Contemporary Review of Multi-Modality Cardiac Imaging Evaluation of Infective Endocarditis
Abstract
1. Introduction
2. Clinical Perspectives
2.1. Presentation
2.2. Risk Factors
2.3. Microbiology
2.4. Management
2.5. Antibiotic Prophylaxis
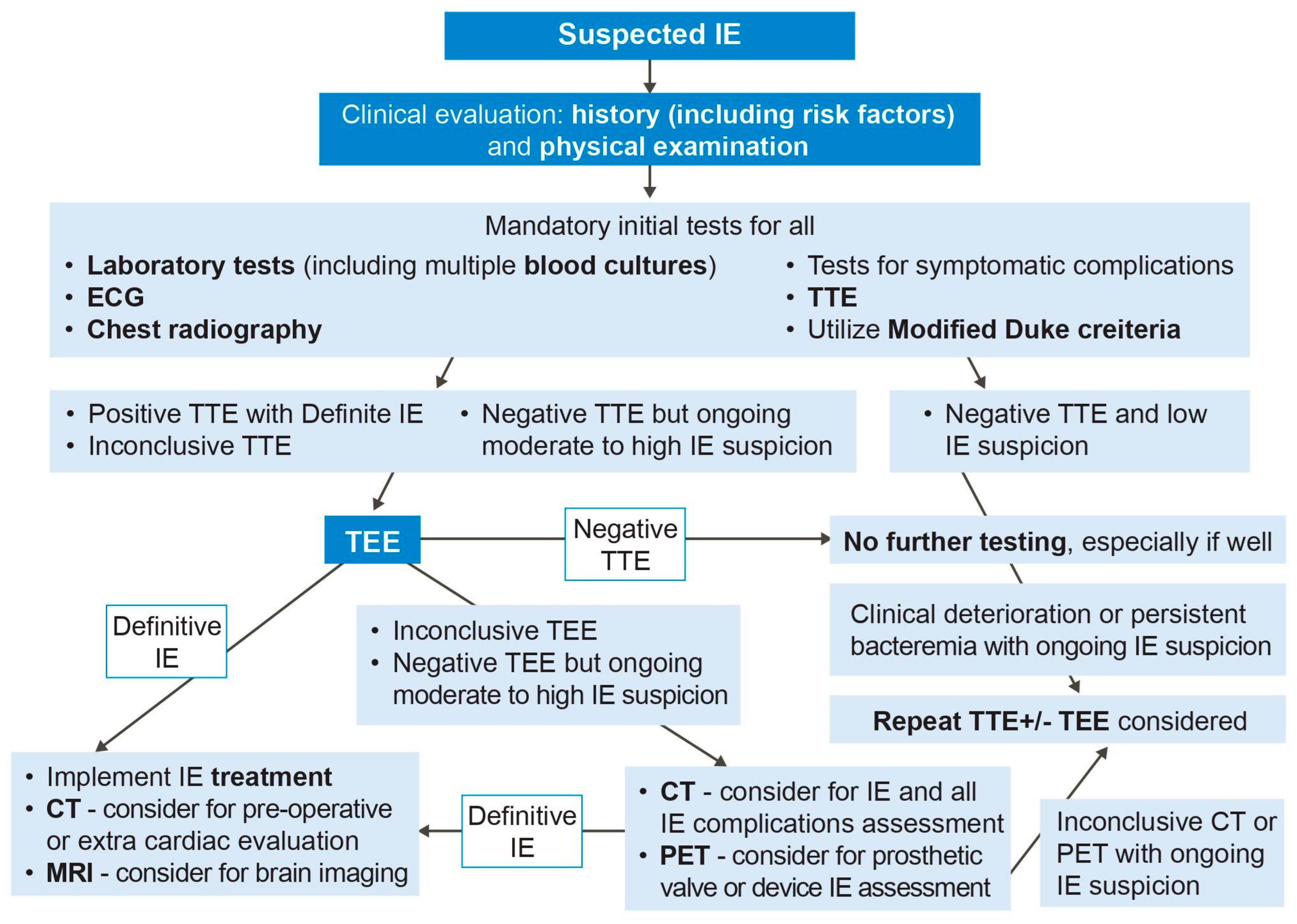
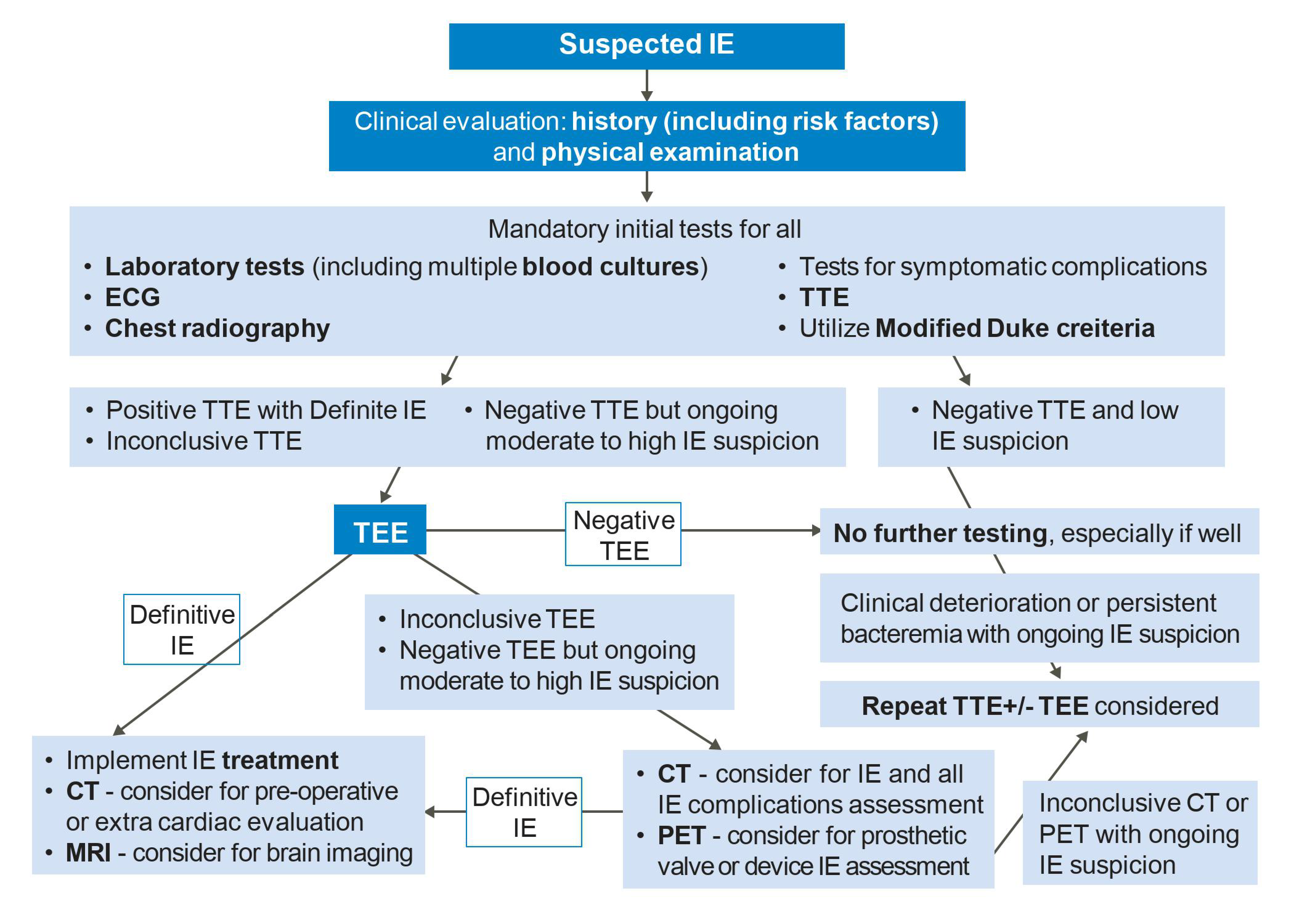
2.6. Endocarditis Features on Imaging
3. Echocardiography
3.1. Transthoracic Echocardiography
3.2. Transesophageal Echocardiography
3.3. Three-Dimensional Echocardiography
3.4. Comparisons of Echocardiography Modalities
3.5. Repeating Echocardiography
4. Cardiac Computed Tomography
4.1. CT Techniques
4.2. CT Evaluation of IE
4.3. Other Roles of CT in IE
5. Cardiac Magnetic Resonance Imaging
6. Nuclear Imaging
6.1. 18F-FDG PET/CT
6.2. WBC SPECT/CT
7. Future Directions of Multimodality Imaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, E.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Thomas, B.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Liesenborghs, L.; Meyers, S.; Lox, M.; Criel, M.; Claes, J.; Peetermans, M.; Trenson, S.; Velde, G.V.; Berghe, P.V.; Baatsen, P.; et al. Staphylococcus aureus endocarditis: Distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur. Heart J. 2019, 40, 3248–3259. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Hernández-Meneses, M.; Muñoz, P.; Martínez-Sellés, M.; Álvarez-Uria, A.; de Alarcón, A.; Gutiérrez-Carretero, E.; Goenaga, M.A.; Zarauza, M.J.; Falces, C.; et al. Characteristics and Outcome of Acute Heart Failure in Infective Endocarditis: Focus on Cardiogenic Shock. Clin. Infect. Dis. 2021, 73, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Siddique, M.S. Subacute Bacterial Endocarditis Prophylaxis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vincent, L.L.; Otto, C.M. Infective Endocarditis: Update on Epidemiology, Outcomes, and Management. Curr. Cardiol. Rep. 2018, 20, 86. [Google Scholar] [CrossRef]
- Kouijzer, J.J.P.; Noordermeer, D.J.; van Leeuwen, W.J.; Verkaik, N.J.; Lattwein, K.R. Native valve, prosthetic valve, and cardiac device-related infective endocarditis: A review and update on current innovative diagnostic and therapeutic strategies. Front. Cell Dev. Biol. 2022, 10, 995508. [Google Scholar] [CrossRef] [PubMed]
- Bin Abdulhak, A.A.; Qazi, A.H.; Tleyjeh, I.M. Workup and Management of Native and Prosthetic Valve Endocarditis. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 73. [Google Scholar] [CrossRef]
- Chambers, H.F.; Bayer, A.S. Native-Valve Infective Endocarditis. N. Engl. J. Med. 2020, 383, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Niebauer, J. Infective endocarditis. In Cardiology Explained; Remedica: London, UK, 2004. [Google Scholar]
- Fournier, P.E.; Thuny, F.; Richet, H.; Lepidi, H.; Casalta, J.P.; Arzouni, J.P.; Maurin, M.; Célard, M.; Mainardi, J.-L.; Caus, T.; et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: A prospective study of 819 new cases. Clin. Infect. Dis. 2010, 51, 131–140. [Google Scholar] [CrossRef]
- Raoult, D.; Casalta, J.P.; Richet, H.; Khan, M.; Bernit, E.; Rovery, C.; Branger, S.; Gouriet, F.; Imbert, G.; Bothello, E.; et al. Contribution of systematic serological testing in diagnosis of infective endocarditis. J. Clin. Microbiol. 2005, 43, 5238–5242. [Google Scholar] [CrossRef]
- Pretet, V.; Blondet, C.; Ruch, Y. Advantages of 18F-FDG PET/CT Imaging over Modified Duke Criteria and Clinical Presumption in Patients with Challenging Suspicion of Infective Endocarditis. Diagnostics 2021, 11, 720. [Google Scholar] [CrossRef]
- Horgan, S.J.; Mediratta, A.; Gillam, L.D. Cardiovascular Imaging in Infective Endocarditis: A Multimodality Approach. Circ. Cardiovasc. Imaging 2020, 13, e008956. [Google Scholar] [CrossRef]
- Erba, P.A.; Pizzi, M.N.; Roque, A.; Salaun, E.; Lancellotti, P.; Tornos, P.; Habib, G. Multimodality Imaging in Infective Endocarditis: An Imaging Team within the Endocarditis Team. Circulation 2019, 140, 1753–1765. [Google Scholar] [CrossRef]
