1. Introduction
Thoracic ultrasound (TUS) has gradually been accepted as a chest imaging modality with high diagnostic and monitoring accuracy for a variety of respiratory diseases and conditions and thus is being increasingly used by a broad range of clinical specialties within respiratory medicine, intensive care, and emergency medicine [
1]. Not least, TUS has gained further popularity, secondary to its growing use during the COVID-19 pandemic as part of the risk stratification to predict respiratory failure and admission to intensive care units, including prognostication in those patients suffering from acute respiratory distress syndrome [
2,
3].
In a clinical setting, satisfactory TUS performance necessitates the theoretical and practical skills obtained from several available certified TUS education possibilities, where the involvement of or add-on simulation-based training is recommended to improve clinical performance [
4,
5]. Such training also contributes to understanding the commonly used terminology and knowledge of normal and pathological findings of the lung parenchyma, pleura, and thoracic wall, which is an essential prerequisite for the sufficient interpretation of TUS observations [
6,
7]. Several TUS scanning protocols with different scanning areas and zones are described, but the most frequently used in prospective clinical studies is the 14-zone approach, which accommodates the scanning of the thorax’s anterior, lateral, and posterior surfaces [
8,
9,
10,
11,
12] (
Figure 1).
Though TUS might be regarded as a novel modality for clinical guidance in the management of lung transplantation (LTx), its wide range of applications within the different fields of LTx has received increased recognition [
13,
14]. However, due to LTx being a rare condition, the evidence for TUS use in all stages of LTx is restricted to a few studies that are very often based on observations from a limited number of lung transplant recipients. Nonetheless, these observations qualify TUS as a pivotal and valid bedside tool to detect LTx-related conditions where intrathoracic pathological findings may appear [
1]. The TUS observations may relate to the time from the LTx operation being conducted and why the course of LTx can be differentiated into the following stages: (1) a pre-LTx stage (e.g., where the quality of the donor lungs can be assessed prior to surgery); (2) a short-term post-operative LTx-stage; (3) a late or long-term post-operative LTx-stage. Common for the postoperative LTx stages is an ongoing surveillance need for the continuous assessment of both acute lung allograft dysfunction (ALAD) [
15] and chronic lung allograft dysfunction (CLAD) [
16]. Generally, TUS is believed to possess still unused and unexploited potential in the LTx setting, which indirectly calls for TUS’s benefits to be clarified.
2. Aim
The aim of this current opinion review is to review the existing literature on the role of TUS in all stages of LTx, from in-donor lung evaluation to graft assessment on ex vivo lung perfusion and in the short- and long-term follow-up after LTx.
3. Knowledge of TUS and LTx
3.1. The Use of TUS in Pre-LTx Stages
The evaluation of lungs for LTx, using TUS, both “in-donor” and on an ex vivo lung perfusion system (EVLP), has been attempted in different settings. In a small case series that included six potential donors, Lebovitz et al. performed “in-donor” lung evaluation using TUS on neurologically deceased donors and compared the TUS findings with the findings on conventional chest X-rays in anterior and posterior planes. Of the six neurologically deceased potential donors, five of them were used for LTx, whereas one was unsuitable. They found that 6/6 evaluated potential donors had bilateral consolidations, pleural effusion was observed in 4/6 potential donors, and edema was seen in 5/6 potential donors. In contrast, a conventional chest X-ray found consolidations/atelectasis in 3/6 of potential donors, pleural effusion in 1/6 potential donors and no edema, and on the basis of these findings, it was concluded that TUS might be a more accurate and efficient tool to evaluate lungs for potential donation [
17]. To our knowledge, no other studies have been published on this subject, but the potential utility of TUS to rule out pleural effusion and edema, more in a pre-LTx setting, is greater than conventional X-ray. TUS can also be used to track dynamic changes over time in a donor management situation.
