Diagenetic and Biological Overprints in Geochemical Signatures of the Gigantoproductus Tertiary Layer (Brachiopoda): Assessing the Paleoclimatic Interpretation
Abstract
1. Introduction
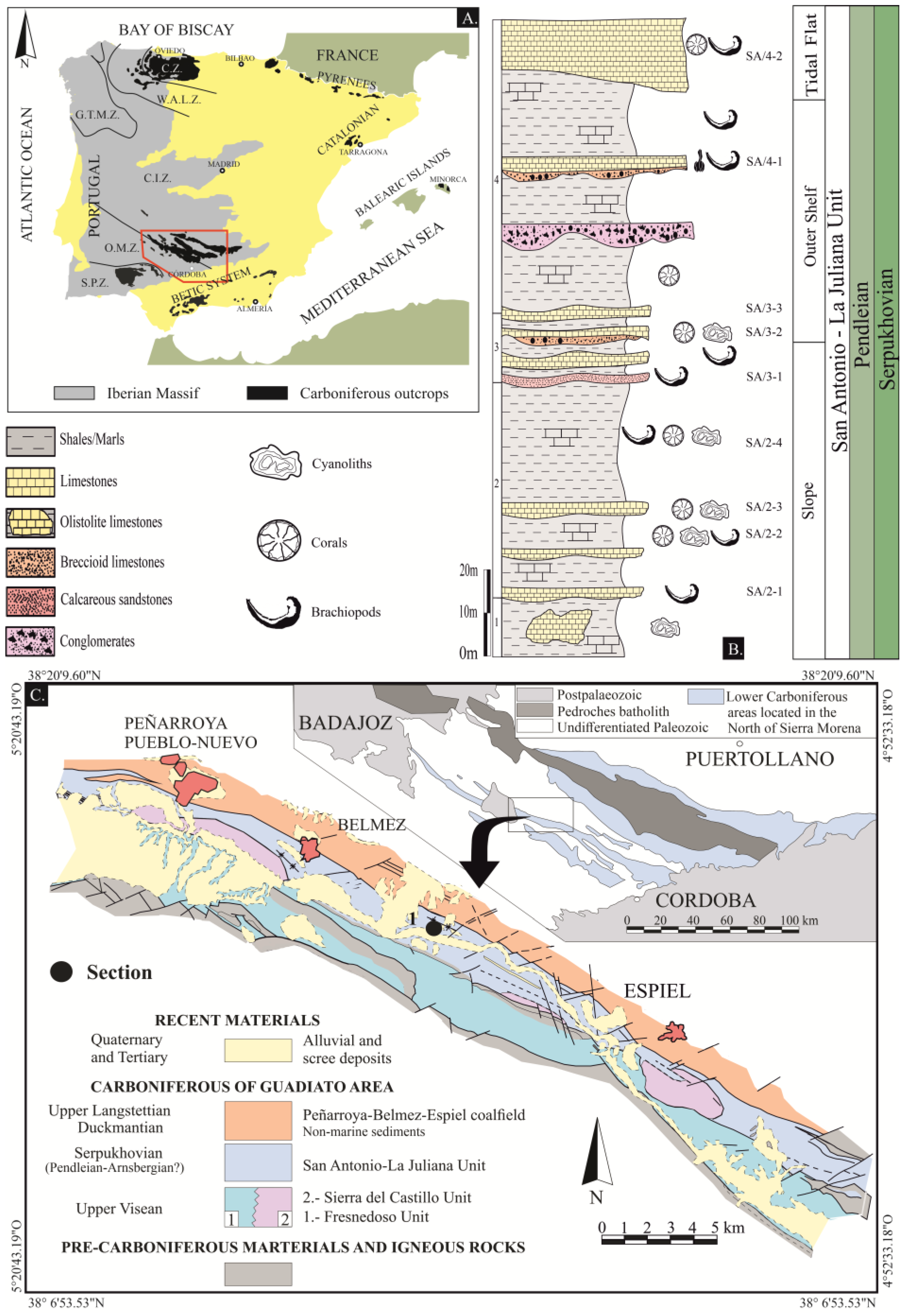
2. Geological Setting
3. Materials and Methods
3.1. Studied Material
3.2. Microscopy Methods
3.3. Trace Elements and Isotope Analyses
4. Results
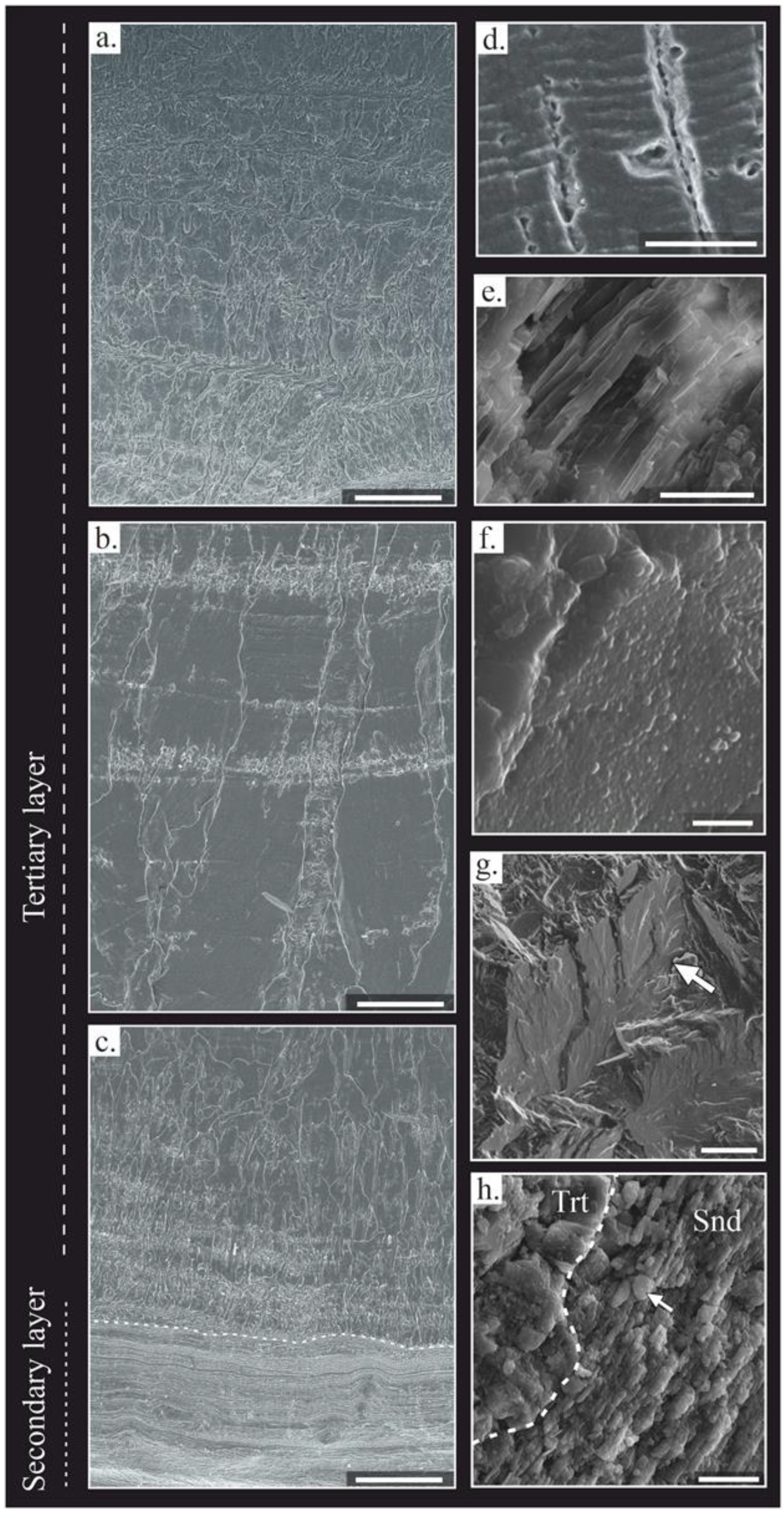
4.1. Shell Microstructure
4.2. Fossil-Shell Preservation
4.2.1. Textural Changes
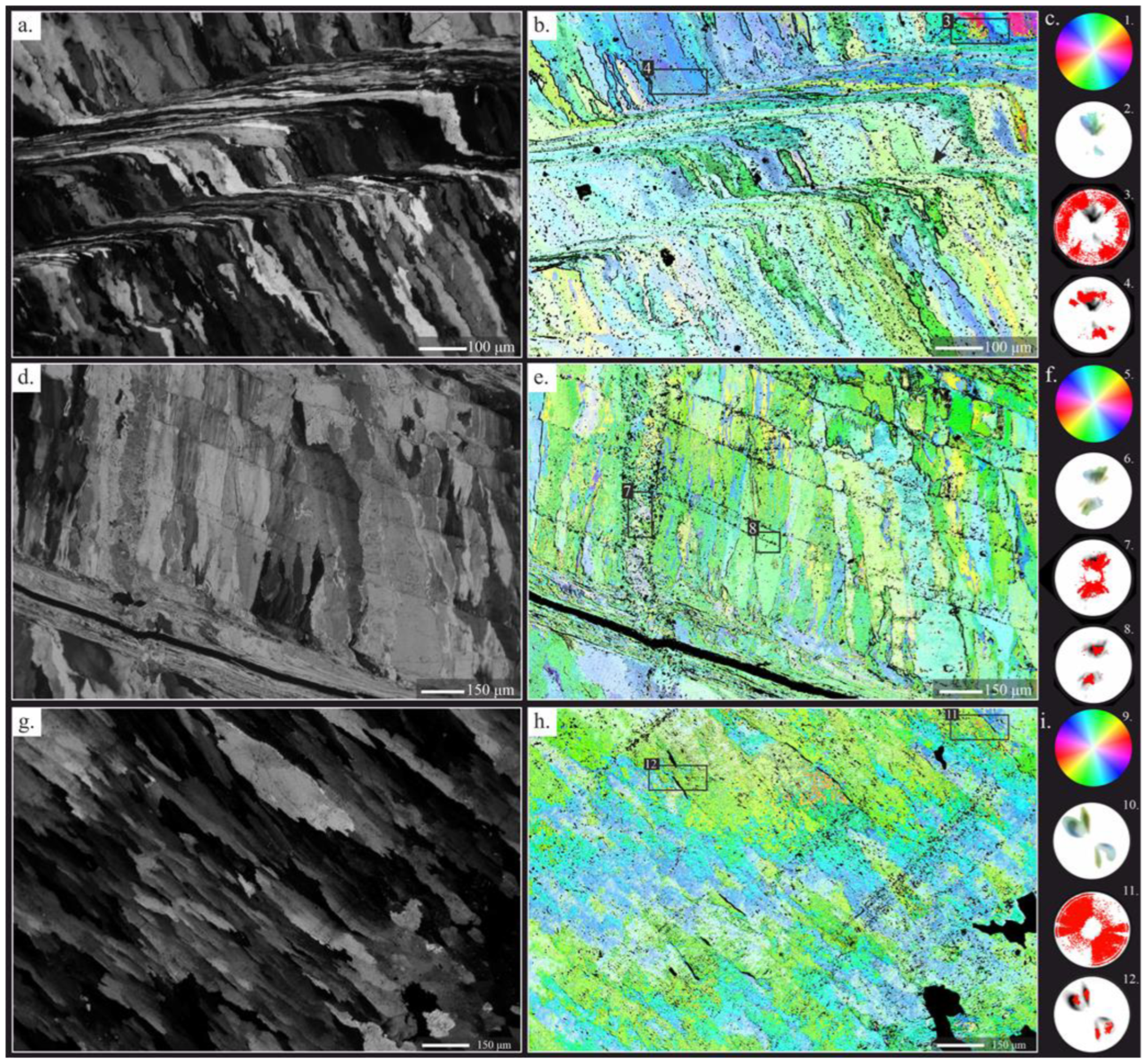
4.2.2. Crystallographic Arrangement
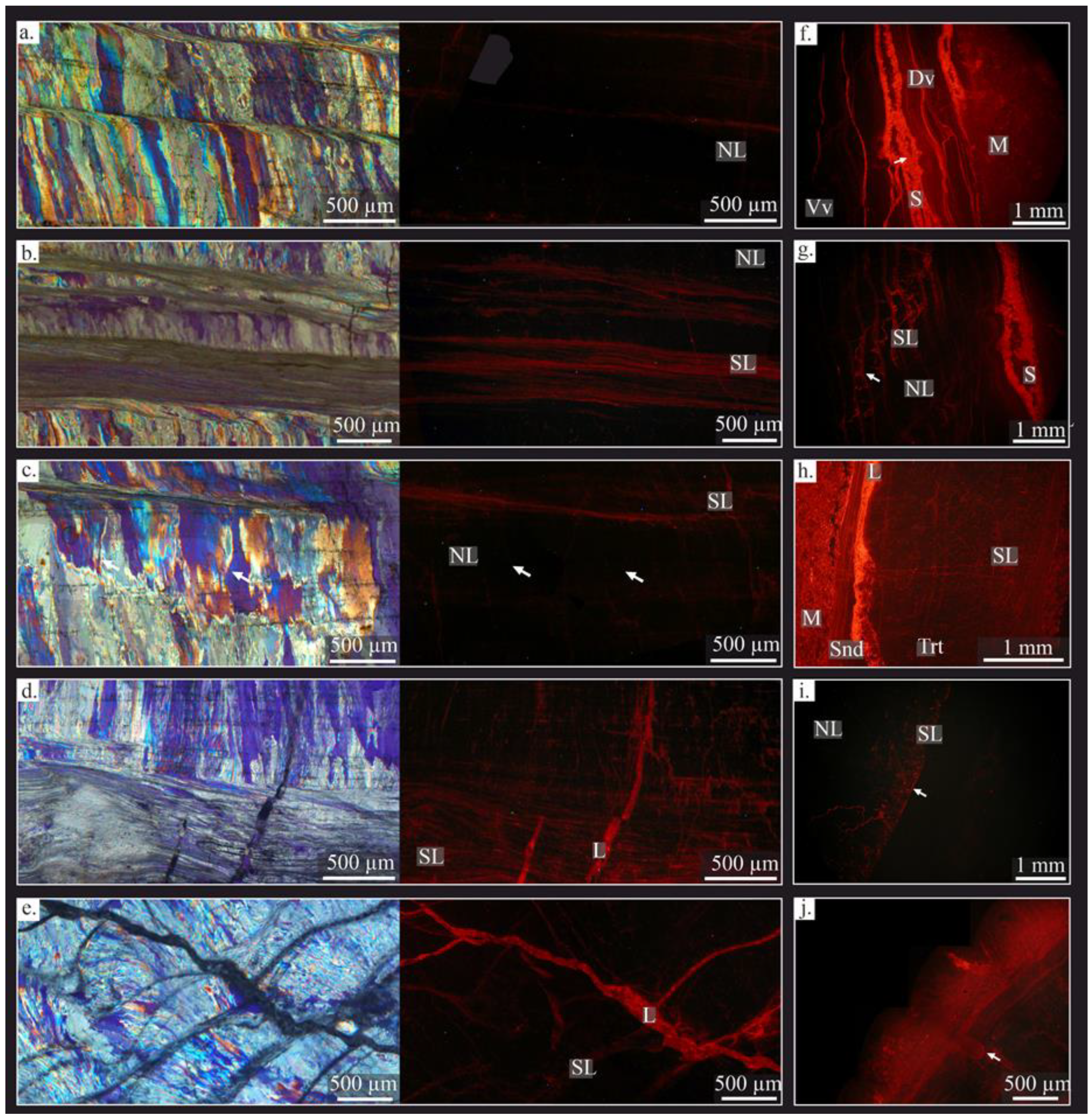
4.2.3. Cathodoluminescence (CL)
4.3. Geochemical Characterisation
4.3.1. Major/Minor and Trace Elements (MTE)
4.3.2. Isotope Geochemistry
5. Discussion
5.1. Fossil Preservation
5.2. Biological Overprint of Geochemical Signatures
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isbell, J.L.; Henry, L.C.; Gulbranson, E.L.; Limarino, C.O.; Fraiser, M.L.; Koch, Z.J.; Ciccioli, P.L.; Dineen, A.A. Glacial paradoxes during the late Paleozoic ice age: Evaluating the equilibrium line altitude as a control on glaciation. Gondwana Res. 2012, 22, 1–19. [Google Scholar] [CrossRef]
- Saltzman, M.R. Late Paleozoic Ice Age: Oceanic Gateway or pCO2? Geology 2003, 31, 151–154. [Google Scholar] [CrossRef]
- Powell, M.G. Climatic basis for sluggish macroevolution during the late Paleozoic ice age. Geology 2005, 33, 381–384. [Google Scholar] [CrossRef]
- Fielding, C.R.; Frank, T.D.; Birgenheier, L.P.; Rygel, M.C.; Jones, A.T.; Roberts, J. Stratigraphic imprint of the Late Palaeozoic Ice Age in eastern Australia: A record of alternating glacial and nonglacial climate regime. J. Geol. Soc. Lond. 2008, 165, 129–140. [Google Scholar] [CrossRef]
- Shi, G.R.; Waterhouse, J.B. Late Palaeozoic global changes affecting high-latitude environments and biotas: An introduction. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 298, 1–16. [Google Scholar] [CrossRef]
- Montañez, I.P.; Poulsen, C.J. The Late Paleozoic Ice Age: An Evolving Paradigm. Annu. Rev. Earth Planet. Sci. 2013, 41, 629–656. [Google Scholar] [CrossRef]
- Qiao, L.; Shen, S.-Z. Global paleobiogeography of brachiopods during the Mississippian—Response to the global tectonic reconfiguration, ocean circulation, and climate changes. Gondwana Res. 2014, 26, 1173–1185. [Google Scholar] [CrossRef]
- Goddéris, Y.; Donnadieu, Y.; Carretier, S.; Aretz, M.; Dera, G.; Macouin, M.; Regard, V. Onset and ending of the late Palaeozoic ice age triggered by tectonically paced rock weathering. Nat. Geosci. 2017, 10, 382–386. [Google Scholar] [CrossRef]
- Armendáriz, M.; Rosales, I.; Quesada, C. Oxygen isotope and Mg/Ca composition of Late Viséan (Mississippian) brachiopod shells from SW Iberia: Palaeoclimatic and palaeogeographic implications in northern Gondwana. Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 268, 65–79. [Google Scholar] [CrossRef]
- McGhee, G.R.; Sheehan, P.M.; Bottjer, D.J.; Droser, M.L. Ecological ranking of Phanerozoic biodiversity crises: The Serpukhovian (early Carboniferous) crisis had a greater ecological impact than the end-Ordovician. Geology 2012, 40, 147–150. [Google Scholar] [CrossRef]
- Stanley, S.M.; Powell, M.G. Depressed rates of origination and extinction during the late Paleozoic ice age: A new state for the global marine ecosystem. Geology 2003, 31, 877–880. [Google Scholar] [CrossRef]
- Brand, U. The oxygen and carbon isotope composition of Carboniferous fossil components: Sea-water effects. Sedimentology 1982, 29, 139–147. [Google Scholar] [CrossRef]
- Grossman, E.L.; Yancey, T.E.; Jones, T.E.; Bruckschen, P.; Chuvashov, B.; Mazzullo, S.J.; Mii, H.-S. Glaciation, aridification, and carbon sequestration in the Permo-Carboniferous: The isotopic record from low latitudes. Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 268, 222–233. [Google Scholar] [CrossRef]
- Stephenson, M.; Angiolini, L.; Cózar, P.; Jadoul, F.; Leng, M.; Millward, D.; Chenery, S. Northern England Serpukhovian (early Namurian) farfield responses to southern hemisphere glaciation. J. Geol. Soc. 2010, 167, 1171–1184. [Google Scholar] [CrossRef]
