Current Toolset in Predicting Acute Coronary Thrombotic Events: The “Vulnerable Plaque” in a “Vulnerable Patient” Concept
Abstract
1. Introduction
2. Pathophysiology of Coronary Atherosclerosis
3. Identifying the Vulnerable Plaque
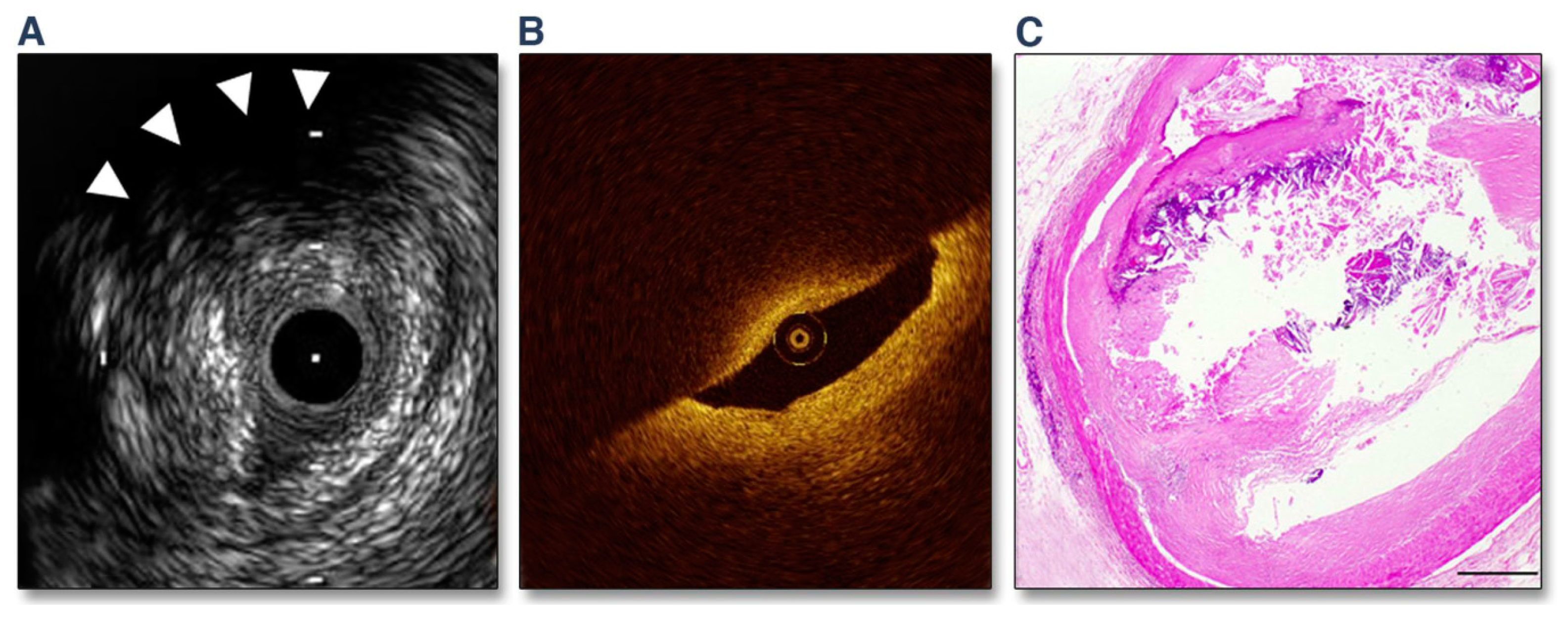
3.1. Invasive Imaging in Medical Practice
3.2. Novel Invasive Imaging Modalities
| Modality | Study * | N | Independent Predictor(s) | Endpoint(s) | Mean Follow-Up | Hazard Ratio | p-Value |
|---|---|---|---|---|---|---|---|
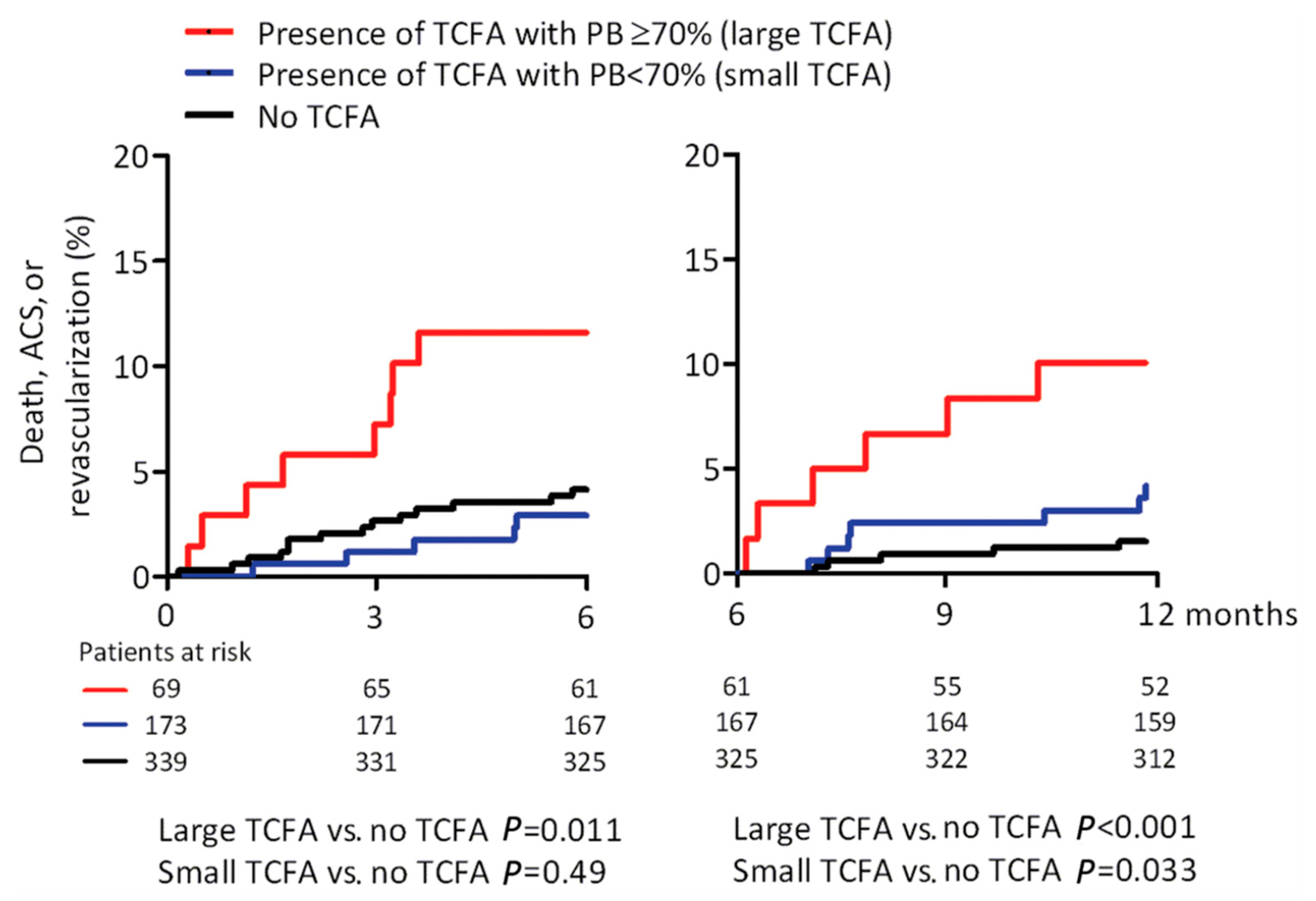
| IVUS & VH | PROSPECT (“Providing Regional Observations to Study Predictors of Events in the Coronary Tree”) Stone et al. [13] | 697 | Plaque burden ≥ 70% | Non-culprit MACE | 3.4 years | 5.03 (2.51–10.11) | <0.001 |
| Minimal lumen area ≤ 4 mm2 | 3.35 (1.77–6.36) | <0.001 | |||||
| Thin cap fibroatheromas | 3.21 (1.61–6.42) | 0.001 | |||||
| Inaba et al. [56] | 697 | Negative remodeling index | Non-culprit MACE | 3 years | 2.39 (1.07–5.34) | 0.033 | |
| Positive remodeling index | 2.34 (1.00–5.44) | 0.049 | |||||
| Zheng et al. [57] | 697 | Distance from ostium to max necrotic core site | Plaque rupture | NA | OR 0.86 (0.76–0.98) | 0.02 | |
| External elastic membrane area | OR 1.14 (1.11–1.17) | <0.0001 | |||||
| Plaque burden | OR 2.05 (1.63–2.58) | <0.0001 | |||||
| Right coronary artery location | OR 2.16 (1.25–3.27) | 0.006 | |||||
| Calcium | OR 0.09 (0.05–0.18) | <0.0001 | |||||
| Radiofrequency -IVUS | AtheroRemoIVUS (“The European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis—Intravascular Ultrasound Study”) [58] | 581 | Minimal lumen area ≤ 4 mm2 | MACE | 4.7 years | 1.49 (1.07–2.08) | 0.020 |
| Plaque burden ≥ 70% | Non-culprit MACE | 1.66 (1.06–2.58) | 0.026 | ||||
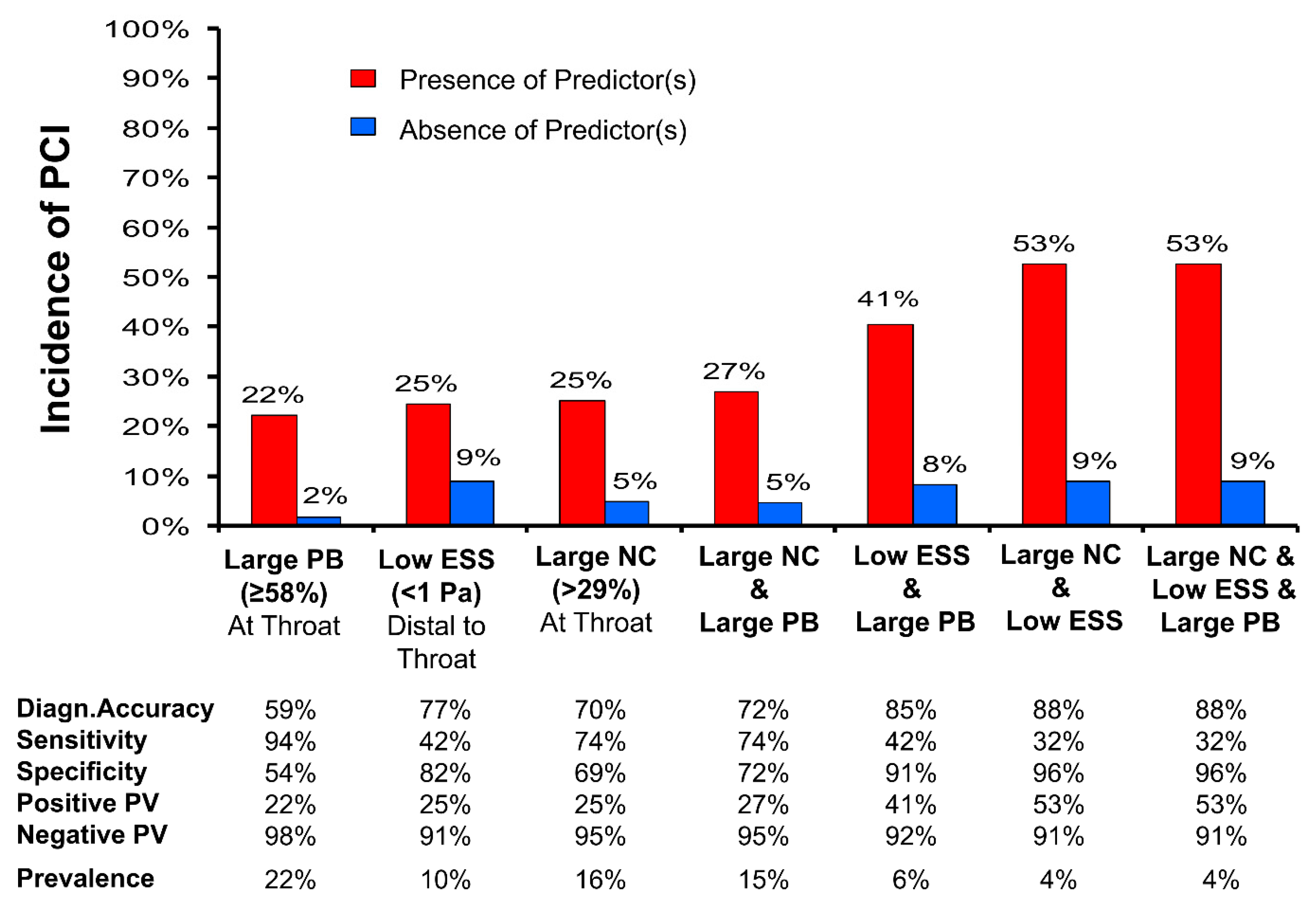
| Angiography & IVUS plus CFD | PREDICTION (“Prediction of Progression of Coronary Artery Disease and Clinical Outcome Using Vascular Profiling of Shear Stress and Wall Morphology”) Stone et al. [12] | 506 | Plaque burden ≥ 58% | PCI | 1 year | 17.57 (3.67–84.20) | <0.001 |
| ESS < 0.98 Pa | 3.18 (1.20–8.43) | 0.020 | |||||
| NIRS | AtheroRemo-NIRS Oemrawsingh et al. [59] | 203 | LCBI ≥ 43% | Non-culprit MACE | 1 year | 4.04 (1.33–12.29) | 0.01 |
| IVUS & NIRS | ATHEROREMO-NIRS and Integrated Biomarker Imaging Study 3 (IBIS-3) studies [46] | 286 | Max LCBI4mm (per 100-unit increase) | Non-culprit MACE | 4.1 years | 1.22 (1.10–1.35) | <0.001 |
| Spectrum NIRS-IVUS registry [48] | 202 | MaxLCBI4mm (per 100-unit increase) | Target vessel failure | 3.5 years | 1.6 (1.2–2.1) | 0.0040 | |
| LRP (Lipid Rich Plaque) Study [60] | 1563 | MaxLCBI4mm (per 100-unit increase) | Non-culprit MACE | 2 years | 1.21 (1.09–1.35) | 0.0004 | |
| OCT, NIRS, IVUS & VH | PREVENT (“The Preventive Coronary Intervention on Stenosis With Functionally Insignificant Vulnerable Plaque”, ClinicalTrials.gov Identifier: NCT02316886) | 1600 | Target vessel failure | 2 years | Recruiting | ||
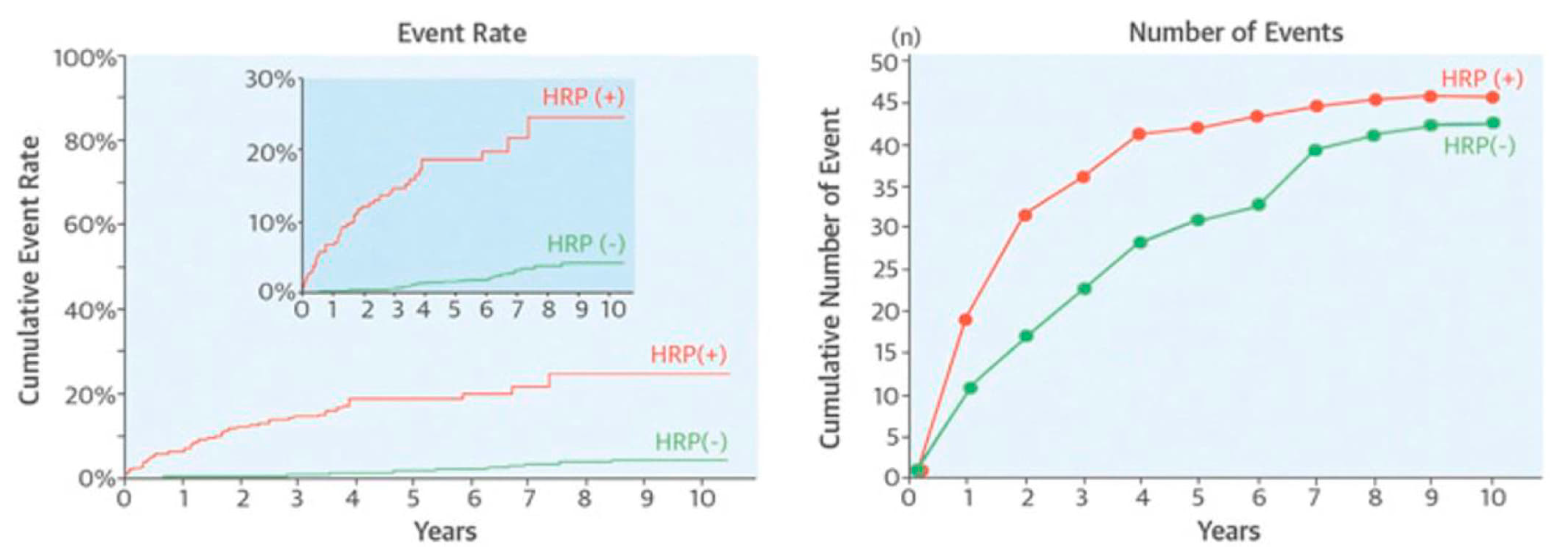
| NIRS & IVUS | PROSPECT II (“Providing Regional Observations to Study Predictors of Events in the Coronary Tree”) [47] | 898 | High lipid content | Non-culprit MACE | 3.7 years | OR 3.80 (1.87–7.70) | 0.0002 |
| Plaque burden ≥ 70% | OR 5.37 (2.42–11.89) | <0.0001 | |||||
| MaxLCBI4mm ≥ 324.7 Plaque burden ≥ 70% | OR 11.33 (6.10–21.03) |
3.3. Biomechanical Regulators of Atherothrombosis
4. Challenges of the “Vulnerable Plaque” Concept
5. Identifying the “Vulnerable Patient”
5.1. Risk Scores
5.2. Biomarkers, Antibodies, and Genetics
6. Non-Invasive Imaging
6.1. Indications
6.2. Subclinical Atherosclerosis
6.3. Computed Tomography and Positron Emission Tomography
7. Challenges of the “Vulnerable Patient” Concept
8. Future Implications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar]