EVLP is a rapidly growing procurement technique used worldwide to evaluate the suitability of donor lungs. The potential donor lungs are removed from the donor and placed on the EVLP system, allowing transplant surgeons and physicians to evaluate the suitability of the donor organ for LTx in marginal donors. In a recently published study, Ayyat et al. performed an ultrasound on 45 lungs from 23 donors placed on an EVLP system to evaluate any applied therapeutic approach during EVLP [
18]. A CLUE (direCt Lung Ultrasound Evaluation) score was developed as the number of Grade 1 images × 1 number of Grade 2 images × 2+ numbers of Grade 3 images × 3+ numbers of Grade 4 images × 4+ numbers of consolidation images × 5 in relation to the total number of images taken. Grade 0 was defined as no B-lines on TUS; grade 1 was defined as 1–25% B-lines; grade 2 was defined as 26–50% B-lines; grade 3 was defined as 51–75% B-lines; and grade 4 was defined as a 76–100% B-line coverage or white-out (i.e., coalescence of B-lines generating a typical white lung image). The final total CLUE score had the highest area under the curve (AUC) of 0.98 (sensitivity 100% and specificity 86%) compared with other EVLP evaluation parameters, such as the ratio of arterial oxygen partial pressure (i.e., PaO
2 in mmHg) to fractional inspired oxygen (i.e., P/F ratio) of 0.58, a change in PaO
2 of 0.61, pulmonary artery pressure of 0.71, pulmonary vascular resistance of 0.64, peak airway pressure of 0.83, and dynamic compliance of 0.85. The TUS-related CLUE scores were calculated and used as adjunctive information for the final consensus decision on suitability for LTx, and therefore, the CLUE score actively impacted the suitability decision, which is a limitation of this study. Post-transplantation follow-up was not reported in this paper.
3.2. TUS Use in Short-Term Post-LTx Stages
Acute lung allograft dysfunction (ALAD) refers to a condition of acute graft dysfunction, presenting in the early course after LTx with an often-used time window of within 90 days in which the graft only retains marginal function [
19]. ALAD has a different etiology; however, immediate allograft dysfunction may develop almost in prolongation of the operative procedure, and in up to 25–50% of cases, the underlying cause relates to primary graft dysfunction (PGD), defined as an acute non-immune-mediated injury to the transplanted lung occurring within the first 72 h postoperatively and which is caused by a combination of ischemia, reperfusion, cold organ preservation, and pretransplant pulmonary hypertension [
20,
