Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Study Design and Diagnostic Criteria
- ADDE type: participants presented a TMH-SL ≤ 0.20 mm and an LLP > Closed Meshwork. The participants who formed the ADDE group were also subclassified into Mild-moderate ADDE if the TMH-SL was ≥0.10 mm or Moderate-severe ADDE if the TMH-SL was ≤0.10 mm.
- EDE type: participants presented a THM-SL ≥ 0.20 mm and an LLP ≤ Closed Meshwork.
2.3. Evaluation Procedures
2.3.1. Symptomatology Assessment
2.3.2. Tear Film Osmolarity
2.3.3. Tear Meniscus Height
2.3.4. Lipid Layer Pattern Evaluation
2.3.5. Fluorescein Break-Up Time and Corneal Staining
2.3.6. Statistical Analysis
3. Results
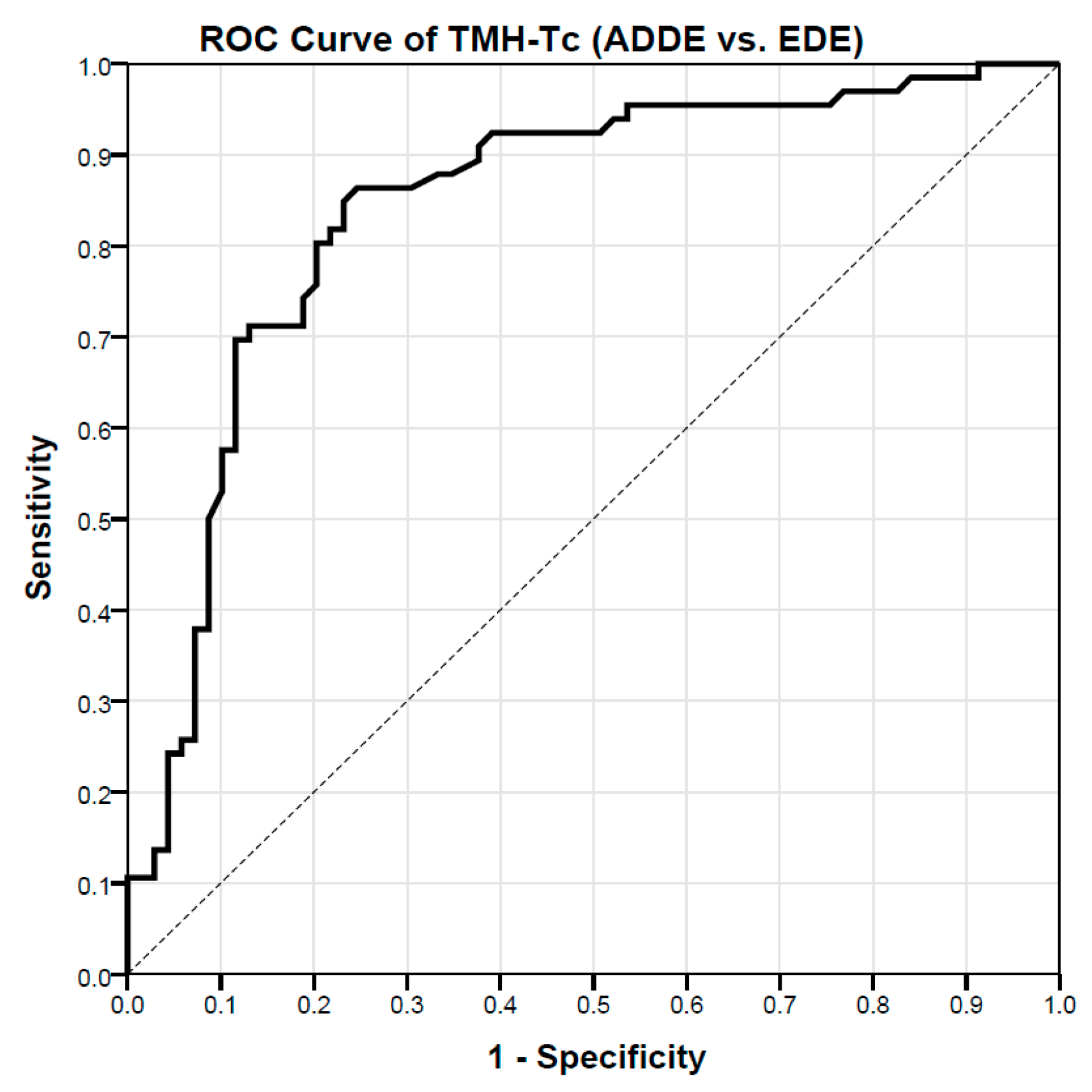
3.1. Assessment of TMH-Tc Cut-Off Values to Differentiate between ADDE and EDE Participants
3.2. Assessment of TMH-Tc Cut-Off Values to Differentiate between Mild-Moderate and Moderate-Severe ADDE Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Yokoi, N.; Georgiev, G.A. Tear-film-oriented diagnosis for dry eye. Jpn. J. Ophthalmol. 2019, 63, 127–136. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Mainstone, J.C.; Bruce, A.S.; Golding, T.R. Tear meniscus measurement in the diagnosis of dry eye. Curr. Eye Res. 1996, 15, 653–661. [Google Scholar] [CrossRef]
- Shen, M.; Li, J.; Wang, J.; Ma, H.; Cai, C.; Tao, A.; Yuan, Y.; Lu, F. Upper and lower tear menisci in the diagnosis of dry eye. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2722–2726. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Resua, C.; Santodomingo-Rubido, J.; Lira, M.; Giraldez, M.J.; Vilar, E.Y. Clinical assessment of the lower tear meniscus height. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2009, 29, 487–496. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Wolffsohn, J.; Gilmartin, B. Comparison between graticule and image capture assessment of lower tear film meniscus height. Contact Lens Anterior Eye 2006, 29, 169–173. [Google Scholar] [CrossRef]
- Lam, A.K.; Tai, S.K.; Chan, J.K.; Ng, R.W. Lower Tear Meniscus Height Measurements Using Keratography and Swept-Source Optical Coherence Tomography and Effect of Fluorescein Instillation Methods. Curr. Eye Res. 2019, 44, 1203–1208. [Google Scholar] [CrossRef]
- Niedernolte, B.; Trunk, L.; Wolffsohn, J.S.; Pult, H.; Bandlitz, S. Evaluation of tear meniscus height using different clinical methods. Clin. Exp. Optom. 2021, 104, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qu, J.H.; Zhang, X.Y.; Sun, X.G. Repeatability and Reproducibility of Noninvasive Keratograph 5M Measurements in Patients with Dry Eye Disease. J. Ophthalmol. 2016, 2016, 8013621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, Z.; Gong, Q.; Ren, W.; Chen, L.; Lu, F.; Hu, L. The Role of Different Tear Volume Detection Methods in the Evaluation and Diagnosis of Mild Dry Eye Disease. Transl. Vis. Sci. Technol. 2022, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Fodor, E.; Hagyo, K.; Resch, M.; Somodi, D.; Nemeth, J. Comparison of Tearscope-plus versus slit lamp measurements of inferior tear meniscus height in normal individuals. Eur. J. Ophthalmol. 2010, 20, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; Garcia-Resua, C.; Barreira, N.; Giraldez, M.J.; Yebra-Pimentel, E. Interobserver variability of an open-source software for tear meniscus height measurement. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2016, 39, 249–256. [Google Scholar] [CrossRef]
