1. Introduction
Breast carcinoma with neuroendocrine features consists of a group of diseases with high heterogeneity. It was reported that the incidence rate of breast carcinoma with neuroendocrine features ranged from 0.1% to 20% [
1,
2,
3]. Breast carcinoma with neuroendocrine features included neuroendocrine neoplasm (NEN) of the breast and invasive breast cancer (IBC) with neuroendocrine differentiation. According to the latest World Health Organization (WHO) classification of breast tumors, NEN was divided into neuroendocrine tumor (NET) and neuroendocrine carcinoma (NEC) based on the degree of differentiation [
3]. IBC with neuroendocrine differentiation was classed into breast carcinoma of no special type and breast carcinoma of special types, such as solid papillary carcinoma and the hypercellular subtype of mucinous carcinoma.
Since the third edition of the WHO classification of breast tumors was published, the definition and classification of this disease had changed greatly in different editions. As a result, there have been controversies surrounding the definition and classification of breast carcinoma with neuroendocrine features. The diagnostic criteria for subjects included in existing studies were not identical. As well, the research results were not completely consistent or were even contradictory [
4,
5]. Further, due to the rarity of the disease, few studies had been conducted, and those which have were mainly case reports [
6,
7,
8]. At present, the treatment strategy of IBC of no special type is used directly in breast carcinoma with neuroendocrine features. The general practice guidelines for breast carcinoma with neuroendocrine features are still not formed. The TNM stage of breast carcinoma with neuroendocrine features was defined by the eighth version of the America joint committee on cancer staging systems [
9]. According to the guidelines of the Chinese Society of Clinical Oncology published in 2020 [
10], the minimum positive threshold of estrogen receptor (ER), progesterone receptor (PR), and Ki-67 are 1%, 1%, and 14%, respectively, and human epidermal growth factor receptor 2 (Her-2) (3+) or ISH positivity meant Her-2 positivity. Breast carcinoma with neuroendocrine features was divided into Luminal A (ER/PR positive, Her-2 negative with low Ki-67 index) disease, Luminal B (ER/PR positive, Her-2 negative with high Ki-67 index, or ER/PR positive, Her-2 positive) disease, Her-2 positive (ER and PR negative, Her-2 positive) disease, and Triple-negative (ER, PR, and Her-2 negative) disease according to molecular subtyping. To investigate the clinicopathological features and prognosis of this disease under the fifth edition of the WHO classification of breast tumors, we designed this study.
2. Materials and Methods
2.1. Study Groups
The data of 87 patients with breast carcinoma with neuroendocrine features treated in the First Medical Center, Chinese PLA General Hospital from January 2001 to January 2022 were retrospectively collected. Patients with breast carcinoma derived from other organs were excluded. Pregnant patients and patients who were breastfeeding were excluded as well. There were no patients without definite pathological diagnosis or without complete medical records. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Chinese PLA General Hospital (NO.: S2022-746). Individual consent for this retrospective analysis was waived.
2.2. Study Variables
General information on the patients was collected, such as age at diagnosis, gender, laterality, smoking history, drinking history, body mass index (BMI), family history, menopause status, hypertension, diabetes, hyperlipidemia, T stage, Her-2 status, Ki-67, ER, PR, molecular typing, vessel carcinoma embolus, N stage, skin or chest wall invasion, distant metastasis, and stage.
Unique clinical features of the patients were analyzed, including clinical symptoms and history of thyroid diseases. Pathological characteristics of the patients were also described, such as detailed classification, expression of neuroendocrine markers, and ductal carcinoma in situ composition. The treatment strategy of the patients, such as neoadjuvant chemotherapy, surgery, and adjuvant therapy, was discussed. All patients enrolled in this study were followed up. The 5-year overall survival (OS), 5-year progression-free survival (PFS), and 5-year disease-specific survival (DSS) of patients in the study group were described. Finally, the factors related to 5-year PFS were analyzed.
2.3. Statistical Analysis
All statistical analyses of this study were performed using Stata Statistical Software version 15.1 (StataCorp LLC, College Station, TX, USA). The measurement data were described by median (inter-quartile range, IQR). Frequency was used to show the counting data. Comparison of counting data between two groups was conducted by Pearson chi-square test. Kruskal–Wallis H test was used to examine multiple comparisons of ranked counting data between groups. Kaplan–Meier method was used in survival analysis, and Log-rank test was used to compare different survival curves. Univariate and multivariate analysis were performed using Cox model. A two-tailed p < 0.05 was considered statistically significant, and all confidence intervals (CI) were expressed at 95% confidence level.
4. Discussion
Breast carcinoma with neuroendocrine features is a group of heterogeneous tumors. Its definitions and diagnostic criteria have varied with the revisions of the WHO classification of breast tumors. As a result, the results of some studies on the clinicopathological characteristics of this disease have been controversial for a long time. Breast carcinoma with neuroendocrine features is a group of tumors that exhibit morphological features similar to those of neuroendocrine tumors of the gastrointestinal tract and lung [
11]. Before 2003, there were no criteria for the definition and diagnosis of this disease. With the further study of breast carcinoma, the consensus on breast carcinoma with neuroendocrine features has gradually been formed. The third version of the WHO criteria of breast tumors defined it as >50% tumor cells with neuroendocrine differentiation confirmed by immunohistochemical staining [
12]. From then on, it was recognized as single breast carcinoma entities named “neuroendocrine breast carcinomas”. In 2012, the WHO classification used the category of “carcinoma with neuroendocrine features” and described this disease as tumors expressing neuroendocrine markers to any extent [
13]. It included well-differentiated NET, poorly differentiated NEC, and carcinoma with neuroendocrine differentiation. In this version, small cell neuroendocrine carcinoma (SCNEC) was brought into the NEC group. The current WHO classification adopted the term “NEN”, including well-differentiated (NET) and poorly-differentiated (NEC) tumors with predominant neuroendocrine differentiation [
3]. The main distinction between the latest classification and the past version is that carcinoma with neuroendocrine differentiation without distinct or uniform enough neuroendocrine histological features and neuroendocrine marker expression is no longer classified as NET or NEC. In this version, large cell neuroendocrine carcinoma (LCNEC) was classified into NEC as well. All the criteria mentioned above are used for the classification of primary breast carcinoma with neuroendocrine features; however, before a diagnosis of primary NEN is made, the possibility of metastasis from other organs should be carefully ruled out. Immunohistochemistry staining is conducive to distinction between NEN derived from other organs from invasive mammary carcinoma with neuroendocrine features [
14]. This study only discussed primary breast carcinoma with neuroendocrine features.