- Hove, D.T.; Slart, R.; Sinha, B.; Glaudemans, A.; Budde, R.P.J. 18F-FDG PET/CT in Infective Endocarditis: Indications and Approaches for Standardization. Curr Cardiol. Rep. 2021, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Rouzet, F.; Brochet, E.; Duval, X. Cardiac Imaging of Infective Endocarditis, Echo and Beyond. Curr. Infect. Dis. Rep. 2017, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.-U.; Sicari, R.; Scientific Committee; Cosyns, B.; et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Jagen, M.A.; Tunick, P.A.; Kronzon, I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J. Am. Soc. Echocardiogr. 2003, 16, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.U.; Kort, S.; Mehran, R.; Schoenhagen, P.; Soman, P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2017, 70, 1647–1672. [Google Scholar] [PubMed]
- Sordelli, C.; Fele, N.; Mocerino, R.; Weisz, S.H.; Ascione, L.; Caso, P.; Carrozza, A.; Tascini, C.; De Vivo, S.; Severino, S. Infective Endocarditis: Echocardiographic Imaging and New Imaging Modalities. J. Cardiovasc. Echogr. 2019, 29, 149–155. [Google Scholar] [CrossRef]
- Pérez-García, C.N.; Olmos, C.; Islas, F.; Marcos-Alberca, P.; Pozo, E.; Ferrera, C.; García-Arribas, D.; De Isla, L.P.; Vilacosta, I. Morphological characterization of vegetation by real-time three-dimensional transesophageal echocardiography in infective endocarditis: Prognostic impact. Echocardiography 2019, 36, 742–751. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, M.A.; Cortés, M.; García-Robles, J.A.; de Diego, J.J.G.; Perez-David, E.; García, E. Utility of real-time three-dimensional transesophageal echocardiography in evaluating the success of percutaneous transcatheter closure of mitral paravalvular leaks. J. Am. Soc. Echocardiogr. 2010, 23, 26–32. [Google Scholar] [CrossRef]
- Berdejo, J.; Shibayama, K.; Harada, K.; Tanaka, J.; Mihara, H.; Gurudevan, S.V.; Siegel, R.J.; Shiota, T. Evaluation of vegetation size and its relationship with embolism in infective endocarditis: A real-time 3-dimensional transesophageal echocardiography study. Circ. Cardiovasc. Imaging 2014, 7, 149–154. [Google Scholar] [CrossRef]
- Shapiro, S.M.; Young, E.; De Guzman, S.; Ward, J.; Chiu, C.Y.; Ginzton, L.E.; Bayer, A.S. Transesophageal echocardiography in diagnosis of infective endocarditis. Chest 1994, 105, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Rohmann, S.; Drexler, M.; Mohr-Kahaly, S.; Gerharz, C.D.; Iversen, S.; Oelert, H.; Meyer, J. Improved diagnostic value of echocardiography in patients with infective endocarditis by transoesophageal approach. A prospective study. Eur. Heart J. 1988, 9, 43–53. [Google Scholar] [CrossRef]
- Shively, B.K.; Gurule, F.T.; Roldan, C.A.; Leggett, J.H.; Schiller, N.B. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. J. Am. Coll. Cardiol. 1991, 18, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.G.; Mügge, A.; Martin, R.P.; Lindert, O.; Hausmann, D.; Nonnast-Daniel, B.; Laas, J.; Lichtlen, P.R. Improvement in the diagnosis of abscesses associated with endocarditis by transesophageal echocardiography. N. Engl. J. Med. 1991, 324, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Karalis, D.G.; Bansal, R.C.; Hauck, A.J.; Ross, J.J., Jr.; Applegate, P.M.; Jutzy, K.R.; Mintz, G.S.; Chandrasekaran, K. Transesophageal echocardiographic recognition of subaortic complications in aortic valve endocarditis. Clinical and surgical implications. Circulation 1992, 86, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Bin Saeedan, M.; Chan, N.; Obuchowski, N.A.; Shrestha, N.; Xu, B.; Unai, S.; Cremer, P.; Grimm, R.A.; Griffin, B.P.; et al. Complementary Diagnostic and Prognostic Contributions of Cardiac Computed Tomography for Infective Endocarditis Surgery. Circ. Cardiovasc. Imaging 2020, 13, e011126. [Google Scholar] [CrossRef]
- Jain, V.; Wang, T.K.M.; Bansal, A.; Farwati, M.; Gad, M.; Montane, B.; Kaur, S.; Bolen, M.A.; Grimm, R.; Griffin, B.; et al. Diagnostic performance of cardiac computed tomography versus transesophageal echocardiography in infective endocarditis: A contemporary comparative meta-analysis. J. Cardiovasc. Comput. Tomogr. 2021, 15, 313–321. [Google Scholar] [CrossRef]