- Brand, U.; Jiang, G.; Azmy, K.; Bishop, J.; Montañez, I.P. Diagenetic evaluation of a Pennsylvanian carbonate succession (Bird Spring Formation, Arrow Canyon, Nevada, U.S.A.)—1: Brachiopod and whole rock comparison. Chem. Geol. 2012, 308-309, 26–39. [Google Scholar] [CrossRef]
- Roark, A.; Grossman, E.L.; Lebold, J. Low seasonality in central equatorial Pangea during a late Carboniferous highstand based on high-resolution isotopic records of brachiopod shells. Bull. Geol. Soc. Am. 2016, 128, 597–608. [Google Scholar] [CrossRef]
- Popp, B.; Anderson, T.F.; Sandberg, P.A.B.N. Brachiopods as indicators of original isotopic compositions in some Paleozoic limestones. Geol. Soc. Am. Bull. 1986, 97, 1262–1269. [Google Scholar] [CrossRef]
- Banner, J.L.; Kaufman, J. The isotopic record of ocean chemistry and diagenesis preserved in non-luminescent brachiopods from Mississippian carbonate rocks, Illinois and Missouri. Geol. Soc. Am. Bull. 1994, 106, 1074–1082. [Google Scholar] [CrossRef]
- Bruckschen, P.; Bruhn, F.; Veizer, J.; Buhl, D. 87Sr86Sr isotopic evolution of Lower Carboniferous seawater: Dinantian of western Europe. Sediment. Geol. 1995, 100, 63–81. [Google Scholar] [CrossRef]
- Bruckschen, P.; Oesmann, S.; Veizer, J. Isotope stratigraphy of the European Carboniferous: Proxy signals for ocean chemistry, climate and tectonics. Chem. Geol. 1999, 161, 127–163. [Google Scholar] [CrossRef]
- Bruckschen, P.; Veizer, J. Oxygen and carbon isotopic composition of Dinantian brachiopods: Paleoenvironmental implications for the Lower Carboniferous of western Europe. Palaeogeogr. Palaeoclim. Palaeoecol. 1997, 132, 243–264. [Google Scholar] [CrossRef]
- Mii, H.-S.; Grossman, E.L.; Yancey, T.E. Carboniferous isotope stratigraphies of North America: Implications for Carboniferous paleoceanography and Mississippian glaciation. Bull. Geol. Soc. Am. 1999, 111, 960–973. [Google Scholar] [CrossRef]
- Grossman, E.L.; Bruckschen, P.; Mii, H.-S.; Chuvashov, B.I.; Yancey, T.E.; Veizer, J. Carboniferous Paleoclimate and Global Change: Isotopic Evidence from the Russian Platform. In Carboniferous Stratigraphy and Paleogeography in Eurasia; Texas A&M University: College Station, TX, USA, 2002; pp. 61–71. [Google Scholar]
- Batt, L.S.; Montañez, I.P.; Isaacson, P.; Pope, M.C.; Butts, S.H.; Abplanalp, J. Multi-carbonate component reconstruction of mid-carboniferous (Chesterian) seawater δ13C. Palaeogeogr. Palaeoclim. Palaeoecol. 2007, 256, 298–318. [Google Scholar] [CrossRef]
- Brand, U. Biogeochemistry of Late Paleozoic North American brachiopods and secular variation of seawater composition. Biogeochemistry 1989, 7, 159–193. [Google Scholar] [CrossRef]
- Veizer, J.; Fritz, P.; Jones, B. Geochemistry of brachiopods: Oxygen and carbon isotopic records of Paleozoic oceans. Geochim. Cosmochim. Acta 1986, 50, 1679–1696. [Google Scholar] [CrossRef]
- Veizer, J.; Bruckschen, P.; Pawellek, F.; Diener, A.; Podlaha, O.G.; Carden, G.A.F.; Jasper, T.; Korte, C.; Strauss, H.; Azmy, K.; et al. Oxygen isotope evolution of Phanerozoic seawater. Palaeogeogr. Palaeoclim. Palaeoecol. 1997, 132, 159–172. [Google Scholar] [CrossRef]
- Mii, H.-S.; Grossman, E.L.; Yancey, T.E.; Chuvashov, B.; Egorov, A. Isotopic records of brachiopod shells from the Russian Platform—Evidence for the onset of mid-Carboniferous glaciation. Chem. Geol. 2001, 175, 133–147. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Coronado, I.; Hegna, T.A. Understanding biomineralization in the fossil record. Earth-Science Rev. 2018, 179, 95–122. [Google Scholar] [CrossRef]
- Muir-Wood, H.; Cooper, G.A. Morphology, Classification and Life Habits of the Productoidea (Brachiopoda); Geological Society of America: Boulder, CO, USA, 1960; ISBN 9780813710815. [Google Scholar]
- Ferguson, J. Some aspects of the ecology and growth of the carboniferous gigantoproductids. Proc. Yorks. Geol. Soc. 1978, 42, 41–54. [Google Scholar] [CrossRef]
- Nolan, L.S.P.; Angiolini, L.; Jadoul, F.; Della Porta, G.; Davies, S.J.; Banks, V.J.; Stephenson, M.H.; Leng, M.J. Sedimentary context and palaeoecology of Gigantoproductus shell beds in the Mississippian Eyam Limestone Formation, Derbyshire carbonate platform, central England. Proc. Yorks. Geol. Soc. 2017, 61, 239–257. [Google Scholar] [CrossRef]