- Bourantas, C.V.; Garcia-Garcia, H.M.; Torii, R.; Zhang, Y.J.; Westwood, M.; Crake, T.; Serruys, P.W. Vulnerable plaque detection: An unrealistic quest or a feasible objective with a clinical value? Heart 2016, 102, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P. Coronary Thrombosis Linked to Fissure in Atherosclerotic Vessel Wall. JAMA 1964, 188, 35–37. [Google Scholar]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. S8), C13–C18. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Kubo, T.; Nakamura, N.; Matsuo, Y.; Okumoto, Y.; Wu, X.; Choi, S.Y.; Komukai, K.; Tanimoto, T.; Ino, Y.; Kitabata, H.; et al. Virtual histology intravascular ultrasound compared with optical coherence tomography for identification of thin-cap fibroatheroma. Int. Heart J. 2011, 52, 175–179. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Braunwald, E. Coronary plaque erosion: Recognition and management. JACC Cardiovasc. Imaging 2013, 6, 288–289. [Google Scholar] [CrossRef]
- Sano, K.; Kawasaki, M.; Ishihara, Y.; Okubo, M.; Tsuchiya, K.; Nishigaki, K.; Zhou, X.; Minatoguchi, S.; Fujita, H.; Fujiwara, H. Assessment of vulnerable plaques causing acute coronary syndrome using integrated backscatter intravascular ultrasound. J. Am. Coll. Cardiol. 2006, 47, 734–741. [Google Scholar] [CrossRef]
- Stone, P.H.; Saito, S.; Takahashi, S.; Makita, Y.; Nakamura, S.; Kawasaki, T.; Takahashi, A.; Katsuki, T.; Nakamura, S.; Namiki, A.; et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: The PREDICTION Study. Circulation 2012, 126, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Fuster, V. The myth of the “vulnerable plaque”: Transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J. Am. Coll. Cardiol. 2015, 65, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Fan, J.; Watanabe, T. Inflammatory reactions in the pathogenesis of atherosclerosis. J. Atheroscler. Thromb. 2003, 10, 63–71. [Google Scholar] [CrossRef]
- Nakagawa, K.; Nakashima, Y. Pathologic intimal thickening in human atherosclerosis is formed by extracellular accumulation of plasma-derived lipids and dispersion of intimal smooth muscle cells. Atherosclerosis 2018, 274, 235–242. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Nakazawa, G.; Virmani, R. Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arterioscler. Thromb. Vasc. Biol. 2007, 27, 986–989. [Google Scholar] [CrossRef]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Della Schiava, N.; Long, A.; Chirico, E.N.; Pialoux, V.; Millon, A. Carotid intraplaque haemorrhage: Pathogenesis, histological classification, imaging methods and clinical value. Ann. Transl. Med. 2020, 8, 1273. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Hao, H.; Shibuya, M.; Imanaka, T.; Fukunaga, M.; Miki, K.; Tamaru, H.; Sawada, H.; Naito, Y.; Ohyanagi, M.; et al. Accuracy of OCT, grayscale IVUS, and their combination for the diagnosis of coronary TCFA: An ex vivo validation study. JACC Cardiovasc. Imaging 2015, 8, 451–460. [Google Scholar] [CrossRef]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Burke, A.P.; Tang, A.L.; Liang, T.Y.; Mannan, P.; Smialek, J.; Virmani, R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996, 93, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, C.; Zhe, C.; Zhu, Y.; Yonetsu, T.; Jia, H.; Hou, J.; Zhang, S.; Jang, I.K.; Yu, B. Plaque erosion delays vascular healing after drug eluting stent implantation in patients with acute coronary syndrome: An In Vivo Optical Coherence Tomography Study. Catheter. Cardiovasc. Interv. 2017, 89, 592–600. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Prati, F.; Uemura, S.; Souteyrand, G.; Virmani, R.; Motreff, P.; Di Vito, L.; Biondi-Zoccai, G.; Halperin, J.; Fuster, V.; Ozaki, Y.; et al. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc. Imaging 2013, 6, 283–287. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, Y.; Zhang, Y.; Dai, J.; Li, L.; Dauerman, H.; Soeda, T.; Wang, Z.; Lee, H.; Wang, C.; et al. Management and Outcome of Patients With Acute Coronary Syndrome Caused by Plaque Rupture Versus Plaque Erosion: An Intravascular Optical Coherence Tomography Study. J. Am. Heart Assoc. 2017, 6, e004730. [Google Scholar] [CrossRef]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion): A 1-Year Follow-Up Report. Circ. Cardiovasc. Interv. 