tibiae | BV/TV (ratio) | 0.139 ± 0.018 | 0.138 ± 0.018 | 0.96 |
| Tb.N (#/mm) | 2.924 ± 0.200 | 3.166 ± 0.434 | 0.99 | |
| Tb.Th (mm) | 0.075 ± 0.002 | 0.074 ± 0.004 | 0.23 | |
| Tb.Sp (mm) | 0.341 ± 0.029 | 0.317 ± 0.033 | 0.95 | |
| Conn.D (#/mm3) | 48.7 ± 7.2 | 66.9 ± 24.5 | 0.95 | |
| TMD (mg HA/cm3) | 854.518 ± 5.577 | 835.526 ± 11.546 | 0.13 |
| Parameter | Scramble | Nmu-KD | p Value | |
|---|---|---|---|---|
| Tibiae | TV (mm3) | 5.49 ± 0.22 | 5.49 ± 0.17 | 0.98 |
| BV/TV (ratio) | 0.975 ± 0.001 | 0.972 ± 0.002 | 0.13 | |
| Ct.Th (mm) | 0.639 ± 0.012 | 0.583 ± 0.028 | 0.10 | |
| MaV (mm3) | 2.01 ± 0.14 | 2.44 ± 0.35 | 0.28 |
| Parameter | Scramble | Nmu-KD | p Value | |
|---|---|---|---|---|
| Osteoblast Analyses | Osteoblast Number (N.Ob/B.Pm) | 49.39 ± 7.42 | 47.62 ± 2.30 | 0.83 |
| Osteoblast Surface (Ob.S/BS) | 41.38 ± 5.14 | 41.07 ± 1.81 | 0.96 | |
| Osteoid Surface (OS/BS) | 16.22 ± 3.47 | 15.66 ± 1.82 | 0.89 | |
| Osteoclast Analyses | Osteoclast Number (N.Oc/B.Pm) | 2.61 ± 1.07 | 2.57 ± 0.63 | 0.95 |
| Osteoclast Surface (Oc.S/BS) | 9.97 ± 0.60 | 10.38 ± 2.67 | 0.88 | |
| Eroded Surface (ES/BS) | 11.06 ± 0.78 | 10.94 ± 2.76 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Born-Evers, G.; Orr, A.L.; Hulsey, E.Q.; Squire, M.E.; Hum, J.M.; Plotkin, L.; Sampson, C.; Hommel, J.; Lowery, J.W. Examining the Role of Hypothalamus-Derived Neuromedin-U (NMU) in Bone Remodeling of Rats. Life 2023, 13, 918. https://doi.org/10.3390/life13040918
Born-Evers G, Orr AL, Hulsey EQ, Squire ME, Hum JM, Plotkin L, Sampson C, Hommel J, Lowery JW. Examining the Role of Hypothalamus-Derived Neuromedin-U (NMU) in Bone Remodeling of Rats. Life. 2023; 13(4):918. https://doi.org/10.3390/life13040918
Chicago/Turabian StyleBorn-Evers, Gabriella, Ashley L. Orr, Elizabeth Q. Hulsey, Maria E. Squire, Julia M. Hum, Lilian Plotkin, Catherine Sampson, Jonathan Hommel, and Jonathan W. Lowery. 2023. "Examining the Role of Hypothalamus-Derived Neuromedin-U (NMU) in Bone Remodeling of Rats" Life 13, no. 4: 918. https://doi.org/10.3390/life13040918
APA StyleBorn-Evers, G., Orr, A. L., Hulsey, E. Q., Squire, M. E., Hum, J. M., Plotkin, L., Sampson, C., Hommel, J., & Lowery, J. W. (2023). Examining the Role of Hypothalamus-Derived Neuromedin-U (NMU) in Bone Remodeling of Rats. Life, 13(4), 918. https://doi.org/10.3390/life13040918