- Brunton, C.H.C.; Lazarev, S.S.; Grant, R.E. A Review and New Classification of the Brachiopod Order Productida. Palaeontology 1995, 38, 915–936. [Google Scholar]
- Angiolini, L.; Stephenson, M.; Leng, M.J.; Jadoul, F.; Millward, D.; Aldridge, A.; Andrews, J.; Chenery, S.; Williams, G. Heterogeneity, cyclicity and diagenesis in a Mississippian brachiopod shell of palaeoequatorial Britain. Terra Nova 2012, 24, 16–26. [Google Scholar] [CrossRef]
- Angiolini, L.; Crippa, G.; Azmy, K.; Capitani, G.; Confalonieri, G.; Della Porta, G.; Griesshaber, E.; Harper, D.A.T.; Leng, M.J.; Nolan, L.; et al. The giants of the phylum Brachiopoda: A matter of diet? Palaeontology 2019, 62, 889–917. [Google Scholar] [CrossRef]
- Grossman, E.L.; Mii, H.-S.; Zhang, C.; Yancey, T.E. Chemical Variation in Pennsylvanian Brachiopod Shells--Diagenetic, Taxonomic, Microstructural, and Seasonal Effects. J. Sediment. Res. 1996, 66, 1011–1022. [Google Scholar] [CrossRef]
- Rush, P.F.; Chafetz, H. Fabric-retentive, Non-luminescent Brachiopods as Indicators of Original 13C and 18O Composition: A Test. J. Sediment. Petrol. 1990, 60, 968–981. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Cusack, M.; Janousch, M.; Finch, A.A. Influence of crystallographic orientation of biogenic calcite on in situ Mg XANES analyses. J. Synchrotron Radiat. 2008, 15, 572–575. [Google Scholar] [CrossRef]
- Urey, H.C.; Lowenstam, H.A.; Epstein, S.; McKinney, C.R. Measurement of Paleotemperatures and Temperatures and the Southeastern United States. Bull. Geol. Soc. Am. 1951, 62, 399–416. [Google Scholar] [CrossRef]
- Carpenter, S.J.; Lohmann, K.C. δ18O and δ13C values of modern brachiopod shells. Geochim. Cosmochim. Acta 1995, 59, 3749–3764. [Google Scholar] [CrossRef]
- Brand, U.; Azmy, K.; Griesshaber, E.; Bitner, M.A.; Logan, A.; Zuschin, M.; Ruggiero, E.; Colin, P.L. Carbon isotope composition in modern brachiopod calcite: A case of equilibrium with seawater? Chem. Geol. 2015, 411, 81–96. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Saulnier, S.; Vigier, N.; Schumacher, A.; Chaussidon, M.; Lécuyer, C. Variability in magnesium, carbon and oxygen isotope compositions of brachiopod shells: Implications for paleoceanographic studies. Chem. Geol. 2016, 423, 49–60. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Milner Garcia, S.; Burckel, P.; Angiolini, L.; Jurikova, H.; Tomašových, A.; Henkel, D. Assessing the biomineralization processes in the shell layers of modern brachiopods from oxygen isotopic composition and elemental ratios: Implications for their use as paleoenvironmental proxies. Chem. Geol. 2019, 524, 49–66. [Google Scholar] [CrossRef]
- Riechelmann, S.; Mavromatis, V.; Buhl, D.; Dietzel, M.; Eisenhauer, A.; Immenhauser, A. Impact of diagenetic alteration on brachiopod shell magnesium isotope (δ26Mg) signatures: Experimental versus field data. Chem. Geol. 2016, 440, 191–206. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Fred, T.A.C.; Andrus, F.T. Vital Effects in the Context of Biomineralization. In Workshop on Biominerals and Biomineralization Processes; Fernández-Díaz, L., Astilleros, J.M., Eds.; Sociedad Española de Mienralogía: Madrid, Spain, 2010; Volume 7, pp. 35–45. [Google Scholar]
- Weiner, S.; Dove, P.M. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Miner. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Coronado, I.; Fine, M.; Bosellini, F.R.; Stolarski, J. Impact of ocean acidification on crystallographic vital effect of the coral skeleton. Nat. Commun. 2019, 10, 2896. [Google Scholar] [CrossRef]
- Auclair, A.-C.; Joachimski, M.M.; Lécuyer, C. Deciphering kinetic, metabolic and environmental controls on stable isotope fractionations between seawater and the shell of Terebratalia transversa (Brachiopoda). Chem. Geol. 2003, 202, 59–78. [Google Scholar] [CrossRef]
- Parkinson, D.; Curry, G.B.; Cusack, M.; Fallick, A.E. Shell structure, patterns and trends of oxygen and carbon stable isotopes in modern brachiopod shells. Chem. Geol. 2005, 219, 193–235. [Google Scholar] [CrossRef]