- Vilacosta, I.; Graupner, C.; San Román, J.A.; Sarriá, C.; Ronderos, R.; Fernández, C.; Mancini, L.; Sanz, O.; Sanmartín, L.; Stoermann, W. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J. Am. Coll. Cardiol. 2002, 39, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Steckelberg, J.M.; Murphy, J.G.; Ballard, D.; Bailey, K.; Tajik, A.J.; Taliercio, C.P.; Giuliani, E.R.; Wilson, W.R. Emboli in infective endocarditis: The prognostic value of echocardiography. Ann. Intern. Med. 1991, 114, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Di Salvo, G.; Belliard, O.; Avierinos, J.F.; Pergola, V.; Rosenberg, V.; Casalta, J.-P.; Gouvernet, J.; Derumeaux, G.; Iarussi, D.; et al. Risk of embolism and death in infective endocarditis: Prognostic value of echocardiography: A prospective multicenter study. Circulation 2005, 112, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Habib, G.; Pergola, V.; Avierinos, J.F.; Philip, E.; Casalta, J.P.; Vailloud, J.-M.; Derumeaux, G.; Gouvernet, J.; Ambrosi, P.; et al. Echocardiography predicts embolic events in infective endocarditis. J. Am. Coll. Cardiol. 2001, 37, 1069–1076. [Google Scholar] [CrossRef]
- Hubert, S.; Thuny, F.; Resseguier, N.; Giorgi, R.; Tribouilloy, C.; Le Dolley, Y.; Casalta, J.-P.; Riberi, A.; Chevalier, F.; Rusinaru, D.; et al. Prediction of symptomatic embolism in infective endocarditis: Construction and validation of a risk calculator in a multicenter cohort. J. Am. Coll. Cardiol. 2013, 62, 1384–1392. [Google Scholar] [CrossRef]
- García-Cabrera, E.; Fernández-Hidalgo, N.; Almirante, B.; Ivanova-Georgieva, R.; Noureddine, M.; Plata, A.; Lomas, J.M.; Gálvez-Acebal, J.; Hidalgo-Tenorio, C.; Ruíz-Morales, J.; et al. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation 2013, 127, 2272–2284. [Google Scholar] [CrossRef]
- Bai, A.D.; Steinberg, M.; Showler, A.; Burry, L.; Bhatia, R.S.; Tomlinson, G.A.; Bell, C.M.; Morris, A.M. Diagnostic Accuracy of Transthoracic Echocardiography for Infective Endocarditis Findings Using Transesophageal Echocardiography as the Reference Standard: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 639–646.e8. [Google Scholar] [CrossRef]
- Habets, J.; Tanis, W.; Reitsma, J.B.; van den Brink, R.B.; Mali, W.P.; Chamuleau, S.A.J.; Budde, R.P.J. Are novel non-invasive imaging techniques needed in patients with suspected prosthetic heart valve endocarditis? A systematic review and meta-analysis. Eur. Radiol. 2015, 25, 2125–2133. [Google Scholar] [CrossRef]
- Bonzi, M.; Cernuschi, G.; Solbiati, M.; Giusti, G.; Montano, N.; Ceriani, E. Diagnostic accuracy of transthoracic echocardiography to identify native valve infective endocarditis: A systematic review and meta-analysis. Intern. Emerg. Med. 2018, 13, 937–946. [Google Scholar] [CrossRef]
- Lo Presti, S.; Elajami, T.K.; Zmaili, M.; Reyaldeen, R.; Xu, B. Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis: A contemporary narrative review. World J. Cardiol. 2021, 13, 254–270. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Sánchez-Nadales, A.; Igbinomwanhia, E.; Cremer, P.; Griffin, B.; Xu, B. Diagnosis of Infective Endocarditis by Subtype Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: A Contemporary Meta-Analysis. Circ. Cardiovasc. Imaging 2020, 13, e010600. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Eudailey, K.; Lewey, J.; Hahn, R.T.; George, I. Aggressive infective endocarditis and the importance of early repeat echocardiographic imaging. J. Thorac. Cardiovasc. Surg. 2014, 147, e26–e28. [Google Scholar] [CrossRef] [PubMed]
- Saeedan, M.B.; Wang, T.K.M. Role of Cardiac CT in Infective Endocarditis: Current Evidence, Opportunities, and Challenges. Radiol. Cardiothorac. Imaging 2021, 3, e200378. [Google Scholar] [CrossRef] [PubMed]