21]. Other early (>72 h) risk factors or conditions in the short-term post-LTx stage leading to ALAD comprise acute cellular rejection (ACR), mechanical abnormalities (anastomotic strictures, bronchomalacia, diaphragm paralysis, and pleural effusion, including hemothorax), pneumonia (viral, bacterial, and fungal infections), thromboembolic disease, anemia, and gastro-esophageal reflux [
15,
22,
23,
24,
25]. In the immediate post-transplantation setting, the use of computed tomography (CT) and other radiological methods is often challenged by a sedated ICU patient with bandages, chest tubes, and catheters, and although TUS might be challenging, it has the advantage of a bedside approach.
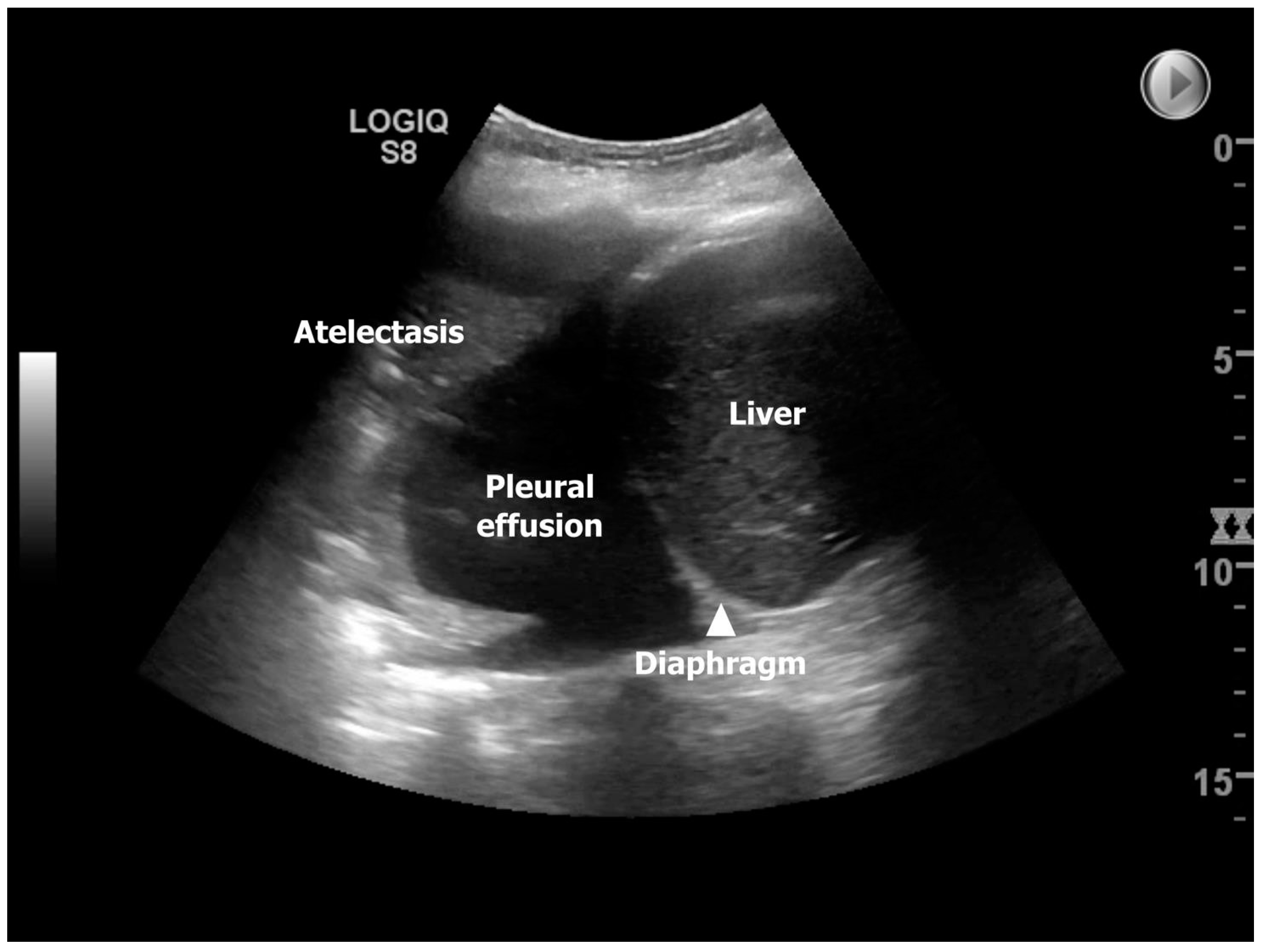
The use of TUS to discover the presence of many of the mentioned underlying conditions for developing ALAD has been explored in one national follow-up study from Denmark, where Davidsen et al. prospectively investigated 14 lung transplant recipients (13/1 double LTX (DLTx)/single LTx (SLTx)) at four time-points corresponding to post-LTx day 3 (TUS #1), 14 (TUS #2), 42 (TUS #3), and 84 (TUS #4) [
11]. In this study, the most frequent pathological finding identified by TUS was pleural effusion, observed in 85.7%, 92.9%, 85.7%, and 78.6% of the lung transplant recipients equivalent to TUS#1-4, which may imitate a high prevalence throughout the entire 84-days observation period (
Figure 2). However, in the study, the presence of pleural effusion was dichotomized, and as TUS can detect pleural effusion below 20 milliliters, this likely explained the findings that are consistent with an overall decreasing presentation over time, going from bilateral to unilateral and large/moderate to small pleural effusion. At TUS#2, the highest prevalence of compression atelectasis was found to be reliable, with the highest prevalence of pleural effusion. Pneumonia was most predominant at TUS#2 (28.6%), with decreasing prevalence at TUS#3-4 (14.3%), and during the observation period, neither lung transplant recipients were diagnosed with pulmonary embolism nor interstitial syndrome. Still, the most profound finding was that TUS as an add-on modality to ordinary LTx surveillance had a clinical impact in 10/14 lung transplant recipients (71.4%) during the observation period due to the detection of diagnoses with diverting interventions, such as re-operation due to sternotomy-related wound infection, pleural drainage, and initiation of/or changed antibiotic strategy. The prevalence of TUS-induced interventions was highest at post-transplant day 14 (TUS#2) and comprised half of the lung transplant recipients (
n = 7 (50%)). The TUS observed findings from this study were assumed to be representative and compatible with the available knowledge on time-dependent pulmonary complications in the short-term post-LTx stages [
26].