- Romanin, M.; Crippa, G.; Ye, F.; Brand, U.; Bitner, M.; Gaspard, D.; Häussermann, V.; Laudien, J. A Sampling Strategy for Recent and Fossil Brachiopods: Selecting the Optimal Shell Segment for Geochemical Analyses. Riv. Ital. Paleontol. Stratigr. 2018, 124, 343–359. [Google Scholar] [CrossRef]
- Bajnai, D.; Fiebig, J.; Tomašových, A.; Milner Garcia, S.; Rollion-Bard, C.; Raddatz, J.; Löffler, N.; Primo-Ramos, C.; Brand, U. Assessing kinetic fractionation in brachiopod calcite using clumped isotopes. Sci. Rep. 2018, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Brand, U.; Logan, A.; Hiller, N.; Richardson, J. Geochemistry of modern brachiopods: Applications and implications for oceanography and paleoceanography. Chem. Geol. 2003, 198, 305–334. [Google Scholar] [CrossRef]
- Cusack, M.; Pérez-Huerta, A.; Janousch, M.; Finch, A.A. Magnesium in the lattice of calcite-shelled brachiopods. Chem. Geol. 2008, 257, 59–64. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Cusack, M.; Jeffries, T.E.; Williams, C.T. High resolution distribution of magnesium and strontium and the evaluation of Mg/Ca thermometry in Recent brachiopod shells. Chem. Geol. 2008, 247, 229–241. [Google Scholar] [CrossRef]
- Buening, N.; Carlson, S.J. Geochemical investigation of growth in selected Recent articulate brachiopods. Lethaia 1992, 25, 331–345. [Google Scholar] [CrossRef]
- Brand, U.; Azmy, K.; Bitner, M.A.; Logan, A.; Zuschin, M.; Came, R.; Ruggiero, E. Oxygen isotopes and MgCO3 in brachiopod calcite and a new paleotemperature equation. Chem. Geol. 2013, 359, 23–31. [Google Scholar] [CrossRef]
- Brand, U.; Bitner, M.A.; Logan, A.; Azmy, K.; Crippa, G.; Angiolini, L.; Colin, P.; Griesshaber, E.; Harper, E.; Haussermann, V. Brachiopod-Based Oxygen-Isotope Thermometer: Update and Review. Riv. Ital. Paleontol. Stratigr. 2019, 125, 775–778. [Google Scholar]
- Cózar, P.; Rodríguez, S. Propuesta de nueva nomenclatura para las unidades del Carbonífero Inferior del sector norte del área del Guadiato (Córdoba). Bol. Geol. Min. 1999, 110, 237–254. [Google Scholar]
- Cózar, P.; Rodríguez, S. Pendleian(early Serpukhovian) marine carbonates from SW Spain: Sedimentology, biostratigraphy and depositional model. Geol. J. 2004, 39, 25–47. [Google Scholar] [CrossRef]
- Medina-Varea, P.; Sarmiento, G.; Rodríguez, S.; Cózar, P. Early Serpukhovian Conodonts from the Guadiato Area (Córdoba, Spain). Coloq. Paleontol. 2005, 55, 21–50. [Google Scholar]
- Cózar, P.; Rodríguez, S.; Somerville, I.D. Large multi-biotic cyanoliths from relatively deep-water facies in the early Serpukhovian of SW Spain. Facies 2003, 49, 31–48. [Google Scholar] [CrossRef]
- Cózar, P. Bioestratigrafía Con Foraminíferos Del Carbonífero Inferior Del Sector Norte Del Área Del Guadiato (Córdoba). Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 1998. [Google Scholar]
- Cózar, P.; Rodríguez, S. Earlandiacea (Foraminiferida) del Carbonífero inferior del Área del Guadiato (suroeste de España). Rev. Soc. Española Paleontol. 2000, 15, 89–99. [Google Scholar] [CrossRef]
- Colmenero, J.R.; Fernandez, L.P.; Moreno, C.; Bahamonde, J.R.; Barba, P.; Heredia, N.; Gonzalez, F. Carboniferous the Geology of Spain; Gibbons, W., Moreno, T., Colmenero, J.R., Eds.; Geological Society, Bath, United Kingdom (GBR): London, UK, 2002; pp. 93–116. ISBN 1862391106. [Google Scholar]
- Coronado, I.; Pérez-Huerta, A.; Rodríguez, S. Primary biogenic skeletal structures in Multithecopora (Tabulata, Pennsylvanian). Palaeogeogr. Palaeoclim. Palaeoecol. 2013, 386, 286–299. [Google Scholar] [CrossRef]
- Heilbronner, R.; Barret, S. Image Analysis in Earth Sciences—Microstructures and Textures of Earth Materials; Springer: London, UK, 2014. [Google Scholar]
- Coronado, I.; Pérez-Huerta, A.; Rodríguez, S. Computer-integrated polarisation (CIP) in the analysis of fossils: A case of study in a Palaeozoic coral (Sinopora, Syringoporicae, Carboniferous). Hist. Biol. 2015, 27, 1098–1112. [Google Scholar] [CrossRef]
- Coronado, I.; Fernández-Martínez, E.; Rodríguez, S.; Tourneur, F. Reconstructing a Carboniferous inferred coral-alcyonarian association using a biomineralogical approach. Geobiology 2015, 13, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Coronado, I.; Rodríguez, S. Biomineral structure and crystallographic arrangement of cerioid and phaceloid growth in corals belonging to the Syringoporicae (Tabulata, Devonian–Carboniferous): A genetic reflection. Geol. Mag. 2016, 153, 718–742. [Google Scholar] [CrossRef]