- Kamani, C.H.; Allenbach, G.; Jreige, M.; Pavon, A.G.; Meyer, M.; Testart, N.; Firsova, M.; Vieira, V.F.; Boughdad, S.; LaLonde, M.N.; et al. Diagnostic Performance of 18F-FDG PET/CT in Native Valve Endocarditis: Systematic Review and Bivariate Meta-Analysis. Diagnostics 2020, 10, 754. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovas. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Mgbojikwe, N.; Jones, S.R.; Leucker, T.M.; Brotman, D.J. Infective endocarditis: Beyond the usual tests. Clevel. Clin. J. Med. 2019, 86, 559–567. [Google Scholar] [CrossRef]
- Rajiah, P.; Moore, A.; Saboo, S. Multimodality Imaging of Complications of Cardiac Valve Surgeries. Radiographics 2019, 39, 932–956. [Google Scholar] [CrossRef] [PubMed]
- Dursun, M.; Yılmaz, S.; Yılmaz, E.; Yılmaz, R.; Onur, İ.; Oflaz, H.; Dindar, A. The utility of cardiac MRI in diagnosis of infective endocarditis: Preliminary results. Diagn. Interv. Radiol. 2015, 21, 28–33. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Griffin, B.; Cremer, P.; Shrestha, N.; Gordon, S.; Pettersson, G.; Desai, M. Diagnostic Utility of CT and MRI for Mycotic Aneurysms: A Meta-Analysis. Am. J. Roentgenol. 2020, 215, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Mikail, N.; Hyafil, F. Nuclear Imaging in Infective Endocarditis. Pharmaceuticals 2021, 15, 14. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, Y.-J.; Kim, S.-H.; Sun, B.J.; Kim, D.-H.; Yun, S.-C.; Song, J.-M.; Choo, S.J.; Chung, C.-H.; Song, J.-K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Glaudemans, A.; Touw, D.J.; van Melle, J.P.; Willems, T.P.; Maass, A.H.; Natour, E.; Prakken, N.; Borra, R.; van Geel, P.P.; et al. Diagnostic value of imaging in infective endocarditis: A systematic review. Lancet Infect. Dis. 2017, 17, e1–e14. [Google Scholar] [CrossRef]
- Sag, S.J.M.; Menhart, K.; Grosse, J.; Hitzenbichler, F.; Hanses, F.; Mohr, A.; Salzberger, B.; Zerdzitzki, M.R.; Hilker, M.; Rupprecht, L.; et al. Diagnostic value of FDG PET/CT imaging in patients with surgically managed infective endocarditis: Results of a retrospective analysis at a tertiary center. J. Nucl. Cardiol. 2022, 29, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.P.; Gouriet, F.; Riberi, A.; Avierinos, J.F.; et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: Increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J. Am. Coll. Cardiol. 2013, 61, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.; Kendi, A.T.; Ajmal, S.; Farid, S.; O’Horo, J.C.; Chareonthaitawee, P.; Baddour, L.M.; Sohail, M.R. Meta-analysis of 18F-FDG PET/CT in the diagnosis of infective endocarditis. J. Nucl. Cardiol. 2019, 26, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Juneau, D.; Golfam, M.; Hazra, S.; Erthal, F.; Zuckier, L.S.; Bernick, J.; Wells, G.A.; Beanlands, R.S.; Chow, B.J. Molecular Imaging for the diagnosis of infective endocarditis: A systematic literature review and meta-analysis. Int. J. Cardiol. 2018, 253, 183–188. [Google Scholar] [CrossRef]
- Boursier, C.; Duval, X.; Mahida, B.; Hoen, B.; Goehringer, F.; Selton-Suty, C.; Chevalier, E.; Roch, V.; Lamiral, Z.; Bourdon, A.; et al. Hypermetabolism of the spleen or bone marrow is an additional albeit indirect sign of infective endocarditis at FDG-PET. Int. Nucl. Cardiol. 2021, 28, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Naya, M. Usefulness of 18F-fluorodeoxyglucose positron emission tomography/computed tomography angiography in a patient with blood culture-negative prosthetic valve endocarditis complicated with perivalvular abscess: A case report. Eur. Heart J.-Case Rep. 2019, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- San, S.; Ravis, E.; Tessonier, L.; Philip, M.; Cammilleri, S.; Lavagna, F.; Norscini, G.; Arregle, F.; Martel, H.; Oliver, L.; et al. Prognostic Value of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Infective Endocarditis. J. Am. Coll. Cardiol. 2019, 74, 1031–1040. [Google Scholar] [CrossRef]