2017, 10, e005860. [Google Scholar] [CrossRef]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective anti-thrombotic therapy without stenting: Intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 2017, 38, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.A.; Obaid, D.R.; O’Sullivan, M.; Shapiro, L.M.; McNab, D.; Densem, C.G.; Schofield, P.M.; Braganza, D.; Clarke, S.C.; Ray, K.K.; et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: The VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc. Imaging 2011, 4, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M.; Garcia-Garcia, H.M.; de Boer, S.P.; Kardys, I.; Heo, J.H.; Akkerhuis, K.M.; Oemrawsingh, R.M.; van Domburg, R.T.; Ligthart, J.; Witberg, K.T.; et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Eur. Heart J. 2014, 35, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Park, S.J.; Dauerman, H.L.; Uemura, S.; Kim, J.S.; Di Mario, C.; Johnson, T.W.; Guagliumi, G.; Kastrati, A.; Joner, M.; et al. Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat. Rev. Cardiol. 2022, 19, 684–703. [Google Scholar] [CrossRef]
- Adriaenssens, T.; Allard-Ratick, M.P.; Thondapu, V.; Sugiyama, T.; Raffel, O.C.; Barlis, P.; Poon, E.K.W.; Araki, M.; Nakajima, A.; Minami, Y.; et al. Optical Coherence Tomography of Coronary Plaque Progression and Destabilization: JACC Focus Seminar Part 3/3. J. Am. Coll. Cardiol. 2021, 78, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Takarada, S.; Imanishi, T.; Kubo, T.; Tanimoto, T.; Kitabata, H.; Nakamura, N.; Tanaka, A.; Mizukoshi, M.; Akasaka, T. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: Assessment by optical coherence tomography study. Atherosclerosis 2009, 202, 491–497. [Google Scholar] [CrossRef]
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K.; et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: The EASY-FIT study. J. Am. Coll. Cardiol. 2014, 64, 2207–2217. [Google Scholar] [CrossRef]
- Ozaki, Y.; Garcia-Garcia, H.M.; Beyene, S.S.; Hideo-Kajita, A.; Kuku, K.O.; Kolm, P.; Waksman, R. Effect of Statin Therapy on Fibrous Cap Thickness in Coronary Plaque on Optical Coherence Tomography- Review and Meta-Analysis. Circ. J. 2019, 83, 1480–1488. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nissen, S.E.; Prati, F.; Windecker, S.; Kataoka, Y.; Puri, R.; Hucko, T.; Kassahun, H.; Liao, J.; Somaratne, R.; et al. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: Rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc. Diagn. Ther. 2021, 11, 120–129. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef]
- Zanchin, C.; Koskinas, K.C.; Ueki, Y.; Losdat, S.; Haner, J.D.; Bar, S.; Otsuka, T.; Inderkum, A.; Jensen, M.R.J.; Lonborg, J.; et al. Effects of the PCSK9 antibody alirocumab on coronary atherosclerosis in patients with acute myocardial infarction: A serial, multivessel, intravascular ultrasound, near-infrared spectroscopy and optical coherence tomography imaging study-Rationale and design of the PACMAN-AMI trial. Am. Heart J. 2021, 238, 33–44. [Google Scholar]
- Raber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Haner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Rodriguez-Porcel, M.; Matsuo, Y.; Cassar, A.; Kwon, T.G.; Franchi, F.; Gulati, R.; Kushwaha, S.S.; Lennon, R.J.; Lerman, L.O.; et al. Evaluation of coronary adventitial vasa vasorum using 3D optical coherence tomography--animal and human studies. Atherosclerosis 2015, 239, 203–208. [Google Scholar] [CrossRef]
- Schuurman, A.S.; Vroegindewey, M.; Kardys, I.; Oemrawsingh, R.M.; Cheng, J.M.; de Boer, S.; Garcia-Garcia, H.M.; van Geuns, R.J.; Regar, E.S.; Daemen, J.; et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur. Heart J. 2018, 39, 295–302. [Google Scholar] [CrossRef]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Botker, H.E.; Maeng, M.; Kjoller-Hansen, L.; Engstrom, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef]
- Madder, R.D.; Kubo, T.; Ino, Y.; Kameyama, T.; Terada, K.; VanOosterhout, S.; Mulder, A.; McNamara, M.; Kenaan, M.; Samani, S.; et al. Target Lesion Lipid Content Detected by Near-Infrared Spectroscopy After Stenting and the Risk of Subsequent Target Lesion Failure. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.J.; Serruys, P.W.; van der Ent, M.; Ligthart, J.; Mastik, F.; Garg, S.; Muller, J.E.; Wilder, M.A.; van de Steen, A.F.; Regar, E. First-in-man clinical use of combined near-infrared spectroscopy and intravascular ultrasound: A potential key to predict distal embolization and no-reflow? J. Am. Coll. Cardiol. 2010, 56, 314. [Google Scholar] [CrossRef] [PubMed]