- Coronado, I.; Rodríguez, S. Microstructure and crystallography in Axophyllinae. Precisions on the genus Morenaphyllum. Span. J. Palaeontol. 2018, 33, 41–56. [Google Scholar] [CrossRef]
- Barret, S. Image Analysis and the Internet. Sci. Data Manag. 1997, 1, 18–25. [Google Scholar]
- Vischer, N.O.E.; Huls, P.G.; Woldringh, C.L. Object-Image—An Interactive Image-Analysis Program Using Structured Point Collection. Bin. Microbiol. 1994, 6, 160–166. [Google Scholar]
- Abràmofff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ Part II. Biophotonics Int. 2005, 11, 36–43. [Google Scholar]
- Pereira, M.F.; Gama, C.; dias da Silva, D.; Fuenlabrada, J.M.; Silva, J.B.; Medina, J. Isotope geochemistry evidence for Laurussian-type sources of South Portuguese Zone Carboniferous turbidites (Variscan Orogeny). Geol. Soc. Lond. Spéc. Publ. 2021, 503, 619–642. [Google Scholar] [CrossRef]
- Garbelli, C.; Angiolini, L.; Shen, S.-Z. Biomineralization and global change: A new perspective for understanding the end-Permian extinction. Geology 2016, 45, 19–22. [Google Scholar] [CrossRef]
- Garbelli, C.; Angiolini, L.; Jadoul, F.; Brand, U. Micromorphology and differential preservation of Upper Permian brachiopod low-Mg calcite. Chem. Geol. 2012, 298, 1–10. [Google Scholar] [CrossRef]
- Griesshaber, E.; Schmahl, W.W.; Neuser, R.; Pettke, T.; Blüm, M.; Mutterlose, J.; Brand, U. Crystallographic texture and microstructure of terebratulide brachiopod shell calcite: An optimized materials design with hierarchical architecture. Am. Miner. 2007, 92, 722–734. [Google Scholar] [CrossRef]
- Casella, L.A.; He, S.; Griesshaber, E.; Fernández-Díaz, L.; Greiner, M.; Harper, E.M.; Jackson, D.J.; Ziegler, A.; Mavromatis, V.; Dietzel, M.; et al. Hydrothermal alteration of aragonitic biocarbonates: Assessment of micro- and nanostructural dissolution–reprecipitation and constraints of diagenetic overprint from quantitative statistical grain-area analysis. Biogeosciences 2018, 15, 7451–7484. [Google Scholar] [CrossRef]
- Cusack, M.; Parkinson, D.; Pérez-Huerta, A.; England, J.; Curry, G.B.; Fallick, A.E. Relationship between δ18O and minor element composition of Terebratalia transversa. Earth Environ. Sci. Trans. R. Soc. Edinb. 2007, 98, 443–449. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Reed, H. Preliminary assessment of coupling the analysis of shell microstructures and microtextures as palaeoecological indicator in fossil brachiopods. Span. J. Palaeontol. 2018, 33, 129–138. [Google Scholar] [CrossRef]
- Goetz, A.J.; Griesshaber, E.; Neuser, R.D.L.; Harper, E.; Schmahl, W.W. Calcite morphology, texture and hardness in the distinct layers of rhynchonelliform brachiopod shells. Eur. J. Miner. 2009, 21, 303–315. [Google Scholar] [CrossRef]
- Schmahl, W.W. The Hierarchical Organization in Biomaterials: From Nanoparticles via Mesocrystals to Functionality Citation. In Workshop on Biominerals and Biomineralization Processes; Fernández Díaz, L., Astilleros García-Monge, J.M., Eds.; Sociedad Española de Mineralogía: Madrid, Spain, 2010; Volume 7, pp. 5–21. [Google Scholar]
- Gilbert, P.U.P.A.; Porter, S.M.; Sun, C.-Y.; Xiao, S.; Gibson, B.M.; Shenkar, N.; Knoll, A.H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. USA 2019, 116, 17659–17665. [Google Scholar] [CrossRef] [PubMed]
- Cusack, M.; Dauphin, Y.; Chung, P.; Pérez-Huerta, A.; Cuif, J.-P. Multiscale structure of calcite fibres of the shell of the brachiopod Terebratulina retusa. J. Struct. Biol. 2008, 164, 96–100. [Google Scholar] [CrossRef]
- Cusack, M.; Pérez-Huerta, A.; Chung, P.; Parkinson, D.; Dauphin, Y.; Cuif, J.-P. Oxygen isotope equilibrium in brachiopod shell fibres in the context of biological control. Miner. Mag. 2008, 72, 239–242. [Google Scholar] [CrossRef]
- Grossman, E.; Mii, H.-S.; Yancey, T.E. Stable isotopes in Late Pennsylvanian brachiopods from the United States: Implications for Carboniferous paleoceanography. Geol. Soc. Am. Bull. 1993, 105, 1284–1296. [Google Scholar] [CrossRef]