- Jerónimo, A.; Olmos, C.; Vilacosta, I.; Ortega-Candil, A.; Rodríguez-Rey, C.; Pérez-Castejón, M.J.; Fernández-Pérez, C.; Pérez-García, C.N.; García-Arribas, D.; Ferrera, C.; et al. Accuracy of 18F-FDG PET/CT in patients with the suspicion of cardiac implantable electronic device infections. J. Nucl. Cardiol. 2022, 29, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Tlili, G.; Amraoui, S.; Mesguich, C.; Rivière, A.; Bordachar, P.; Hindié, E.; Bordenave, L. High performances of 18F-fluorodeoxyglucose PET-CT in cardiac implantable device infections: A study of 40 patients. J. Nucl. Cardiol. 2015, 22, 787–798. [Google Scholar] [CrossRef]
- Granados, U.; Fuster, D.; Pericas, J.M.; Llopis, J.L.; Ninot, S.; Quintana, E.; Almela, M.; Paré, C.; Tolosana, J.M.; Falces, C.; et al. Diagnostic Accuracy of 18F-FDG PET/CT in Infective Endocarditis and Implantable Cardiac Electronic Device Infection: A Cross-Sectional Study. J. Nucl. Med. 2016, 57, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Amraoui, S.; Tlili, G.; Sohal, M.; Berte, B.; Hindié, E.; Ritter, P.; Ploux, S.; Denis, A.; Derval, N.; Rinaldi, C.A.; et al. Contribution of PET Imaging to the Diagnosis of Septic Embolism in Patients with Pacing Lead Endocarditis. JACC Cardiovasc. Imaging 2016, 9, 283–290. [Google Scholar] [CrossRef]
- Hove, D.T.; Treglia, G.; Slart, R.; Damman, K.; Wouthuyzen-Bakker, M.; Postma, D.F.; Gheysens, O.; Borra, R.J.H.; Mecozzi, G.; van Geel, P.P.; et al. The value of 18F-FDG PET/CT for the diagnosis of device-related infections in patients with a left ventricular assist device: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Lwin, M.T.; Tsoi, V. Blood culture negative infective endocarditis in adult congenital heart disease patients with prosthetic grafts: A case series. Eur. Heart J.-Case Rep. 2021, 5, ytab106. [Google Scholar] [CrossRef]
- Mikail, N.; Benali, K.; Dossier, A.; Bouleti, C.; Hyafil, F.; Le Guludec, D.; Rouzet, F.; Ou, P. Additional Diagnostic Value of Combined Angio-Computed Tomography and 18F-Fluorodeoxyglucose Positron Emission Tomography in Infectious Aortitis. JACC Cardiovasc. Imaging 2018, 11 Pt 2, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Lauri, C.; Iezzi, R.; Rossi, M.; Tinelli, G. Imaging Modalities for the Diagnosis of Vascular Graft Infections: A Consensus Paper amongst Different Specialists. J. Clin. Med. 2020, 9, 1510. [Google Scholar] [CrossRef] [PubMed]
- Folmer, E.I.R.; von Meijenfeldt, G.C.I.; Scholten, R.S.T.R.O.G.; van der Laan, M.J.; Glaudemans, A.; Slart, R.; Iezzi, R.; Prakken, M.H.J.; Debus, E.S.; Honig, S.; et al. A systematic review and meta-analysis of 18F-fluoro-d-deoxyglucose positron emission tomography interpretation methods in vascular graft and endograft infection. J. Vasc. Surg. 2020, 72, 2174–2185.e2. [Google Scholar] [CrossRef] [PubMed]
- Mikail, N.; Benali, K.; Mahida, B.; Vigne, J.; Hyafil, F.; Rouzet, F.; Le Guludec, D. 18F-FDG-PET/CT Imaging to Diagnose Septic Emboli and Mycotic Aneurysms in Patients with Endocarditis and Cardiac Device Infections. Curr. Cardiol. Rep. 2018, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, A.M.; van Aarnhem, E.E.; Budde, R.P. Effect of antibiotics on FDG-PET/CT imaging of prosthetic heart valve endocarditis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1223. [Google Scholar] [CrossRef]
- Swart, L.E.; Gomes, A.; Scholtens, A.M.; Sinha, B.; Tanis, W.; Lam, M.G.; van der Vlugt, M.J.; Streukens, S.A.F.; Aarntzen, E.H.; Bucerius, J.; et al. Improving the Diagnostic Performance of 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography in Prosthetic Heart Valve Endocarditis. Circulation 2018, 138, 1412–1427. [Google Scholar] [CrossRef]
- Calais, J.; Touati, A.; Grall, N.; Laouénan, C.; Benali, K.; Mahida, B.; Vigne, J.; Hyafil, F.; Iung, B.; Duval, X.; et al. Diagnostic Impact of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and White Blood Cell SPECT/Computed Tomography in Patients with Suspected Cardiac Implantable Electronic Device Chronic Infection. Circ. Cardiovasc. Imaging 2019, 12, e007188. [Google Scholar] [CrossRef]