- Kuku, K.O.; Singh, M.; Ozaki, Y.; Dan, K.; Chezar-Azerrad, C.; Waksman, R.; Garcia-Garcia, H.M. Near-Infrared Spectroscopy Intravascular Ultrasound Imaging: State of the Art. Front. Cardiovasc. Med. 2020, 7, 107. [Google Scholar] [CrossRef]
- Fard, A.M.; Vacas-Jacques, P.; Hamidi, E.; Wang, H.; Carruth, R.W.; Gardecki, J.A.; Tearney, G.J. Optical coherence tomography--near infrared spectroscopy system and catheter for intravascular imaging. Opt. Express 2013, 21, 30849–30858. [Google Scholar] [CrossRef]
- Muller, J.; Madder, R. OCT-NIRS Imaging for Detection of Coronary Plaque Structure and Vulnerability. Front. Cardiovasc. Med. 2020, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Verjans, J.W.; Osborn, E.A.; Ughi, G.J.; Calfon Press, M.A.; Hamidi, E.; Antoniadis, A.P.; Papafaklis, M.I.; Conrad, M.F.; Libby, P.; Stone, P.H.; et al. Targeted Near-Infrared Fluorescence Imaging of Atherosclerosis: Clinical and Intracoronary Evaluation of Indocyanine Green. JACC Cardiovasc. Imaging 2016, 9, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, J.W.; Shishkov, M.; Namati, E.; Morse, T.; Shubochkin, R.; McCarthy, J.R.; Ntziachristos, V.; Bouma, B.E.; Jaffer, F.A.; et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med. 2011, 17, 1680–1684. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Jaffer, F.A.; Gijsen, F.J.; van Soest, G.; Madden, S.P.; Courtney, B.K.; Fard, A.M.; Tenekecioglu, E.; Zeng, Y.; van der Steen, A.F.W.; et al. Hybrid intravascular imaging: Recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur. Heart J. 2017, 38, 400–412. [Google Scholar] [CrossRef]
- Inaba, S.; Mintz, G.S.; Farhat, N.Z.; Fajadet, J.; Dudek, D.; Marzocchi, A.; Templin, B.; Weisz, G.; Xu, K.; de Bruyne, B.; et al. Impact of positive and negative lesion site remodeling on clinical outcomes: Insights from PROSPECT. JACC Cardiovasc. Imaging 2014, 7, 70–78. [Google Scholar] [CrossRef]
- Zheng, B.; Mintz, G.S.; McPherson, J.A.; De Bruyne, B.; Farhat, N.Z.; Marso, S.P.; Serruys, P.W.; Stone, G.W.; Maehara, A. Predictors of Plaque Rupture Within Nonculprit Fibroatheromas in Patients With Acute Coronary Syndromes: The PROSPECT Study. JACC Cardiovasc. Imaging 2015, 8, 1180–1187. [Google Scholar] [CrossRef]
- Schuurman, A.S.; Vroegindewey, M.M.; Kardys, I.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; van Geuns, R.J.; Regar, E.; Van Mieghem, N.M.; Ligthart, J.; Serruys, P.W.; et al. Prognostic Value of Intravascular Ultrasound in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 72, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Oemrawsingh, R.M.; Cheng, J.M.; Garcia-Garcia, H.M.; van Geuns, R.J.; de Boer, S.P.; Simsek, C.; Kardys, I.; Lenzen, M.J.; van Domburg, R.T.; Regar, E.; et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Di Mario, C.; Torguson, R.; Ali, Z.A.; Singh, V.; Skinner, W.H.; Artis, A.K.; Cate, T.T.; Powers, E.; Kim, C.; et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: A prospective, cohort study. Lancet 2019, 394, 1629–1637. [Google Scholar] [CrossRef]
- Papafaklis, M.I.; Takahashi, S.; Antoniadis, A.P.; Coskun, A.U.; Tsuda, M.; Mizuno, S.; Andreou, I.; Nakamura, S.; Makita, Y.; Hirohata, A.; et al. Effect of the local hemodynamic environment on the de novo development and progression of eccentric coronary atherosclerosis in humans: Insights from PREDICTION. Atherosclerosis 2015, 240, 205–211. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Papafaklis, M.I.; Athanasiou, L.; Kalatzis, F.G.; Naka, K.K.; Siogkas, P.K.; Takahashi, S.; Saito, S.; Fotiadis, D.I.; Feldman, C.L.; et al. A new methodology for accurate 3-dimensional coronary artery reconstruction using routine intravascular ultrasound and angiographic data: Implications for widespread assessment of endothelial shear stress in humans. EuroIntervention 2013, 9, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Papafaklis, M.I.; Bourantas, C.V.; Yonetsu, T.; Vergallo, R.; Kotsia, A.; Nakatani, S.; Lakkas, L.S.; Athanasiou, L.S.; Naka, K.K.; Fotiadis, D.I.; et al. Anatomically correct three-dimensional coronary artery reconstruction using frequency domain optical coherence tomographic and angiographic data: Head-to-head comparison with intravascular ultrasound for endothelial shear stress assessment in humans. EuroIntervention 2015, 11, 407–415. [Google Scholar] [CrossRef]