- Long, J.V.P.; Agrell, S.O. The cathodo-luminescence of minerals in thin section. Miner. Mag. J. Miner. Soc. 1965, 34, 318–326. [Google Scholar] [CrossRef]
- Frank, J.R.; Carpenter, A.B.; Oglesby, T.W. Cathodoluminescence and Composition of Calcite Cement in the Taum Sauk Limestone (Upper Cambrian), Southeast Missouri. J. Sediment. Res. 1982, 52, 631–638. [Google Scholar] [CrossRef]
- Machel, H.-G. Cathodoluminescence in Calcite and Dolomite and Its Chemical Interpretation. Geosci. Can. 1985, 12, 139–147. [Google Scholar]
- Boggs, S.; Krinsley, D. Application of Cathodoluminescence Imaging to the Study of Sedimentary Rocks; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Barbin, V.; Gaspard, D. Cathodoluminescence of recentarticulate brachiopod shells. Implications for growth stages and diagenesis evaluation. Geobios 1995, 28, 39–45. [Google Scholar] [CrossRef]
- Barbin, V. Cathodoluminescence of Carbonate Shells: Biochemical vs Diagenetic Process. In Cathodoluminescence in Geosciences; Pagel, M., Barbin, V., Blanc, P., Ohnenstetter, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 303–329. ISBN 978-3-642-08526-0. [Google Scholar]
- Barbin, V. Application of cathodoluminescence microscopy to recent and past biological materials: A decade of progress. Miner. Pet. 2013, 107, 353–362. [Google Scholar] [CrossRef]
- Tomašových, A.; Farkaš, J. Cathodoluminescence of Late Triassic terebratulid brachiopods: Implications for growth patterns. Palaeogeogr. Palaeoclim. Palaeoecol. 2005, 216, 215–233. [Google Scholar] [CrossRef]
- Benito, M.I.; Reolid, M. Belemnite taphonomy (Upper Jurassic, Western Tethys) part II: Fossil–diagenetic analysis including combined petrographic and geochemical techniques. Palaeogeogr. Palaeoclim. Palaeoecol. 2012, 358-360, 89–108. [Google Scholar] [CrossRef]
- Lutz, R.A.; Rhoads, D.C. Anaerobiosis and a Theory of Growth Line Formation. Science 1977, 198, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.W.; Rosenberg, G.D.; Tkachuck, R.D. Growth increments in the shell of the living brachiopod Terebratalia transversa. Mar. Biol. 1988, 98, 511–518. [Google Scholar] [CrossRef]
- Curry, G. Shell Growth and Ecology of the Recent Brachiopod Terebratulina from Scotland. Palaeontology 1982, 25, 227–246. [Google Scholar]
- Müller, T.; Tomašových, A.; Correa, M.L.; Mertz-Kraus, R.; Mikuš, T. Mapping intrashell variation in Mg/Ca of brachiopods to external growth lines: Mg enrichment corresponds to seasonal growth slowdown. Chem. Geol. 2022, 593, 120758. [Google Scholar] [CrossRef]
- Busenberg, E.; Niel Plummer, L. Kinetic and thermodynamic factors controlling the distribution of SO32− and Na+ in calcites and selected aragonites. Geochim. Cosmochim. Acta 1985, 49, 713–725. [Google Scholar] [CrossRef]
- Rollion-Bard, C.; Blamart, D. Possible controls on Li, Na, and Mg incorporation into aragonite coral skeletons. Chem. Geol. 2015, 396, 98–111. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ichikuni, M. Uptake of sodium and potassium by calcite. Chem. Geol. 1984, 42, 137–146. [Google Scholar] [CrossRef]
- Yoshimura, T.; Suzuki, A.; Tamenori, Y.; Kawahata, H. Micro-X-ray fluorescence-based comparison of skeletal structure and P, Mg, Sr, O and Fe in a fossil of the cold-water coral Desmophyllum sp., NW Pacific. Geo-Marine Lett. 2014, 34, 1–9. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tamenori, Y.; Suzuki, A.; Kawahata, H.; Iwasaki, N.; Hasegawa, H.; Nguyen, L.T.; Kuroyanagi, A.; Yamazaki, T.; Kuroda, J.; et al. Altervalent substitution of sodium for calcium in biogenic calcite and aragonite. Geochim. Cosmochim. Acta 2017, 202, 21–38. [Google Scholar] [CrossRef]
- Mii, H.-S.; Grossman, E.L. Late Pennsylvanian seasonality reflected in the 18O and elemental composition of a brachiopod shell. Geology 1994, 22, 661. [Google Scholar] [CrossRef]
- Mucci, A. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. 1983, 283, 780–799. [Google Scholar] [CrossRef]
- McConnaughey, T. 13C and 18O isotopic disequilibrium in biological carbonates: I. Patterns. Geochim. Cosmochim. Acta 1989, 53, 151–162. [Google Scholar] [CrossRef]


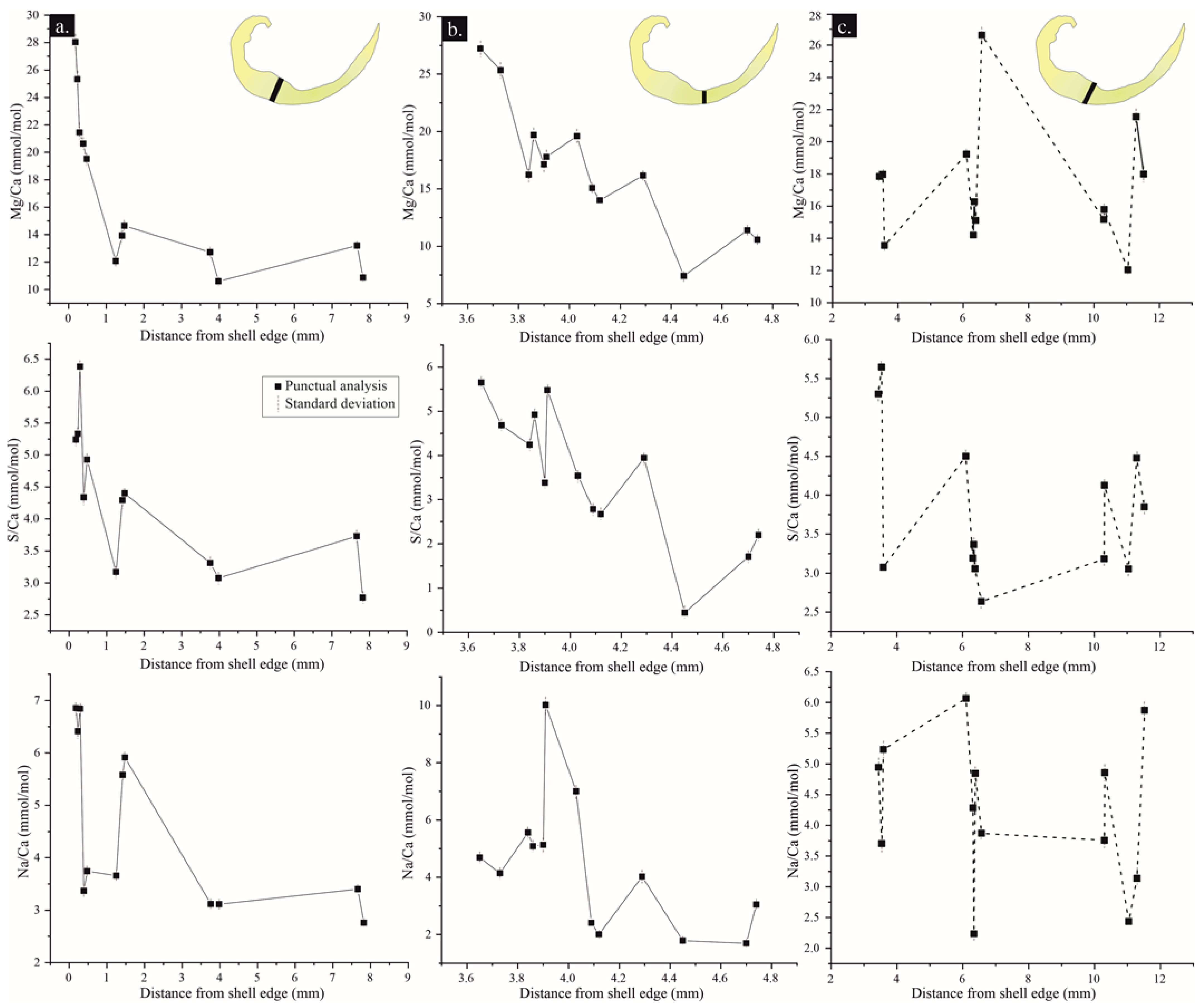
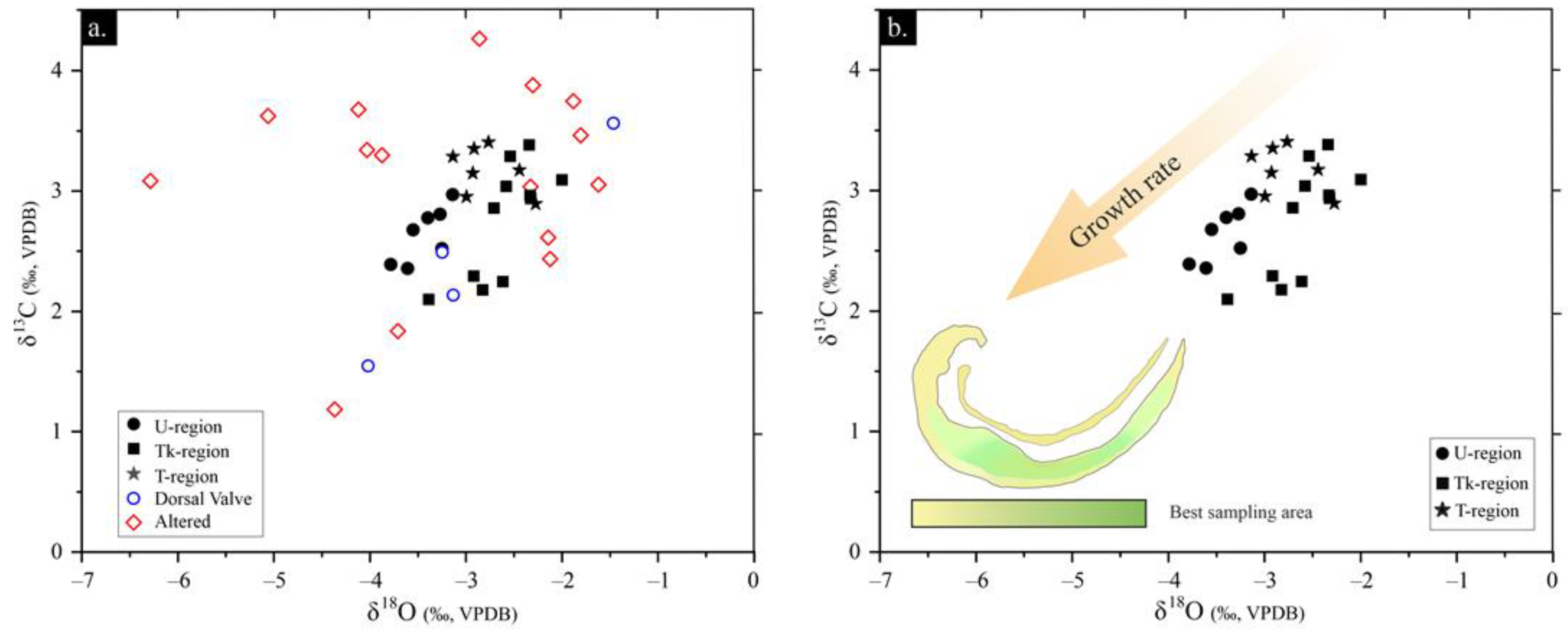