- Slart, R.; Glaudemans, A.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural recommendations of cardiac PET/CT imaging: Standardization in inflammatory-, infective-, infiltrative-, and innervation- (4Is) related cardiovascular diseases: A joint collaboration of the EACVI and the EANM. Eur. Heart J. Cardiovas. Imaging 2020, 21, 1320–1330. [Google Scholar] [CrossRef]
- Tanis, W.; Scholtens, A.; Habets, J.; van den Brink, R.B.; van Herwerden, L.A.; Chamuleau, S.A.; Budde, R.P. CT angiography and 18F-FDG-PET fusion imaging for prosthetic heart valve endocarditis. JACC Cardiovasc. Imaging 2013, 6, 1008–1013. [Google Scholar] [CrossRef]
- Wahadat, A.R.; Tanis, W.; Swart, L.E.; Scholtens, A.; Krestin, G.P.; van Mieghem, N.M.D.A.; Schurink, C.A.M.; van der Spoel, T.I.G.; Brink, F.S.V.D.; Vossenberg, T.; et al. Added value of 18F-FDG-PET/CT and cardiac CTA in suspected transcatheter aortic valve endocarditis. J. Nucl. Cardiol. 2021, 28, 2072–2082. [Google Scholar] [CrossRef]
- Pizzi, M.N.; Dos-Subirà, L.; Roque, A.; Fernández-Hidalgo, N.; Cuéllar-Calabria, H.; Domènech, A.P.; Gonzàlez-Alujas, M.T.; Subirana-Domènech, M.; Miranda-Barrio, B.; Ferreira-González, I.; et al. 18F-FDG-PET/CT angiography in the diagnosis of infective endocarditis and cardiac device infection in adult patients with congenital heart disease and prosthetic material. Int. J. Cardiol. 2017, 248, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.N.; Roque, A.; Cuéllar-Calabria, H.; Fernández-Hidalgo, N.; Ferreira-González, I.; González-Alujas, M.T.; Igual-Barceló, A.; Garcia-Dorado, D.; Almirante, B.; Castell-Conesa, J.; et al. 18F-FDG-PET/CTA of Prosthetic Cardiac Valves and Valve-Tube Grafts: Infective versus Inflammatory Patterns. JACC Cardiovasc. Imaging 2016, 9, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.N.; Roque, A.; Fernández-Hidalgo, N.; Cuéllar-Calabria, H.; Ferreira-González, I.; Gonzàlez-Alujas, M.T.; Oristrell, G.; Gracia-Sánchez, L.; González, J.J.; Rodríguez-Palomares, J.; et al. Improving the Diagnosis of Infective Endocarditis in Prosthetic Valves and Intracardiac Devices with 18F-Fluordeoxyglucose Positron Emission Tomography/Computed Tomography Angiography: Initial Results at an Infective Endocarditis Referral Center. Circulation 2015, 132, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Conti, U.; Lazzeri, E.; Sollini, M.; Doria, R.; De Tommasi, S.M.; Bandera, F.; Tascini, C.; Menichetti, F.; Dierckx, R.A.J.O.; et al. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J. Nucl. Med. 2012, 53, 1235–1243. [Google Scholar] [CrossRef]
- Hyafil, F.; Rouzet, F.; Lepage, L.; Benali, K.; Raffoul, R.; Duval, X.; Hvass, U.; Iung, B.; Nataf, P.; Lebtahi, R.; et al. Role of radiolabelled leucocyte scintigraphy in patients with a suspicion of prosthetic valve endocarditis and inconclusive echocardiography. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 586–594. [Google Scholar] [CrossRef]
- Schenone, A.L.; Hutt, E.; Cremer, P.; Jaber, W.A. Utility of nuclear cardiovascular imaging in the cardiac intensive care unit. J. Nucl. Cardiol. 2021, 1–17. [Google Scholar] [CrossRef]
- Holcman, K.; Małecka, B.; Rubiś, P.; Ząbek, A.; Szot, W.; Boczar, K.; Leśniak-Sobelga, G.; Hlawaty, M.; Wiśniowska-Śmiałek, S.; Stępień, A.; et al. The role of 99mTc-HMPAO-labelled white blood cell scintigraphy in the diagnosis of cardiac device-related infective endocarditis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1022–1030. [Google Scholar] [CrossRef]
- de Vaugelade, C.; Mesguich, C.; Nubret, K.; Camou, F.; Greib, C.; Dournes, G.; Debordeaux, F.; Hindie, E.; Barandon, L.; Tlili, G.; et al. Infections in patients using ventricular-assist devices: Comparison of the diagnostic performance of 18F-FDG PET/CT scan and leucocyte-labeled scintigraphy. J. Nucl. Cardiol. 2019, 26, 42–55. [Google Scholar] [CrossRef]
- Holcman, K.; Rubiś, P.; Ząbek, A.; Ćmiel, B.; Szot, W.; Boczar, K.; Wiśniowska-Śmiałek, S.; Stępień, A.; Małecka, B.; Podolec, P.; et al. The Prognostic Value of 99mTc-HMPAO-Labeled Leucocyte SPECT/CT in Cardiac Device-Related Infective Endocarditis. JACC Cardiovasc. Imaging 2020, 13, 1739–1751. [Google Scholar] [CrossRef]