3.3. TUS Use in Long-Term Post-LTx Stages
The majority of lung transplant recipients will have obtained close to maximal post-LTx lung function around one year after LTx [
27]. Nonetheless, underlying conditions, as described for ALAD, may still complicate the LTx course, giving rise to CLAD, which occurs with increasing prevalence one year after LTx, supporting the demand for long-term follow-up [
16,
22,
28]. CLAD is defined as a persistent decline (>three months) in forced expiratory volume in one second (FEV1) of >20% compared to the best post-transplant baseline value. This is further phenotyped into either bronchiolitis obliterans syndrome (BOS) with an obstructive ventilation pattern, restricted allograft syndrome (RAS) with a restrictive ventilation pattern (i.e., a decline in total lung capacity (TLC) of < 90% of the best post-transplant baseline value) in combination with the presence of pleura-parenchymal opacities on a high-resolution computed tomography (HRCT), which is often manifested as apical pleura-parenchymal fibroelastosis (PPFE), or a mixed-type CLAD [
16,
28,
29].
One prospective French study by Droneau et al. aimed to validate whether TUS could be used in a long-term post-LTx setting, with the performance of TUS in lung transplant recipients who had undergone LTx on an average of 23 months prior to inclusion (median: 9 months) [
30]. The study concluded that an ordinary TUS approach, including standard variables (e.g., the presence of B-lines, lung sliding, lung pulse, and seashore sign), was feasible in lung transplant recipients. However, as the cohort comprised 22 presumable lung healthy transplant recipients, most TUS findings were expectedly normal, which is why it was emphasized that using TUS needed further exploration in long-term post-LTx settings.
In 2017, Davidsen et al. published a case report proposing that TUS could be used as a novel tool to phenotype CLAD [
31]. The same study group further investigated this hypothesis in a later observational study with the prospective inclusion of 25 lung transplant recipients with new-onset CLAD (n(BOS):n(RAS) = 19:6), who were examined with TUS and HRCT, performed within an average time window of ten days prior to or after TUS [
12]. HRCT was used as the gold standard for the PPFE findings that would correspond to the TUS findings demonstrating pleural thickening. It was found that the RAS patients were presenting differently from the BOS patients, with a significant difference in pleural thickening, as measured by a TUS of 5.6 mm and 2.9 mm (normal pleura thickness is 1 mm), respectively, and was consistent with a significantly higher prevalence of PPFE findings on HRCT in the RAS compared to BOS patients. Importantly, the study proved a high diagnostic accuracy of TUS to identify apical pleural thickening in either the anterior or posterior apical zones in RAS patients as a surrogate marker of HRCT-verified PPFE in lung transplant recipients (i.e., with a sensitivity of 100% (95% CI; 54–100%), specificity of 100% (95% CI; 82–100%), positive predictive value (PPV) of 100% (95% CI; 54–100%), and a negative predictive value (NPV) of 100% (95% CI; 82–100%)) (
Figure 3). Hence, confirmation of this novel so-called RAS-sign in lung transplant recipients with developed CLAD increased the probability of RAS over BOS. As such, it was concluded that TUS could discriminate RAS from BOS and thus be used as an up-front tool for CLAD phenotyping, which clinically impacts the reduction of time for diagnosing RAS, which is crucial since its prognosis is inferior to BOS [
32,
33,
34].
3.4. Other Associations to LTx
Various studies have attempted to describe the association between ACR and computed tomography (CT) abnormalities. For example, Gotway et al. found limited accuracy in the use of CT to diagnose ACR [
35], while Park et al. found an association between ACR and bilateral ground-glass opacities and septal thickening with lower predominance, which has not been proven afterwards [
36]. However, no studies have evaluated TUS as a possible method to diagnose ACR, which might be due to the divergent results and the limited accuracy of CT in diagnosing ACR.
Post-procedure pneumothorax identification in lung transplant recipients has been investigated in one study by Bensted et al. [
37]. They performed TUS on 165 patients and found eight pneumothoraces after conducting transbronchial biopsies. Using the chest X-ray as the gold standard in their study, TUS was shown to have a sensitivity of 75%, specificity of 93%, PPV of 35%, and NPV of 99% in their study. These results parallel the findings from a previous meta-analysis on TUS being used to detect pneumothorax [
38].