- Vergallo, R.; Papafaklis, M.I.; Yonetsu, T.; Bourantas, C.V.; Andreou, I.; Wang, Z.; Fujimoto, J.G.; McNulty, I.; Lee, H.; Biasucci, L.M.; et al. Endothelial shear stress and coronary plaque characteristics in humans: Combined frequency-domain optical coherence tomography and computational fluid dynamics study. Circ. Cardiovasc. Imaging 2014, 7, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Papafaklis, M.I.; Mizuno, S.; Takahashi, S.; Coskun, A.U.; Antoniadis, A.P.; Tsuda, M.; Feldman, C.L.; Saito, S.; Stone, P.H. Incremental predictive value of combined endothelial shear stress, plaque necrotic core, and plaque burden for future cardiac events: A post-hoc analysis of the PREDICTION study. Int. J. Cardiol. 2016, 202, 64–66. [Google Scholar] [CrossRef]
- Stone, P.H.; Maehara, A.; Coskun, A.U.; Maynard, C.C.; Zaromytidou, M.; Siasos, G.; Andreou, I.; Fotiadis, D.; Stefanou, K.; Papafaklis, M.; et al. Role of Low Endothelial Shear Stress and Plaque Characteristics in the Prediction of Nonculprit Major Adverse Cardiac Events: The PROSPECT Study. JACC Cardiovasc. Imaging 2018, 11, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.H.; Maehara, A.; Coskun, A.U.; Maynard, C.; Andreou, I.; Siasos, G.; Zaromitidou, M.; Fotiadis, D.I.; Stefanou, K.; Papafaklis, M.I.; et al. TCT-317 Local Low Endothelial Shear Stress (ESS) Provides Incremental Prediction of Non-culprit MACE in Addition to Plaque Burden, Minimal Lumen Area, and Plaque Morphology: The PROSPECT Study. J. Am. Coll. Cardiol. 2015, 66, B126. [Google Scholar] [CrossRef]
- Choi, G.; Lee, J.M.; Kim, H.J.; Park, J.B.; Sankaran, S.; Otake, H.; Doh, J.H.; Nam, C.W.; Shin, E.S.; Taylor, C.A.; et al. Coronary Artery Axial Plaque Stress and its Relationship With Lesion Geometry: Application of Computational Fluid Dynamics to Coronary CT Angiography. JACC Cardiovasc. Imaging 2015, 8, 1156–1166. [Google Scholar] [CrossRef]
- Papafaklis, M.; Vergallo, R.; Andreou, I.; Yonetsu, T.; Bourantas, C.V.; Sakellarios, A.; Fotiadis, D.I.; Feldman, C.L.; Michalis, L.; Jang, I.K.; et al. Prediction of culprit lesions in patients with acute coronary syndrome by assessing the hemodynamic forces acting on plaques: A three-dimensional frequency-domain optical coherence tomography study. Eur. Heart J. 2016, 37, 600. [Google Scholar]
- Rioufol, G.; Finet, G.; Ginon, I.; Andre-Fouet, X.; Rossi, R.; Vialle, E.; Desjoyaux, E.; Convert, G.; Huret, J.F.; Tabib, A. Multiple atherosclerotic plaque rupture in acute coronary syndrome: A three-vessel intravascular ultrasound study. Circulation 2002, 106, 804–808. [Google Scholar] [CrossRef]
- Libby, P. How does lipid lowering prevent coronary events? New insights from human imaging trials. Eur. Heart J. 2015, 36, 472–474. [Google Scholar] [CrossRef]
- Zhou, M.; Ma, C.; Liu, W.; Liu, H.; Wang, N.; Kang, Q.; Li, P. Valsartan Promoting Atherosclerotic Plaque Stabilization by Upregulating Renalase: A Potential-Related Gene of Atherosclerosis. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Shah, B.R.; Volz, E.M.; Horton, J.R.; Shaw, L.K.; Newby, L.K.; Granger, C.B.; Mark, D.B.; Califf, R.M.; Becker, R.C. Evolution of the coronary care unit: Clinical characteristics and temporal trends in healthcare delivery and outcomes. Crit. Care Med. 2010, 38, 375–381. [Google Scholar] [CrossRef] [PubMed]
- van Lammeren, G.W.; den Ruijter, H.M.; Vrijenhoek, J.E.; van der Laan, S.W.; Velema, E.; de Vries, J.P.; de Kleijn, D.P.; Vink, A.; de Borst, G.J.; Moll, F.L.; et al. Time-dependent changes in atherosclerotic plaque composition in patients undergoing carotid surgery. Circulation 2014, 129, 2269–2276. [Google Scholar] [CrossRef]