- Kircher, M.; Lapa, C. Novel Noninvasive Nuclear Medicine Imaging Techniques for Cardiac Inflammation. Curr. Cardiovasc. Imaging Rep. 2017, 10, 6. [Google Scholar] [CrossRef]


| Major Criteria | |
| I. Positive blood cultures for IE | |
| a. | Two separate blood cultures with typical microorganism consistent with infective endocarditis, in the absence of a primary focus (viridans streptococci, Streptococcus bovis, Staphylococcus aureus, HACEK group or a community-acquired enterococci). |
| b. | Microorganisms consistent with IE from persistently positive blood cultures defined as follows: at least 2 positive cultures of blood samples drawn >12 h apart; or all of 3 or a majority of ≥4 separate cultures of blood (with first and last sample drawn at least 1 h apart). |
| c. | Single positive blood culture for Coxiella burnetii or antiphase I IgG antibody titer > 1:800. |
| II. Evidence of endocardial involvement | |
| Positive echocardiogram for IE, i.e., vegetation, abscess, new partial dehiscence of prosthetic valve, new valvular regurgitation (worsening, changing, or preexisting murmur is not sufficient). | |
| Minor Criteria | |
| 1 | Predisposition, predisposing heart condition, or history of drug injection. |
| 2 | Fever, temperature >38 °C or >100.4 °F. |
| 3 | Vascular phenomena, major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, and Janeway’s lesions, petechiae, or purpura |
| 4 | immunologic phenomena such as: glomerulonephritis, Osler’s nodes, Roth’s spots, and rheumatoid factors. |
| 5 | Microbiological evidence not fitting major criteria: positive blood culture but does not meet a major criterion as noted above or serological evidence of active infection with organism consistent with IE. |
| Definite IE | 2 major criteria or, 1 major + 3 minor criteria or, 5 minor criteria |
| Possible IE | 1 major criterion + 1 minor criterion or, 3 minor criteria |
| Diagnostic Findings | Echo Findings | MDCT Characteristics |
|---|---|---|
| Vegetation | Intracardiac oscillating or non-oscillating mass seen attached to valvular surface, chamber walls, or any intracardiac device. | Hypodense, homogenous, irregular with low-to-intermediate attenuation mass attached to the endocardial surface, native or prosthetic valve, or any other cardiac implantable device. |
| Abscess | Irregular shaped, perivalvular, non-homogenous mass. | Perivalvular collection of a low attenuated area with peripheral contrast enhancement. |
| Pseudoaneurysm | Pulsatile perivalvular space which is echo free with communication with cardiac chambers. | Contrast-filled cavity in the perivalvular area with a direct communication with cardiac chamber. |
| Leaflet perforation | Defect in the leaflets with flow through the defect. | Defect in continuity of the valve leaflets. |
| Prosthetic valve dehiscence | Paravalvular regurgitation with or without rocking motion of the prosthetic device. | Presence of contrast material in the tissue defect between the annulus and the misaligned prosthetic valve. |
| Fistula | Connection between two adjacent intracardiac cavities. | Contrast material-filled tract between two cardiac chambers. |
| Aneurysm | Leaflets with saccular outpouchings with embarrassment of the leaflet curvature. | Leaflet outpouching. |
| Paravalvular leak | Abnormal Doppler flow in the perivalvular region. | Distinctive presence of the contrast agent column in the periannular region. |
| Imaging Modality | Strengths | Limitations |
|---|---|---|
| Transthoracic echocardiography (TTE) | Widely available, first-line modality, safe with no radiation exposure, portable, high temporal resolution, assesses hemodynamics, valve function, endocarditis features, and chamber function. | Operator and patient dependent on imaging windows, creates artifacts, lower sensitivity than more advanced modalities in identifying most endocarditis features including small vegetations, periannular complications, prosthetic valve, and device-related endocarditis. |