- Nichols, K.K.; Mitchell, G.L.; Zadnik, K. The repeatability of clinical measurements of dry eye. Cornea 2004, 23, 272–285. [Google Scholar] [CrossRef]
- Li, J.; Shen, M.; Wang, J.; Ma, H.; Tao, A.; Xu, S.; Lu, F. Clinical significance of tear menisci in dry eye. Eye Contact Lens 2012, 38, 183–187. [Google Scholar] [CrossRef]
- Wang, J.; Palakuru, J.R.; Aquavella, J.V. Correlations among upper and lower tear menisci, noninvasive tear break-up time, and the Schirmer test. Am. J. Ophthalmol. 2008, 145, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Le, Q.; Hong, J.; Wang, W.; Wang, F.; Xu, J. Assessment of Lower Tear Meniscus. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2016, 93, 1420–1425. [Google Scholar] [CrossRef]
- Tong, L.; Teng, L.S. Review of Literature on Measurements of Non-invasive Break Up Times, Lipid Morphology and Tear Meniscal Height Using Commercially Available Hand-held Instruments. Curr. Eye Res. 2018, 43, 567–575. [Google Scholar] [CrossRef]
- Miller, K.L.; Walt, J.G.; Mink, D.R.; Satram-Hoang, S.; Wilson, S.E.; Perry, H.D.; Asbell, P.A.; Pflugfelder, S.C. Minimal clinically important difference for the ocular surface disease index. Arch. Ophthalmol. 2010, 128, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; O’Day, D.M. Statistical analysis of multi-eye data in ophthalmic research. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1186–1188. [Google Scholar]
- Garcia-Resua, C.; Pena-Verdeal, H.; Minones, M.; Giraldez, M.J.; Yebra-Pimentel, E. Interobserver and intraobserver repeatability of lipid layer pattern evaluation by two experienced observers. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2014, 37, 431–437. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; Benitez Del Castillo, J.M.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 2011, 151, 792–798 e791. [Google Scholar] [CrossRef]
- Benelli, U.; Nardi, M.; Posarelli, C.; Albert, T.G. Tear osmolarity measurement using the TearLab Osmolarity System in the assessment of dry eye treatment effectiveness. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2010, 33, 61–67. [Google Scholar] [CrossRef]
- Keech, A.; Senchyna, M.; Jones, L. Impact of time between collection and collection method on human tear fluid osmolarity. Curr. Eye Res. 2013, 38, 428–436. [Google Scholar] [CrossRef]
- Guillon, J.P. Use of the Tearscope Plus and attachments in the routine examination of the marginal dry eye contact lens patient. Adv. Exp. Med. Biol. 1998, 438, 859–867. [Google Scholar]
- Guillon, J.P. Abnormal lipid layers. Observation, differential diagnosis, and classification. Adv. Exp. Med. Biol. 1998, 438, 309–313. [Google Scholar]