4. TUS Future Research—Advantages and Disadvantages of TUS
Despite the fact that only a few TUS studies are carried out within the field of LTx, TUS has manifested itself as a valuable and indispensable bedside tool to detect and monitor pathological conditions in relation to LTx, in addition to being used as a tool with impact to influence further clinical decision making prior to or after LTx. In this context, TUS can be used to exclude LTx-related conditions/complications, thereby avoiding the waste of otherwise unnecessary investigations required to clarify a clinical suspicion. Still, more studies are required on larger LTx cohorts and with longer observation periods (>24 months post-LTx) to obtain a more profound understanding and acquire more qualified data on when, and on which focus areas TUS should be used in the LTx setting, considering the dynamic pathological changes and exposures (e.g., LTx immunosuppression) that will inevitably appear over time. In this perspective, gold standard examinations (preferable chest CT/HRCT) should optimally be performed alongside TUS to achieve the most reliable comparison and to prevent under- or over-diagnostics of pathological findings/LTx-complications that may disappear or appear when accepting a long time window between TUS and gold standard testing. Based on such knowledge, future studies should focus on TUS as an integrated part of short-term as well as long-term LTx surveillance follow-up. This should aim to investigate whether TUS implications may optimize the earlier detection of LTx-complications and avoid laborious follow-up set-ups, including CT, which would be in favor of lung transplant recipients but also regarding a reduction in health care costs (e.g., an RCT comparing follow-up, including TUS vs. a standard follow-up). TUS use for the selection of potential LTx candidates has not been investigated, but it presumably could contribute to excluding candidates with obvious intrathoracic pathology, such as severe PPFE with lung shrinkage with pleural adhesions and thickening, which would challenge LTx surgery.
Importantly, TUS should be regarded as an adjunction to the existing examinations regarding selection for and follow-up of LTx, including phenotyping CLAD, and cannot stand alone. However, the immediate advantages and disadvantages of TUS are illustrated in
Table 1.
5. Conclusions
TUS has a huge potential within the field of diagnostics and monitoring of more respiratory diseases, and recently its use has also been shown to have a clinical impact on rare respiratory conditions, such as LTx. Several studies have investigated the use of TUS in different stages of LTx. When evaluating “in-donor” lungs, TUS is a helpful tool with a higher accuracy than conventional chest X-rays. When using the ex vivo evaluation of lungs for transplantation, TUS is a possible add-on examination with a higher AUC than any of the other evaluation methods used in the EVLP systems; however, it was more labor-intensive. In the immediate postoperative course, TUS offers many advantages and convenience as a point-of-care examination and effectively identifies early complications, such as pleural effusion, consolidation, and pneumothorax. In a long-term follow-up and in the phenotyping of CLAD, TUS has demonstrated its potential as a suitable screening tool with high accuracy when in the hands of trained experts. In conclusion, TUS is believed to possess still unused and unexploited potential in the LTx setting, which indirectly calls for TUS’s benefits to be clarified in future large multicenter and randomized studies.
Author Contributions
H.H.L.S. and J.R.D. have equally contributed to the conceptualization, project administration, writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the journals, Ultrashall in der Medizin (European Journal of Ultrasound) and the Journal of Clinical Medicine for permission to reuse the figures and table from previously published papers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laursen, C.B.; Clive, A.; Hallifax, R.; Pietersen, P.I.; Asciak, R.; Davidsen, J.R.; Bhatnagar, R.; Bedawi, E.O.; Jacobsen, N.; Coleman, C.; et al. European respiratory society statement on thoracic ultrasound. Eur. Respir. J. 2021, 57, 2001519. Available online: https://erj.ersjournals.com/content/erj/57/3/2001519.full.pdf (accessed on 13 January 2023). [CrossRef] [PubMed]
- Skaarup, S.H.; Aagaard, R.; Ovesen, S.H.; Weile, J.; Kirkegaard, H.; Espersen, C.; Lassen, M.C.H.; Skaarup, K.G.; Posth, S.; Laursen, C.B.; et al. Focused lung ultrasound to predict respiratory failure in patients with symptoms of COVID-19: A multicentre prospective cohort study. ERJ Open Res. 2022, 8. Available online: https://openres.ersjournals.com/content/erjor/8/4/00128-2022.full.pdf (accessed on 13 January 2023). [CrossRef] [PubMed]
- Gil-Rodríguez, J.; Martos-Ruiz, M.; Peregrina-Rivas, J.-A.; Aranda-Laserna, P.; Benavente-Fernández, A.; Melchor, J.; Guirao-Arrabal, E. Guirao-Arrabal. Lung ultrasound, clinical and analytic scoring systems as prognostic tools in SARS-CoV-2 pneumonia: A validating cohort. Diagnostics 2021, 11, 2211. Available online: https://mdpi-res.com/d_attachment/diagnostics/diagnostics-11-02211/article_deploy/diagnostics-11-02211-v2.pdf?version=1638171755 (accessed on 13 January 2023). [CrossRef]
- Pietersen, P.I.; Jørgensen, R.; Graumann, O.; Konge, L.; Skaarup, S.H.; Schultz, H.H.L.; Laursen, C.B. Training thoracic ultrasound skills: A randomized controlled trial of simulation-based training versus training on healthy volunteers. Respiration 2021, 100, 34–43. Available online: https://www.karger.com/Article/Abstract/509298 (accessed on 13 January 2023). [CrossRef]
- European Respiratory Society. Thoracic Ultrasound Certified Training Programme. Available online: https://www.ersnet.org/education-and-professional-development/ers-certified-training-programmes/thoracic-ultrasound-certified-training-programme/ (accessed on 13 January 2023).