- Kubo, T.; Maehara, A.; Mintz, G.S.; Doi, H.; Tsujita, K.; Choi, S.Y.; Katoh, O.; Nasu, K.; Koenig, A.; Pieper, M.; et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J. Am. Coll. Cardiol. 2010, 55, 1590–1597. [Google Scholar] [CrossRef]
- Gaba, P.; Gersh, B.J.; Muller, J.; Narula, J.; Stone, G.W. Evolving concepts of the vulnerable atherosclerotic plaque and the vulnerable patient: Implications for patient care and future research. Nat. Rev. Cardiol. 2022, 20, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Hellings, W.E.; Peeters, W.; Moll, F.L.; Pasterkamp, G. From vulnerable plaque to vulnerable patient: The search for biomarkers of plaque destabilization. Trends Cardiovasc. Med. 2007, 17, 162–171. [Google Scholar] [CrossRef]
- Raggi, P.; Pontone, G.; Andreini, D. Role of new imaging modalities in pursuit of the vulnerable plaque and the vulnerable patient. Int. J. Cardiol. 2018, 250, 278–283. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Tomaniak, M.; Katagiri, Y.; Modolo, R.; de Silva, R.; Khamis, R.Y.; Bourantas, C.V.; Torii, R.; Wentzel, J.J.; Gijsen, F.J.H.; van Soest, G.; et al. Vulnerable plaques and patients: State-of-the-art. Eur. Heart J. 2020, 41, 2997–3004. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyorala, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetiere, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. Am. J. Epidemiol. 1989, 129, 687–702. [Google Scholar] [CrossRef]
- Fried, L.P.; Borhani, N.O.; Enright, P.; Furberg, C.D.; Gardin, J.M.; Kronmal, R.A.; Kuller, L.H.; Manolio, T.A.; Mittelmark, M.B.; Newman, A.; et al. The Cardiovascular Health Study: Design and rationale. Ann. Epidemiol. 1991, 1, 263–276. [Google Scholar] [CrossRef]
- Friedman, G.D.; Cutter, G.R.; Donahue, R.P.; Hughes, G.H.; Hulley, S.B.; Jacobs, D.R., Jr.; Liu, K.; Savage, P.J. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J. Clin. Epidemiol. 1988, 41, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Feinleib, M.; McNamara, P.M.; Garrison, R.J.; Castelli, W.P. An investigation of coronary heart disease in families. The Framingham offspring study. Am. J. Epidemiol. 1979, 110, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Andrus, B.; Lacaille, D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J. Am. Coll. Cardiol. 2014, 63, 2886. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63 Pt B, 2889–2934. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Pankow, J.S.; Decker, P.A.; Berardi, C.; Hanson, N.Q.; Sale, M.; Tang, W.; Kanaya, A.M.; Larson, N.B.; Tsai, M.Y.; Wassel, C.L.; et al. Circulating cellular adhesion molecules and risk of diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabet. Med. 2016, 33, 985–991. [Google Scholar] [CrossRef]
- Hwang, S.J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M., Jr.; Boerwinkle, E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef]
- Pai, J.K.; Pischon, T.; Ma, J.; Manson, J.E.; Hankinson, S.E.; Joshipura, K.; Curhan, G.C.; Rifai, N.; Cannuscio, C.C.; Stampfer, M.J.; et al. Inflammatory markers and the risk of coronary heart disease in men and women. N. Engl. J. Med. 2004, 351, 2599–2610. [Google Scholar] [CrossRef]
- van Holten, T.C.; Waanders, L.F.; de Groot, P.G.; Vissers, J.; Hoefer, I.E.; Pasterkamp, G.; Prins, M.W.; Roest, M. Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS ONE 2013, 8, e62080. [Google Scholar] [CrossRef]
- Yuan, D.; Chu, J.; Lin, H.; Zhu, G.; Qian, J.; Yu, Y.; Yao, T.; Ping, F.; Chen, F.; Liu, X. Mechanism of homocysteine-mediated endothelial injury and its consequences for atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 1109445. [Google Scholar] [CrossRef]
- Stamatelopoulos, K.; Pol, C.J.; Ayers, C.; Georgiopoulos, G.; Gatsiou, A.; Brilakis, E.S.; Khera, A.; Drosatos, K.; de Lemos, J.A.; Stellos, K. Amyloid-Beta (1-40) Peptide and Subclinical Cardiovascular Disease. J. Am. Coll. Cardiol. 2018, 72, 1060–1061. [Google Scholar] [CrossRef]