| Preservation State | Element | N total | Mean | S.D. | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|---|
| WP-NL | Ca | 205 | 390,449.9 | 6880.8 | 372,632.4 | 391,045.6 | 406,978.5 |
| Mg | 3380.5 | 911.3 | 1218.3 | 3238.6 | 5886.3 | ||
| Sr | 1737.9 | 430.5 | 642.7 | 1767.3 | 2968.1 | ||
| Mn | 155.3 | 214.3 | 0.0 | 0.0 | 836.6 | ||
| Fe | 184.3 | 263.0 | 0.0 | 0.0 | 1026.2 | ||
| S | 1060.2 | 328.8 | 388.5 | 993.2 | 1894.4 | ||
| Na | 1038.2 | 358.4 | 0.0 | 1031.0 | 2202.8 | ||
| PP-NL | Ca | 20 | 372,422.6 | 29,616.5 | 269,479.6 | 380,938.4 | 400,052.1 |
| Mg | 4651.7 | 1876.9 | 645.3 | 5020.8 | 7273.4 | ||
| Sr | 1763.1 | 473.2 | 465.1 | 1839.2 | 2443.8 | ||
| Mn | 278.9 | 467.2 | 0.0 | 27.1 | 1828.1 | ||
| Fe | 955.4 | 1710.0 | 0.0 | 427.6 | 6499.1 | ||
| S | 1490.5 | 929.9 | 0.0 | 1620.0 | 3576.5 | ||
| Na | 926.0 | 574.8 | 0.0 | 997.6 | 2017.4 | ||
| WP-SL | Ca | 44 | 382,121.0 | 8596.2 | 365,870.4 | 382,139.2 | 398,808.4 |
| Mg | 4164.8 | 1044.6 | 2291.8 | 3959.4 | 6748.7 | ||
| Sr | 2208.9 | 444.7 | 1166.9 | 2262.0 | 3027.2 | ||
| Mn | 314.9 | 367.3 | 0.0 | 170.4 | 1332.3 | ||
| Fe | 371.2 | 408.2 | 0.0 | 268.2 | 1391.5 | ||
| S | 994.7 | 373.6 | 460.6 | 979.2 | 1974.5 | ||
| Na | 932.9 | 358.0 | 311.5 | 867.8 | 1713.3 | ||
| PP-SL | Ca | 80 | 378,873.3 | 141,25.2 | 313,975.9 | 383,615.3 | 398,608.2 |
| Mg | 4378.5 | 1468.8 | 1724.9 | 4010.6 | 9661.7 | ||
| Sr | 1975.1 | 501.0 | 879.4 | 1894.1 | 3729.1 | ||
| Mn | 125.0 | 220.3 | 0.0 | 0.0 | 1038.0 | ||
| Fe | 451.7 | 880.7 | 0.0 | 139.9 | 6499.1 | ||
| S | 1202.5 | 565.0 | 4.0 | 1125.4 | 3576.5 | ||
| Na | 920.4 | 378.3 | 29.7 | 934.5 | 2010.0 | ||
| PP-L | Ca | 15 | 382,641.0 | 8793.2 | 358,236.3 | 383,947.7 | 394,598.2 |
| Mg | 4829.2 | 1635.3 | 2249.6 | 4589.6 | 7786.0 | ||
| Sr | 1981.5 | 473.8 | 1217.7 | 2232.4 | 2765.1 | ||
| Mn | 582.0 | 292.4 | 302.1 | 457.0 | 1332.3 | ||
| Fe | 56.0 | 89.6 | 0.0 | 0.0 | 287.6 | ||
| S | 1264.5 | 462.3 | 596.7 | 1093.4 | 1918.4 | ||
| Na | 966.7 | 276.5 | 504.4 | 1023.5 | 1550.2 |
| Shell Part | Element | N Total | Mean | S.D. | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|---|
| U | Ca | 33 | 388,430.1 | 6175.9 | 373,604.5 | 389,194.3 | 398,379.5 |
| Mg | 3259.7 | 840.4 | 1899.8 | 3178.3 | 5735.5 | ||
| Sr | 1604.6 | 551.8 | 642.7 | 1648.9 | 2570.6 | ||
| Mn | 227.5 | 255.8 | 0 | 170.4 | 836.6 | ||
| Fe | 205 | 266.8 | 0 | 38.9 | 1026.2 | ||
| S | 930.1 | 303.3 | 440.6 | 893.1 | 1786.2 | ||
| Na | 1131.9 | 337.6 | 526.6 | 1105.1 | 1995.2 | ||
| Tk | Ca | 97 | 393,264.2 | 6774.8 | 372,632.4 | 394,433.8 | 406,978.5 |
| Mg | 3012.1 | 722.7 | 1218.3 | 2943.1 | 4999.7 | ||
| Sr | 1656.4 | 408.8 | 845.6 | 1623.6 | 2460.7 | ||
| Mn | 140.9 | 202.1 | 0 | 0 | 774.6 | ||
| Fe | 219.4 | 282.1 | 0 | 46.6 | 925.1 | ||
| S | 1040.1 | 371.3 | 408.5 | 957.2 | 2367 | ||
| Na | 1063.5 | 401.9 | 0 | 1068 | 2202.8 | ||
| T | Ca | 90 | 386,561.5 | 7115.3 | 366,270.7 | 387,503.8 | 397,164.3 |
| Mg | 4126.5 | 1125.4 | 2056.6 | 4067.9 | 7273.4 | ||
| Sr | 1890.7 | 330.4 | 1107.7 | 1902.6 | 2968.1 | ||
| Mn | 160.1 | 244.8 | 0 | 0 | 1332.3 | ||
| Fe | 161.4 | 264.3 | 0 | 0 | 1041.7 | ||
| S | 1249.2 | 470.2 | 224.3 | 1165.5 | 3576.5 | ||
| Na | 968.8 | 346.5 | 0 | 945.7 | 1579.8 | ||
| Secondary layer | Ca | 30 | 379,749.4 | 8141.1 | 365,870.4 | 378,940.5 | 398,808.4 |
| Mg | 4444.0 | 1076.2 | 2780.3 | 4565.5 | 6748.7 | ||
| Sr | 2283.4 | 360.2 | 1462.9 | 2367.7 | 3027.2 | ||
| Mn | 182.3 | 210.1 | 0.0 | 116.2 | 704.9 | ||
| Fe | 370.0 | 453.7 | 0.0 | 225.4 | 1609.2 | ||
| S | 980.8 | 288.5 | 460.6 | 1039.3 | 1549.9 | ||
| Na | 1013.9 | 385.7 | 311.5 | 997.5 | 1713.3 |
| Shell Region | δ18O | δ13C | 87Sr/86Sr (Std Err) | M.S.D. | Shell Region | δ18O | δ13C | 87Sr/86Sr (Std Err) | M.S.D. | Shell Region | δ18O | δ13C | 87Sr/86Sr (Std Err) | M.S.D. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Umbornal | −3.4 | 2.78 | Thin | −2.91 | 3.35 | 0.70786 | ||||||||
| −3.61 | 2.35 | −2.77 | 3.41 | (4−6) | Poorly preserved | −3.71 | 1.84 | |||||||
| −3.78 | 2.39 | −3.13 | 3.29 | 0.707845 | −2.86 | 4.27 | ||||||||
| −3.55 | 2.68 | −2.99 | 2.95 | (4−6) | −5.06 | 3.62 | 0.707893 | |||||||
| −3.25 | 2.52 | −2.93 | 3.15 | −4.12 | 3.68 | (4−6) | ||||||||
| −3.14 | 2.97 | −2.33 | 3.04 | −2.32 | 3.87 | |||||||||