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Laursen, C.B.; Davidsen, J.R.; Gleeson, F.V. Technique and Protocols. In Thoracic Ultrasund [ERS Monograph]; Laursen, C.B., Rahman, N.M., Volpicelli, G., Eds.; European Respiratory Society: Sheffield, UK, 2018; pp. 14–30. [Google Scholar] [CrossRef]
- Laursen, C.B.; Sloth, E.; Lambrechtsen, J.; Lassen, A.T.; Madsen, P.H.; Henriksen, D.P.; Davidsen, J.R.; Rasmussen, F. Focused sonography of the heart, lungs, and deep veins identifies missed life-threatening conditions in admitted patients with acute respiratory symptoms. Chest 2013, 144, 1868–1875. Available online: http://journal.publications.chestnet.org/data/Journals/CHEST/928990/chest_144_6_1868.pdf (accessed on 13 January 2023). [CrossRef]
- Laursen, C.B.; Sloth, E.; Lassen, A.; Christensen, R.D.; Lambrechtsen, J.; Madsen, P.H.; Henriksen, D.P.; Davidsen, J.R.; Rasmussen, F. Point-of-care ultrasonography in patients admitted with respiratory symptoms: A single-blind, randomised controlled trial. Lancet Respir. Med. 2014, 2, 638–646. Available online: http://www.sciencedirect.com/science/article/pii/S2213260014701353 (accessed on 13 January 2023). [CrossRef]
- Davidsen, J.R.; Bendstrup, E.; Henriksen, D.P.; Graumann, O.; Laursen, C.B. Lung ultrasound has limited diagnostic value in rare cystic lung diseases: A cross-sectional study. Eur. Clin. Respir. 2017, 4, 1330111. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5475293/pdf/zecr-4-1330111.pdf (accessed on 13 January 2023). [CrossRef]
- Davidsen, J.R.; Schultz, H.H.L.; Henriksen, D.P.; Iversen, M.; Kalhauge, A.; Carlsen, J.; Perch, M.; Graumann, O.; Laursen, C.B. Lung ultrasound in the assessment of pulmonary complications after lung transplantation. Ultraschall Med. 2020, 41, 148–156. Available online: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/a-0783-2466.LungenultraschallinderBeurteilungvonKomplikationennachLungentransplantation (accessed on 13 January 2023). [CrossRef]
- Davidsen, J.; Laursen, C.; Højlund, M.; Lund, T.; Jeschke, K.; Iversen, M.; Kalhauge, A.; Bendstrup, E.; Carlsen, J.; Perch, M.; et al. Lung ultrasound to phenotype chronic lung allograft dysfunction in lung transplant recipients. A prospective observational study. J. Clin. Med. 2021, 10, 1078. Available online: https://res.mdpi.com/d_attachment/jcm/jcm-10-01078/article_deploy/jcm-10-01078.pdf (accessed on 13 January 2023). [CrossRef]
- Verleden, S.E.; Verleden, G.M. Novel biomarkers of chronic lung allograft dysfunction: Is there anything reliable? Curr. Opin. Organ Transpl. 2022, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Beaufils, F.; De Brandt, J.; Viney, K.; Bradley, C.; Cottin, V.; Hassan, M.; Cruz, J. European respiratory society international congress 2021: Highlights from best-abstract awardees. Breathe 2022, 18, 210176. Available online: https://breathe.ersjournals.com/content/breathe/18/1/210176.full.pdf (accessed on 13 January 2023). [CrossRef] [PubMed]
- Ahmad, S.; Shlobin, O.A.; Nathan, S.D. Pulmonary complications of lung transplantation. Chest 2011, 139, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-a consensus report from the pulmonary council of the ishlt. J. Heart Lung Transplant. 2019, 38, 493–503. Available online: https://www.jhltonline.org/article/S1053-2498(19)31432-9/pdf (accessed on 13 January 2023). [CrossRef] [PubMed]
- Lebovitz, D.J.; Tabbut, M.; Latifi, S.Q.; Dezelon, L.; Jones, R. Lung ultrasound utility in the management of the neurologically deceased organ donor. Prog. Transplant. 2016, 26, 210–214. [Google Scholar] [CrossRef]