- Stakos, D.A.; Stamatelopoulos, K.; Bampatsias, D.; Sachse, M.; Zormpas, E.; Vlachogiannis, N.I.; Tual-Chalot, S.; Stellos, K. The Alzheimer’s Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Stamatelopoulos, K.; Sibbing, D.; Rallidis, L.S.; Georgiopoulos, G.; Stakos, D.; Braun, S.; Gatsiou, A.; Sopova, K.; Kotakos, C.; Varounis, C.; et al. Amyloid-beta (1–40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J. Am. Coll. Cardiol. 2015, 65, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Stamatelopoulos, K.; Mueller-Hennessen, M.; Georgiopoulos, G.; Sachse, M.; Boeddinghaus, J.; Sopova, K.; Gatsiou, A.; Amrhein, C.; Biener, M.; Vafaie, M.; et al. Amyloid-beta (1–40) and Mortality in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome: A Cohort Study. Ann. Intern. Med. 2018, 168, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Stamatelopoulos, K.; Mueller-Hennessen, M.; Georgiopoulos, G.; Lopez-Ayala, P.; Sachse, M.; Vlachogiannis, N.I.; Sopova, K.; Delialis, D.; Bonini, F.; Patras, R.; et al. Cathepsin S Levels and Survival Among Patients With Non-ST-Segment Elevation Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2022, 80, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Mareti, A.; Kritsioti, C.; Georgiopoulos, G.; Vlachogiannis, N.I.; Delialis, D.; Sachse, M.; Sopova, K.; Koutsoukis, A.; Kontogiannis, C.; Patras, R.; et al. Cathepsin B expression is associated with arterial stiffening and atherosclerotic vascular disease. Eur. J. Prev. Cardiol. 2020, 27, 2288–2291. [Google Scholar] [CrossRef]
- Stellos, K.; Gatsiou, A.; Stamatelopoulos, K.; Perisic Matic, L.; John, D.; Lunella, F.F.; Jae, N.; Rossbach, O.; Amrhein, C.; Sigala, F.; et al. Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nat. Med. 2016, 22, 1140–1150. [Google Scholar] [CrossRef]
- Hao, Y.; Zhou, J.; Zhou, M.; Ma, X.; Lu, Z.; Gao, M.; Pan, X.; Tang, J.; Bao, Y.; Jia, W. Serum levels of fibroblast growth factor 19 are inversely associated with coronary artery disease in chinese individuals. PLoS ONE 2013, 8, e72345. [Google Scholar] [CrossRef]
- Mirza, M.A.; Larsson, A.; Lind, L.; Larsson, T.E. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009, 205, 385–390. [Google Scholar] [CrossRef]
- Udell, J.A.; Morrow, D.A.; Jarolim, P.; Sloan, S.; Hoffman, E.B.; O’Donnell, T.F.; Vora, A.N.; Omland, T.; Solomon, S.D.; Pfeffer, M.A.; et al. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J. Am. Coll. Cardiol. 2014, 63, 2421–2428. [Google Scholar] [CrossRef]
- Matuszek, B.; Lenart-Lipinska, M.; Duma, D.; Solski, J.; Nowakowski, A. Evaluation of concentrations of FGF-21—A new adipocytokine in type 2 diabetes. Endokrynol. Pol. 2010, 61, 50–54. [Google Scholar]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Domouzoglou, E.M.; Lam, K.S.L.; Xu, A. Fibroblast growth factors in cardiovascular disease: The emerging role of FGF21. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1029–H1038. [Google Scholar] [CrossRef] [PubMed]
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Khamis, R.Y.; Hughes, A.D.; Caga-Anan, M.; Chang, C.L.; Boyle, J.J.; Kojima, C.; Welsh, P.; Sattar, N.; Johns, M.; Sever, P.; et al. High Serum Immunoglobulin G and M Levels Predict Freedom From Adverse Cardiovascular Events in Hypertension: A Nested Case-Control Substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. EBioMedicine 2016, 9, 372–380. [Google Scholar] [CrossRef]
- Solow, E.B.; Vongpatanasin, W.; Skaug, B.; Karp, D.R.; Ayers, C.; de Lemos, J.A. Antinuclear Antibodies Are Associated With All-Cause Mortality and Cardiovascular Outcomes in the General Population. J. Am. Coll. Cardiol. 2015, 65, 2669–2670. [Google Scholar] [CrossRef]
- Tada, H.; Melander, O.; Louie, J.Z.; Catanese, J.J.; Rowland, C.M.; Devlin, J.J.; Kathiresan, S.; Shiffman, D. Risk prediction by genetic risk scores for coronary heart disease is independent of self-reported family history. Eur. Heart J. 2016, 37, 561–567. [Google Scholar] [CrossRef]
- Mega, J.L.; Stitziel, N.O.; Smith, J.G.; Chasman, D.I.; Caulfield, M.J.; Devlin, J.J.; Nordio, F.; Hyde, C.L.; Cannon, C.P.; Sacks, F.M.; et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: An analysis of primary and secondary prevention trials. Lancet 2015, 385, 2264–2271. [Google Scholar] [CrossRef]
- Ananthasubramaniam, G.; Ananthasubramaniam, K. Stress electrocardiography testing in coronary artery disease: Is it time for its swan song or to redefine its role in the modern era? Indian Heart J. 2022, 74, 81–85. [Google Scholar] [CrossRef]