- Chen, L.L.; Chen, Z.L.; Xu, G.H.; Zhang, L.Q. Evaluation of the tear film function in diabetic patients using the Keratograph 5M. Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2018, 54, 762–766. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J. The Effect of instilled fluorescein solution volume on the values and repeatability of TBUT measurements. Cornea 2005, 24, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; Garcia-Resua, C.; Ramos, L.; Yebra-Pimentel, E.; Giraldez, M.J. Diurnal variations in tear film break-up time determined in healthy subjects by software-assisted interpretation of tear film video recordings. Clin. Exp. Optom. J. Aust. Optom. Assoc. 2016, 99, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, P.; Brown, B. Review of the tear break-up time and a closer look at the tear break-up time of Hong Kong Chinese. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 1993, 70, 30–38. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Davies, L.N.; Dunne, M.C.; Gilmartin, B. Statistical guidelines for clinical studies of human vision. Ophthalmic Physiol. Opt. 2011, 31, 123–136. [Google Scholar] [CrossRef] [PubMed]
- de Monchy, I.; Gendron, G.; Miceli, C.; Pogorzalek, N.; Mariette, X.; Labetoulle, M. Combination of the Schirmer I and phenol red thread tests as a rescue strategy for diagnosis of ocular dryness associated with Sjogren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5167–5173. [Google Scholar] [CrossRef] [Green Version]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Uchida, A.; Uchino, M.; Goto, E.; Hosaka, E.; Kasuya, Y.; Fukagawa, K.; Dogru, M.; Ogawa, Y.; Tsubota, K. Noninvasive interference tear meniscometry in dry eye patients with Sjogren syndrome. Am. J. Ophthalmol. 2007, 144, 232–237. [Google Scholar] [CrossRef]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Garcia-Resua, C.; Giraldez, M.J.; Yebra-Pimentel, E. Analysis of the Differences in Ocular Surface Damage and Inflammatory Signs between Healthy and Evaporative Dry Eye Participants. Ocul. Immunol. Inflamm. 2022, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Seo, K.Y.; Kim, T.I.; Chin, H.S.; Jung, J.W. Location and pattern of non-invasive keratographic tear film break-up according to dry eye disease subtypes. Acta Ophthalmol. 2019, 97, e1089–e1097. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, J.Y.; Chin, H.S.; Seo, K.Y.; Kim, T.I.; Jung, J.W. Assessment of the Tear Meniscus by Strip Meniscometry and Keratograph in Patients with Dry Eye Disease According to the Presence of Meibomian Gland Dysfunction. Cornea 2017, 36, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vanathi, M.; Kishore, A.; Gupta, N.; Tandon, R. Evaluation of strip meniscometry, tear meniscus height and depth in the diagnosis of dry eye disease in asian Indian eyes. Ocul. Surf. 2019, 17, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gong, L.; Lu, Y.; Jin, H.; Robitaille, M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjogren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012, 90, e359–e366. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Tabuchi, H.; Nakakura, S.; Nagasato, D.; Baba, H.; Kiuchi, Y. Usability and reproducibility of tear meniscus values generated via swept-source optical coherence tomography and the slit lamp with a graticule method. Int. Ophthalmol. 2018, 38, 679–686. [Google Scholar] [CrossRef]


| Age (Years) * | OSDI (Score) ** | Osmolarity (mOsm/L) * | Corneal Staining (Oxford Scheme) ** | FBUT (s) * | TMH-SL (mm) * | LLP (Guillon Scheme) ** | TMH-Tc (mm) * | ||
|---|---|---|---|---|---|---|---|---|---|