| Transesophageal echocardiography (TEE) | Portable, higher sensitivity than TTE for most endocarditis features, preferred modality for vegetations, valve perforation, prosthetic valve dehiscence and paravalvular leak, identifies fistula, high spatial and temporal resolution. | Invasive imaging modality, may still have artifact and lower sensitivity for some prosthetic valves and cardiac devices, avoid in contraindications such as prior gastroesophageal disease and surgery, active bleeding, patient intolerance. |
| Cardiac CT | Short study, excellent for detection of perivalvular complications (pseudoaneurysm, abscess, and fistula) in all types of endocarditis, can also identify other endocarditis features, detect extracardiac complications, high spatial resolution, use for pre-operative workup, and assesses coronaries and major vessels. | Non-portable, lower sensitivity than echocardiography for smaller vegetations, perforations, and paravalvular leaks. Inferior temporal resolution to echocardiography, radiation exposure, iodinated contrast administration (avoid in chronic renal impairment, especially when creatinine clearance below 30). |
| Cardiac Magnetic Resonance | Can identify endocarditis complications in some scenarios, such as using its high sensitivity for cerebral lesions. Reference standard for chamber quantification and can also quantify valve disease and shunts (such as for fistula). | Long study, non-portable, can cause claustrophobia, cost, non-compatible devices, lower temporal resolution than echocardiography, only for stable patients who can lie flat and follow instructions. |
| 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) | Improved sensitivity for prosthetic valve and device-related endocarditis in some scenarios. | Non-portable, low sensitivity for native valve endocarditis, no functional cine imaging, radiation exposure, special pre-test preparation, cost, false-positive results within 3 months after cardiac surgery, false-negative results in patients treated with antimicrobials. |
| All Cases | PVIE | ||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| Vegetations | TTE | 61% [40] | 94% [40] | 29% [41] | 100% [41] |
| TEE [33] | 96% | 83% | 89% | 74% | |
| CCT [33] | 85% | 84% | 78% | 94% | |
| Perivalvular complications | TTE | 28% [30] | 98.6% [30] | 36% [41] | 93% [41] |
| TEE | 70% [33] | 96% [33] | 86% [41] | 98% [41] | |
| CCT [33] | 88% | 93% | |||
| Perforation | TTE | ||||
| TEE [33] | 79% | 93% | |||
| CCT [33] | 48% | 93% | |||
| Dehiscence | TTE | 11% [41] | 100% [41] | ||
| TEE | 67% [33] | 99% [33] | 94% [41] | 97% [41] | |
| CCT [33] | 46% | 97% | |||
| All cases of endocarditis | TTE | 71% [42] | 80% [42] | 33% [41] | 100% [41] |
| TEE | 90% [32] | 96% [32] | 86% [41] | 95% [41] | |
| CCT | ~93% [43] | 95% [43] | |||
| PET [44] | 74% | 88% | 86% | 84% | |
| PET-NVIE | 31% | 98% | |||
| PET-CDRIE | 72% | 83% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arockiam, A.D.; Agrawal, A.; El Dahdah, J.; Honnekeri, B.; Kafil, T.S.; Halablab, S.; Griffin, B.P.; Wang, T.K.M. Contemporary Review of Multi-Modality Cardiac Imaging Evaluation of Infective Endocarditis. Life 2023, 13, 639. https://doi.org/10.3390/life13030639
Arockiam AD, Agrawal A, El Dahdah J, Honnekeri B, Kafil TS, Halablab S, Griffin BP, Wang TKM. Contemporary Review of Multi-Modality Cardiac Imaging Evaluation of Infective Endocarditis. Life. 2023; 13(3):639. https://doi.org/10.3390/life13030639
Chicago/Turabian StyleArockiam, Aro Daniela, Ankit Agrawal, Joseph El Dahdah, Bianca Honnekeri, Tahir S. Kafil, Saleem Halablab, Brian P. Griffin, and Tom Kai Ming Wang. 2023. "Contemporary Review of Multi-Modality Cardiac Imaging Evaluation of Infective Endocarditis" Life 13, no. 3: 639. https://doi.org/10.3390/life13030639
APA StyleArockiam, A. D., Agrawal, A., El Dahdah, J., Honnekeri, B., Kafil, T. S., Halablab, S., Griffin, B. P., & Wang, T. K. M. (2023). Contemporary Review of Multi-Modality Cardiac Imaging Evaluation of Infective Endocarditis. Life, 13(3), 639. https://doi.org/10.3390/life13030639