- Ayyat, K.S.; Okamoto, T.; Niikawa, H.; Sakanoue, I.; Dugar, S.; Latifi, S.Q.; Lebovitz, D.J.; Moghekar, A.; McCurry, K.R. A clue for better assessment of donor lungs: Novel technique in clinical ex vivo lung perfusion. J. Heart Lung Transplant. 2020, 39, 1220–1227. Available online: https://www.jhltonline.org/article/S1053-2498(20)31665-X/fulltext (accessed on 13 January 2023). [CrossRef]
- Pavlisko, E.N.; Neely, M.L.; Kopetskie, H.; Hwang, D.M.; Farver, C.F.; Wallace, W.D.; Arrossi, A.; Illei, P.; Sever, M.L.; Kirchner, J.; et al. Prognostic implications of and clinical risk factors for acute lung injury and organizing pneumonia after lung transplantation: Data from a multicenter prospective cohort study. Am. J. Transplant. 2022, 22, 3002–3011. Available online: https://onlinelibrary.wiley.com/doi/10.1111/ajt.17183 (accessed on 13 January 2023). [CrossRef]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ishlt working group on primary lung graft dysfunction, part i: Definition and grading-a 2016 consensus group statement of the international society for heart and lung transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef]
- Andersen, K.H.; Schultz, H.H.L.; Nyholm, B.; Iversen, M.P.; Gustafsson, F.; Carlsen, J. Pulmonary hypertension as a risk factor of mortality after lung transplantation. Clin. Transplant. 2016, 30, 357–364. Available online: https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/ctr.12692?download=true (accessed on 13 January 2023). [CrossRef]
- Meyer, K.K.; Raghu, G.; Verleden, G.M.; Corris, P.A.; Aurora, P.; Wilson, K.C.; Brozek, J.; Glanville, A.R. An international ishlt/ats/ers clinical practice guideline: Diagnosis and management of bronchiolitis obliterans syndrome. Eur. Respir. J. 2014, 44, 1479–1503. Available online: http://erj.ersjournals.com/content/44/6/1479.full.pdf (accessed on 13 January 2023). [CrossRef]
- Bugge, T.B.; Perch, M.; Rezahosseini, O.; Crone, C.G.; Jensen, K.; Schultz, H.H.; Bredahl, P.; Hornum, M.; Nielsen, S.D.; Lund, T.K. Post-transplantation anemia and risk of death following lung transplantation. Transplant. Proc. 2022, 54, 2329–2336. [Google Scholar] [CrossRef]
- Holm, A.; Schultz, H.; Johansen, H.; Pressler, T.; Lund, T.; Iversen, M.; Perch, M. Bacterial re-colonization occurs early after lung transplantation in cystic fibrosis patients. J. Clin. Med. 2021, 10, 1275. Available online: https://mdpi-res.com/d_attachment/jcm/jcm-10-01275/article_deploy/jcm-10-01275.pdf?version=1616137995 (accessed on 13 January 2023). [CrossRef] [PubMed]
- Crone, C.G.; Rezahosseini, O.; Schultz, H.H.L.; Qvist, T.; Johansen, H.K.; Nielsen, S.D.; Perch, M. Achromobacter spp. In a cohort of non-selected pre- and post-lung transplant recipients. Pathogens 2022, 11, 181. Available online: https://mdpi-res.com/d_attachment/pathogens/pathogens-11-00181/article_deploy/pathogens-11-00181.pdf?version=1643359963 (accessed on 13 January 2023). [CrossRef] [PubMed]
- Brown, A.W.; Kaya, H.; Nathan, S.D. Lung transplantation in iip: A review. Respirology 2016, 21, 1173–1184. Available online: http://onlinelibrary.wiley.com/store/10.1111/resp.12691/asset/resp12691.pdf?v=1&t=j3mwhx4o&s=ae8e8d7e7971d5e524e275fd77ff7840cc22eb96 (accessed on 13 January 2023). [CrossRef] [PubMed]
