Enhancing Clinical Decision-Making in Complex Corneal Disorders: The Role of In-Vivo Confocal Microscopy
Abstract
1. Introduction
2. Case Reports
2.1. Case 1
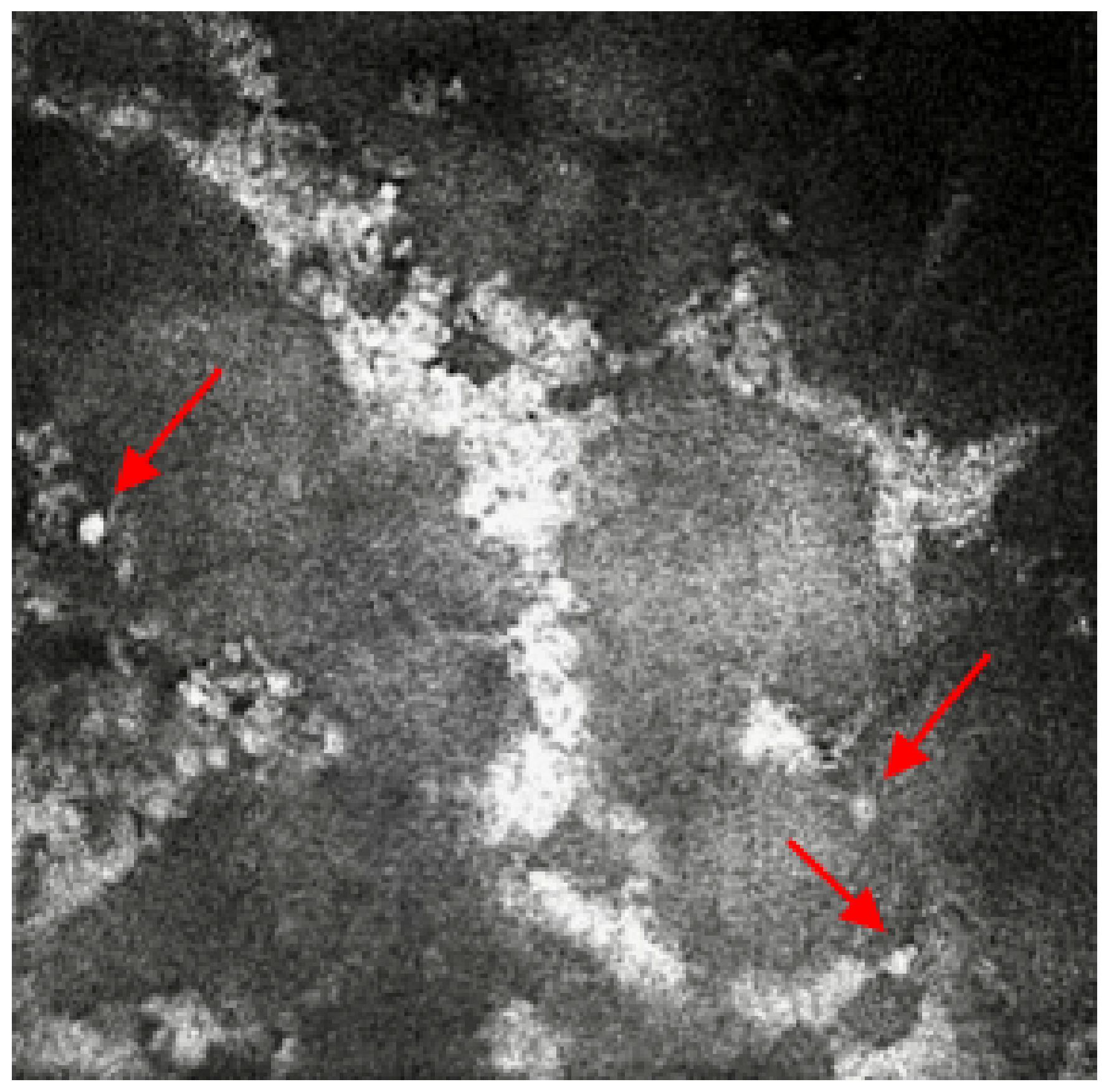
2.2. Case 2
2.3. Case 3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gokul, A.; Vellara, H.R.; Patel, D.V. Advanced anterior segment imaging in keratoconus: A review. Clin. Exp. Ophthalmol. 2018, 46, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Anis, F.S.; Mohammed, I.; Yapa, A.D.S.; Amoaku, W.M.; Dua, H.S.S. In vivo confocal microscopy features and clinicohistological correlation of limbal nerve corpuscles. Br. J. Ophthalmol. 2021, 105, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Slater, J.A.; McGhee, C.N.J.; Pradhan, M.; Braatvedt, G.D. Corneal Confocal Microscopy in Type 1 Diabetes Mellitus: A Six-Year Longitudinal Study. Transl. Vis. Sci. Technol. 2022, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Pezzullo, L.; Streatfeild, J.; Simkiss, P.; Shickle, D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv. Res. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Moussa, G.; Hodson, J.; Gooch, N.; Virdee, J.; Penaloza, C.; Kigozi, J.; Rauz, S. Calculating the economic burden of presumed microbial keratitis admissions at a tertiary referral centre in the UK. Eye 2021, 35, 2146–2154. [Google Scholar] [CrossRef]
- Dart, J.K.; Stapleton, F.; Minassian, D. Contact lenses and other risk factors in microbial keratitis. Lancet 1991, 338, 650–653. [Google Scholar] [CrossRef]
- Stellwagen, A.; MacGregor, C.; Kung, R.; Konstantopoulos, A.; Hossain, P. Personal hygiene risk factors for contact lens-related microbial keratitis. BMJ Open Ophthalmol. 2020, 5, e000476. [Google Scholar] [CrossRef]
- Butt, G.F.; Recchioni, A.; Moussa, G.; Hodson, J.; Wallace, G.R.; Murray, P.I.; Rauz, S. The impact of the COVID-19 pandemic on microbial keratitis presentation patterns. PloS ONE 2021, 16, e0256240. [Google Scholar] [CrossRef]
- Li, Y.; Hong, J.; Wei, A.; Wang, X.; Chen, Y.; Cui, X.; Sun, X.; Liu, Z.; Xu, J. Vision-Related Quality of Life in Patients with Infectious Keratitis. Optom. Vis. Sci. 2014, 91, 278–283. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Dart, J.K.G.; De, S.K.; Carnt, N.; Cleary, G.; Hau, S. Comparison of culture, confocal microscopy and PCR in routine hospital use for microbial keratitis diagnosis. Eye 2022, 36, 2172–2178. [Google Scholar] [CrossRef]
- Hau, S.C.; Dart, J.K.; Vesaluoma, M.; Parmar, D.N.; Claerhout, I.; Bibi, K.; Larkin, D.F. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br. J. Ophthalmol. 2010, 94, 982–987. [Google Scholar] [CrossRef]
- Mun, Y.; Kim, M.K.; Oh, J.Y. Ten-year analysis of microbiological profile and antibiotic sensitivity for bacterial keratitis in Korea. PloS ONE 2019, 14, e0213103. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Galal, M.; Kulkarni, B.; Elalfy, M.S.; Lake, D.; Hamada, S.; Said, D.G.; Dua, H.S. Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011-2020: A 10-Year Study. J. Fungi 2021, 7, 966. [Google Scholar] [CrossRef]