| Total Sample (n = 200) | Mean/Median | 42.84 | 25.00 | 318.40 | 1.00 | 7.76 | 0.186 | Close Meshwork | 0.173 |
| SD/IQR | 16.86 | 18.75–35.42 | 18.51 | 0.00–1.00 | 6.17 | 0.089 | Open Meshwork—Wave | 0.077 | |
| Minimum | 18.00 | 12.50 | 275.00 | 0.00 | 1.45 | 0.060 | Open Meshwork | 0.040 | |
| Maximum | 71.00 | 77.08 | 400.00 | 4.00 | 41.60 | 0.740 | Colour | 0.640 |
| Age (Years) * | OSDI (Score) ** | Osmolarity (mOsm/L) * | Corneal Staining (Oxford Scheme) ** | FBUT (s) * | TMH-SL (mm) * | LLP (Guillon Scheme) ** | TMH-Tc (mm) * | ||
|---|---|---|---|---|---|---|---|---|---|
| ADDE (n = 69) | Mean/Median | 46.14 | 25.00 | 319.83 | 1.00 | 8.55 | 0.140 | Wave | 0.143 |
| SD/IRQ | 14.95 | 18.75–39.58 | 16.51 | 0.00–1.50 | 6.17 | 0.037 | Wave-amorphous | 0.054 | |
| Minimum | 18.00 | 12.50 | 291.00 | 0.00 | 1.45 | 0.060 | Wave | 0.04 | |
| Maximum | 71.00 | 60.42 | 400.00 | 4.00 | 41.60 | 0.190 | Colour | 0.33 | |
| EDE (n = 66) | Mean/Media | 42.86 | 22.57 | 312.50 | 1.00 | 7.39 | 0.270 | Closed Meshwork | 0.228 |
| SD/IQR | 18.66 | 16.67–34.09 | 19.42 | 0.00–1.00 | 4.20 | 0.105 | Open Meshwork—Closed Meshwork | 0.092 | |
| Minimum | 18.00 | 12.50 | 275.00 | 0.00 | 1.83 | 0.200 | Open Meshwork | 0.080 | |
| Maximum | 71.00 | 77.08 | 400.00 | 4.00 | 22.75 | 0.740 | Closed Meshwork | 0.640 | |
| p | 0.260 ‡ | 0.142 † | 0.090 ‡ | 0.300 † | 0.300 ‡ | <0.001 ‡ | <0.001 † | <0.001 ‡ |
| Age (Years) * | OSDI (Score) ** | Osmolarity (mOsm/L) * | Corneal Staining (Oxford Scheme) ** | FBUT (s) * | TMH-SL (mm) * | LLP (Guillon Scheme) ** | TMH-Tc (mm) * | ||
|---|---|---|---|---|---|---|---|---|---|
| ADDE Mild-moderate | Mean/Median | 45.24 | 29.17 | 317.65 | 1.00 | 9.45 | 0.157 | Wave | 0.159 |
| SD/IQR | 15.16 | 18.75–37.50 | 13.35 | 0.00–1.00 | 8.68 | 0.022 | Wave—amorphous | 0.050 | |
| Minimum | 18.00 | 12.50 | 291.00 | 0.00 | 1.75 | 0.011 | Wave | 0.070 | |
| (n = 64) | Maximum | 68.00 | 60.42 | 368.00 | 3.00 | 14.60 | 0.200 | Colour | 0.330 |
| ADDE Moderate-severe (n = 15) | Mean/Median | 48.40 | 25.00 | 327.13 | 1.00 | 4.91 | 0.081 | Wave | 0.228 |
| SD/IQR | 14.45 | 20.00–41.67 | 23.81 | 0.00–2.00 | 3.01 | 0.023 | Wave-Colour | 0.092 | |
| Minimum | 20.00 | 12.50 | 303.00 | 0.00 | 1.45 | 0.060 | Wave | 0.080 | |
| Maximum | 71.00 | 60.42 | 400.00 | 4.00 | 10.45 | 0.100 | Colour | 0.640 | |
| p | 0.513 ‡ | 0.884 † | 0.052 ‡ | 0.056 † | 0.047 ‡ | <0.001 ‡ | 0.222 † | <0.001 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope. Life 2022, 12, 2007. https://doi.org/10.3390/life12122007
Sabucedo-Villamarin B, Pena-Verdeal H, Garcia-Queiruga J, Giraldez MJ, Garcia-Resua C, Yebra-Pimentel E. Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope. Life. 2022; 12(12):2007. https://doi.org/10.3390/life12122007
Chicago/Turabian StyleSabucedo-Villamarin, Belen, Hugo Pena-Verdeal, Jacobo Garcia-Queiruga, Maria Jesus Giraldez, Carlos Garcia-Resua, and Eva Yebra-Pimentel. 2022. "Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope" Life 12, no. 12: 2007. https://doi.org/10.3390/life12122007
APA StyleSabucedo-Villamarin, B., Pena-Verdeal, H., Garcia-Queiruga, J., Giraldez, M. J., Garcia-Resua, C., & Yebra-Pimentel, E. (2022). Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope. Life, 12(12), 2007. https://doi.org/10.3390/life12122007