| −3.27 | 2.81 | −2.44 | 3.17 | −6.28 | 3.08 | |||||||||
| Mean | −3.43 | 2.64 | 0.22/0.23 | −2.34 | 3.38 | −4.37 | 1.18 | |||||||
| Thick | −2.82 | 2.18 | 0.70783 | −2.54 | 3.29 | −1.64 | 3.05 | |||||||
| −3.39 | 2.1 | (4−6) | −2.27 | 2.89 | −1.89 | 3.76 | ||||||||
| −2.62 | 2.25 | Mean | −2.67 | 3.19 | 0.32/0.18 | −3.87 | 3.3 | 0.707807 | ||||||
| −2.92 | 2.29 | Dorsal Valve | −3.25 | 2.49 | −2.16 | 2.61 | (5−6) | |||||||
| −2 | 3.09 | −4.02 | 1.54 | −2.14 | 2.43 | |||||||||
| −2.58 | 3.04 | −1.46 | 3.56 | −1.83 | 3.46 | |||||||||
| −2.33 | 2.96 | 0.707831 | −3.13 | 2.13 | −4.03 | 3.34 | ||||||||
| −2.32 | 2.94 | (4−6) | Mean | −2.97 | 2.43 | 1.08/0.85 | Mean | −3.31 | 3.11 | 1.4/0.84 | ||||
| −2.71 | 2.86 | |||||||||||||
| Mean | −2.63 | 2.63 | 0.40/0.42 |
| Features | Preservation States | |||||||
|---|---|---|---|---|---|---|---|---|
| Well-Preserved | Poorly Preserved | |||||||
| Tertiary Layer | Secondary Layer | Tertiary Layer | Growth Lines | Dorsal Valve | Secondary Layer | |||
| Structural | Microstructure | Prismatic crystals with noticeable microlaminae, preferential orientation | Growth lines: original organic-matrix remains and preferential orientation of the crystals | Lath crystals, original organic-matrix remains and preferential orientation | Recrystallisation (e.g., degrading neomorphism), sparite cements, filled fractures, small patches of crystals without a preferential orientation | Recrystallisation, delamination | Recrystallisation, matrix infilling, micritzation, sparite cements, filled fractures | Recrystallisation, delamination, micritized patches, matrix infilling, filled fractures |
| Cathodoluminescence | Non-luminescent | Slightly luminescent, luminescence pattern in growth lines | Slightly luminescent, luminescence banding | Non-luminescent | Increasing luminescence near fractured areas | Luminescent | Slightly luminescent | |
| Geochemical | Crystallographic | Highly arranged crystals: c-axis oriented perpendicular to shell growth | Highly arranged crystals: c-axis oriented subparallel to shell growth | Highly arranged crystals: c-axis oriented parallel to shell growth | Poorly arranged crystals: c-axis randomly oriented | |||
| MTE (ppm) | Mg: 3380, Sr: 1737, Mn: 155, Fe: 184 | Mg: 4164, Sr: 2208, Mn: 314, Fe: 371 | Mg: 4444 Sr: 2283 Mn: 182 Fe: 370 | Mg: 4651, Sr: 1763, Mn: 278, Fe: 955 | Mg: 3765, Sr: 1922, Mn: 971, Fe: 858 | _ | Mg: 4849, Sr: 2323, Mn: 117, Fe: 838 | |
| δ13C–δ18O | Low dispersion of the ratio δ18O: −3.78‰ to −2‰; δ13C: 2.09‰ to 3.4‰; grouped in clusters depending on ontogeny development | High dispersion of the ratio δ18O: −6.28‰ to −1.83‰; δ13C: 1.18‰ to 4.27‰ | ||||||
| 87Sr/86Sr | Low dispersion of the ratio: 0.707830–0.707860 | High dispersion of the ratio: 0.707807–0.707893 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos-Carralafuente, J.R.; Coronado, I.; Cruz, J.A.; Cózar, P.; Fernández-Martínez, E.; Rodríguez, S. Diagenetic and Biological Overprints in Geochemical Signatures of the Gigantoproductus Tertiary Layer (Brachiopoda): Assessing the Paleoclimatic Interpretation. Life 2023, 13, 714. https://doi.org/10.3390/life13030714
Mateos-Carralafuente JR, Coronado I, Cruz JA, Cózar P, Fernández-Martínez E, Rodríguez S. Diagenetic and Biological Overprints in Geochemical Signatures of the Gigantoproductus Tertiary Layer (Brachiopoda): Assessing the Paleoclimatic Interpretation. Life. 2023; 13(3):714. https://doi.org/10.3390/life13030714
Chicago/Turabian StyleMateos-Carralafuente, José R., Ismael Coronado, Juncal A. Cruz, Pedro Cózar, Esperanza Fernández-Martínez, and Sergio Rodríguez. 2023. "Diagenetic and Biological Overprints in Geochemical Signatures of the Gigantoproductus Tertiary Layer (Brachiopoda): Assessing the Paleoclimatic Interpretation" Life 13, no. 3: 714. https://doi.org/10.3390/life13030714
APA StyleMateos-Carralafuente, J. R., Coronado, I., Cruz, J. A., Cózar, P., Fernández-Martínez, E., & Rodríguez, S. (2023). Diagenetic and Biological Overprints in Geochemical Signatures of the Gigantoproductus Tertiary Layer (Brachiopoda): Assessing the Paleoclimatic Interpretation. Life, 13(3), 714. https://doi.org/10.3390/life13030714