- Muntendam, P.; McCall, C.; Sanz, J.; Falk, E.; Fuster, V.; High-Risk Plaque, I. The BioImage Study: Novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease--study design and objectives. Am. Heart J. 2010, 160, 49–57.e1. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Young, R.; Burke, G.; Jeffrey Carr, J.; Detrano, R.C.; Folsom, A.R.; Kronmal, R.; Lima, J.A.C.; Liu, K.J.; McClelland, R.L.; et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: The multi-ethnic study of atherosclerosis (MESA). Eur. Heart J. 2018, 39, 2401–2408. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Adamson, P.D.; Williams, M.C.; Dweck, M.R.; Mills, N.L.; Boon, N.A.; Daghem, M.; Bing, R.; Moss, A.J.; Mangion, K.; Flather, M.; et al. Guiding Therapy by Coronary CT Angiography Improves Outcomes in Patients With Stable Chest Pain. J. Am. Coll. Cardiol. 2019, 74, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Papadopoulou, S.L.; Serruys, P.W.; Sakellarios, A.; Kitslaar, P.H.; Bizopoulos, P.; Girasis, C.; Zhang, Y.J.; de Vries, T.; Boersma, E.; et al. Noninvasive Prediction of Atherosclerotic Progression: The PROSPECT-MSCT Study. J. Am. Coll. Cardiol. Imaging 2016, 9, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Driessen, R.S.; Bom, M.J.; van Diemen, P.A.; Schumacher, S.P.; Leonora, R.M.; Everaars, H.; van Rossum, A.C.; Raijmakers, P.G.; van de Ven, P.M.; van Kuijk, C.C.; et al. Incremental prognostic value of hybrid [15O]H2O positron emission tomography-computed tomography: Combining myocardial blood flow, coronary stenosis severity, and high-risk plaque morphology. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1105–1113. [Google Scholar] [CrossRef]
- Wang, X.; van den Hoogen, I.J.; Butcher, S.C.; Kuneman, J.H.; de Graaf, M.A.; Kamperidis, V.; Boukes, M.; Maaniitty, T.; Schultz, J.; van Rosendael, A.R.; et al. Importance of plaque volume and composition for the prediction of myocardial ischaemia using sequential coronary computed tomography angiography/positron emission tomography imaging. Eur. Heart J. Cardiovasc. Imaging 2022, jeac130. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, G.; Mavraganis, G.; Delialis, D.; Georgiou, S.; Aivalioti, E.; Patras, R.; Petropoulos, I.; Dimopoulou, M.A.; Angelidakis, L.; Sianis, A.; et al. Carotid ultrasonography improves residual risk stratification in guidelines-defined high cardiovascular risk patients. Eur. J. Prev. Cardiol. 2022, 29, 1773–1784. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Sogaard, R.; Rasmussen, L.M.; Mejldal, A.; Lambrechtsen, J.; Steffensen, F.H.; Frost, L.; Egstrup, K.; Urbonaviciene, G.; Busk, M.; et al. Five-Year Outcomes of the Danish Cardiovascular Screening (DANCAVAS) Trial. N. Engl. J. Med. 2022, 387, 1385–1394. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emfietzoglou, M.; Mavrogiannis, M.C.; García-García, H.M.; Stamatelopoulos, K.; Kanakakis, I.; Papafaklis, M.I. Current Toolset in Predicting Acute Coronary Thrombotic Events: The “Vulnerable Plaque” in a “Vulnerable Patient” Concept. Life 2023, 13, 696. https://doi.org/10.3390/life13030696
Emfietzoglou M, Mavrogiannis MC, García-García HM, Stamatelopoulos K, Kanakakis I, Papafaklis MI. Current Toolset in Predicting Acute Coronary Thrombotic Events: The “Vulnerable Plaque” in a “Vulnerable Patient” Concept. Life. 2023; 13(3):696. https://doi.org/10.3390/life13030696
Chicago/Turabian StyleEmfietzoglou, Maria, Michail C. Mavrogiannis, Hector M. García-García, Kimon Stamatelopoulos, Ioannis Kanakakis, and Michail I. Papafaklis. 2023. "Current Toolset in Predicting Acute Coronary Thrombotic Events: The “Vulnerable Plaque” in a “Vulnerable Patient” Concept" Life 13, no. 3: 696. https://doi.org/10.3390/life13030696
APA StyleEmfietzoglou, M., Mavrogiannis, M. C., García-García, H. M., Stamatelopoulos, K., Kanakakis, I., & Papafaklis, M. I. (2023). Current Toolset in Predicting Acute Coronary Thrombotic Events: The “Vulnerable Plaque” in a “Vulnerable Patient” Concept. Life, 13(3), 696. https://doi.org/10.3390/life13030696