- Schultz, H.H.L.; Møller, C.H.; Møller-Sørensen, H.; Mortensen, J.; Lund, T.K.; Andersen, C.B.; Perch, M.; Carlsen, J.; Iversen, M. Variation in time to peak values for different lung function parameters after double lung transplantation. Transplant. Proc. 2020, 52, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Glanville, A.R.; Verleden, G.M.; Todd, J.L.; Benden, C.; Calabrese, F.; Gottlieb, J.; Hachem, R.R.; Levine, D.; Meloni, F.; Palmer, S.M.; et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-a consensus report from the pulmonary council of the ishlt. J. Heart Lung Transplant. 2019, 38, 483–492. Available online: https://www.jhltonline.org/article/S1053-2498(19)31431-7/pdf (accessed on 13 January 2023). [CrossRef]
- Ofek, E.; Sato, M.; Saito, T.; Wagnetz, U.; Roberts, H.C.; Chaparro, C.; Waddell, T.K.; Singer, L.; Hutcheon, M.A.; Keshavjee, S.; et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod. Pathol. 2013, 26, 350–356. Available online: https://www.nature.com/articles/modpathol2012171.pdf (accessed on 13 January 2023). [CrossRef]
- Droneau, S.; Noel-Savina, E.; Plat, G.; Murris-Espin, M.; Leborgne-Krams, A.; Brouchet, L.; Dahan, M.; Didier, A. Use of ultrasonography for lung transplant recipients on postoperative care. J. Ultrasound Med. 2019, 38, 1101–1108. Available online: https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/jum.14774?download=true (accessed on 13 January 2023). [CrossRef] [PubMed]
- Davidsen, J.R.; Laursen, C.B.; Bendstrup, E.; Schultz, H.H.L. Lung ultrasound—A novel diagnostic tool to phenotype chronic lung allograft dysfunction? Ultrasound Int. 2017, 3, E117–E119. Available online: https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0043-116489.pdf (accessed on 13 January 2023). [CrossRef] [PubMed]
- Vos, R.; Verleden, S.; Verleden, G.M. Chronic lung allograft dysfunction: Evolving practice. Curr. Opin. Organ. Transplant. 2015, 20, 483–491. [Google Scholar] [CrossRef]
- Verleden, S.E.; Todd, J.L.; Sato, M.; Palmer, S.M.; Martinu, T.; Pavlisko, E.N.; Vos, R.; Neyrinck, A.; Van Raemdonck, D.; Saito, T.; et al. Impact of clad phenotype on survival after lung retransplantation: A multicenter study. Am. J. Transplant. 2015, 15, 2223–2230. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/ajt.13281 (accessed on 13 January 2023). [CrossRef] [PubMed]
- Bonniaud, P.; Cottin, V.; Beltramo, G. Pleuroparenchymal fibroelastosis: So many unmet needs. Eur. Respir. J. 2022, 60, 2201798. Available online: https://erj.ersjournals.com/content/60/6/2201798 (accessed on 13 January 2023). [CrossRef]
- Gotway, M.B.; Dawn, S.K.; Sellami, D.; Golden, J.A.; Reddy, G.P.; Keith, F.M.; Webb, W.R. Webb. Acute rejection following lung transplantation: Limitations in accuracy of thin-section ct for diagnosis. Radiology 2001, 221, 207–212. [Google Scholar] [CrossRef]
- Park, C.; Paik, H.; Haam, S.; Lim, B.; Byun, M.; Shin, J.; Kim, H.; Hwang, S.; Kim, T. Hrct features of acute rejection in patients with bilateral lung transplantation: The usefulness of lesion distribution. Transplant. Proc. 2014, 46, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Bensted, K.; McKenzie, J.; Havryk, A.; Plit, M.; Ben-Menachem, E. Lung ultrasound after transbronchial biopsy for pneumothorax screening in post-lung transplant patients. J. Bronchol. Interv. Pulmonol. 2018, 25, 42–47. [Google Scholar] [CrossRef]
- Alrajhi, K.; Woo, M.Y.; Vaillancourt, C. Test characteristics of ultrasonography for the detection of pneumothorax: A systematic review and meta-analysis. Chest 2012, 141, 703–708. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).