- Sim, R.; Yong, K.; Liu, Y.C.; Tong, L. In Vivo Confocal Microscopy in Different Types of Dry Eye and Meibomian Gland Dysfunction. J. Clin. Med. 2022, 11, 2349. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ibrahim, O.M.A. Application of In Vivo Confocal Microscopy in Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES41–DES47. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Infectious keratitis: A review. Clin. Exp. Ophthalmol. 2022, 50, 543–562. [Google Scholar] [CrossRef]
- Hemady, R.K.; Griffin, N.; Aristimuno, B. Recurrent corneal infections in a patient with the acquired immunodeficiency syndrome. Cornea 1993, 12, 266–269. [Google Scholar] [CrossRef]
- Hassan, H.M.; Papanikolaou, T.; Mariatos, G.; Hammad, A.; Hassan, H. Candida albicans keratitis in an immunocompromised patient. Clin. Ophthalmol. 2010, 4, 1211–1215. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Dohlman, T.H.; Amparo, F.; Arnoldner, M.A.; Jamali, A.; Hamrah, P.; Dana, R. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology 2015, 122, 662–668. [Google Scholar] [CrossRef]
- Turgut, A.; Tubbs, R.S.; Turgut, M. Paul Hoffmann (1884–1962 AD) and Jules Tinel (1879–1952 AD), and their legacy to neuroscience: The Hoffmann-Tinel sign. Child’s Nerv. Syst. 2019, 35, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Moein, H.-R.; Akhlaq, A.; Dieckmann, G.; Abbouda, A.; Pondelis, N.; Salem, Z.; Müller, R.T.; Cruzat, A.; Cavalcanti, B.M.; Jamali, A.; et al. Visualization of microneuromas by using in vivo confocal microscopy: An objective biomarker for the diagnosis of neuropathic corneal pain? Ocul. Surf. 2020, 18, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Kheirkhah, A.; Cavalcanti, B.M.; Cruzat, A.; Jamali, A.; Hamrah, P. Correlation of corneal immune cell changes with clinical severity in dry eye disease: An in vivo confocal microscopy study. Ocul. Surf. 2021, 19, 183–189. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recchioni, A.; Barua, A.; Dominguez-Vicent, A. Enhancing Clinical Decision-Making in Complex Corneal Disorders: The Role of In-Vivo Confocal Microscopy. Life 2023, 13, 679. https://doi.org/10.3390/life13030679
Recchioni A, Barua A, Dominguez-Vicent A. Enhancing Clinical Decision-Making in Complex Corneal Disorders: The Role of In-Vivo Confocal Microscopy. Life. 2023; 13(3):679. https://doi.org/10.3390/life13030679
Chicago/Turabian StyleRecchioni, Alberto, Ankur Barua, and Alberto Dominguez-Vicent. 2023. "Enhancing Clinical Decision-Making in Complex Corneal Disorders: The Role of In-Vivo Confocal Microscopy" Life 13, no. 3: 679. https://doi.org/10.3390/life13030679
APA StyleRecchioni, A., Barua, A., & Dominguez-Vicent, A. (2023). Enhancing Clinical Decision-Making in Complex Corneal Disorders: The Role of In-Vivo Confocal Microscopy. Life, 13(3), 679. https://doi.org/10.3390/life13030679