The results of the previous studies in different periods were different or even contradictory. Previous studies found that most patients were 50 years old or older [
15,
16], and that the clinical symptoms of this disease were mainly bloody nipple discharge [
16], which was consistent with the results of this study. NEN can be divided into functional and non-functional tumors according to whether the tumor has hormone activity, and most NENs are non-functional. Functional NEN produces excessive hormones, leading to clinical symptoms such as diarrhea and facial flushing. Non-functional NENs do not produce enough hormones to cause these symptoms [
17]. Paraneoplastic endocrine syndrome may occur in breast cancer with or without neuroendocrine differentiation [
18]. There were few reports on paraneoplastic endocrine syndrome related to breast neuroendocrine tumors. One case with hyperprolactinemia was reported in the previous literature [
19]. Studies have illustrated that patients often had ER/PR positive and Her-2 negative tumors [
5,
20], supporting this study. Another study found that neuroendocrine differentiation was more common in Luminal B breast cancer [
21], which is consistent with the results of this study. However, among them, Her-2 positive patients were rare, only occasionally seen in case reports [
22]. Research showed that breast carcinoma with neuroendocrine features was highly aggressive, with a high rate of local recurrence and distant metastasis, and a poor prognosis [
5]. A study has also shown that its general clinical characteristics are not different from other breast cancers, and its biological behavior was not aggressive; on the contrary, it tended to be an independent good-prognosis subgroup [
20].
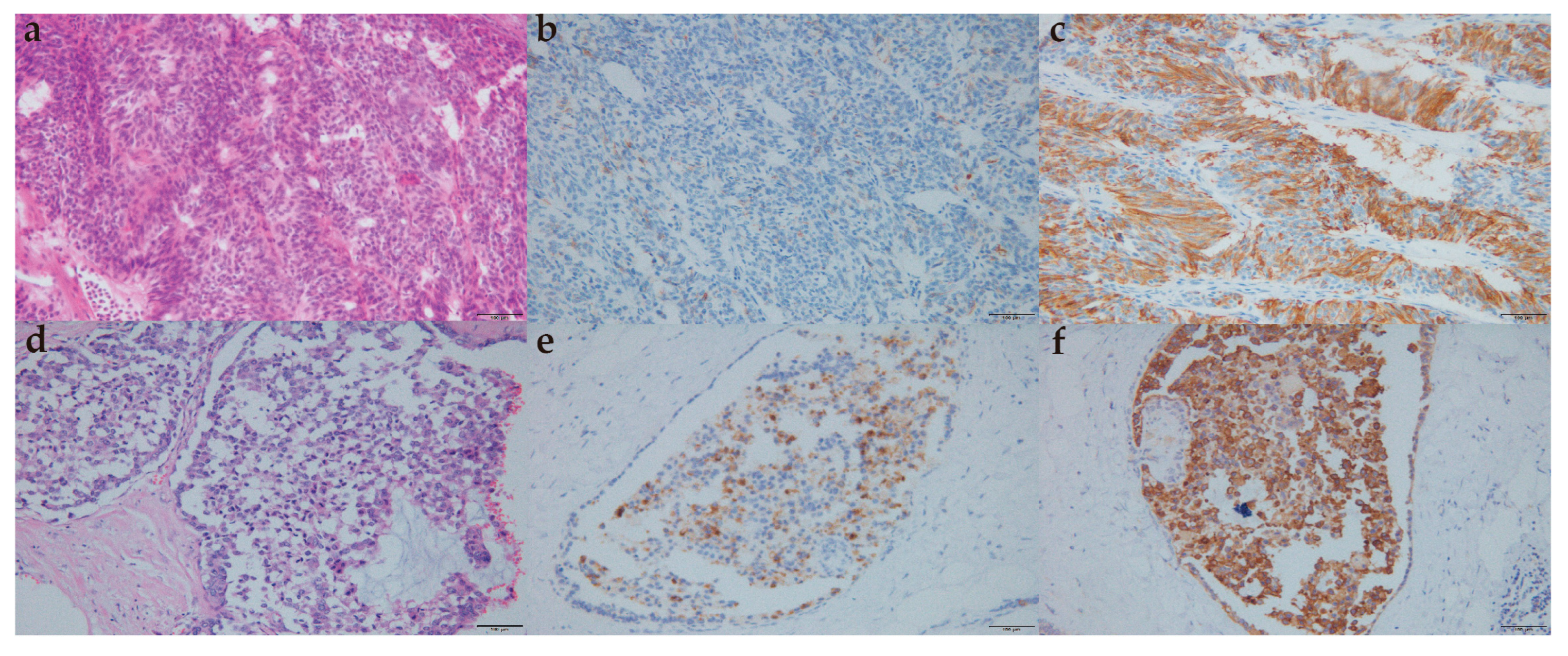
Morphologically, the typical features of the lung/gastrointestinal tract NET, such as ribbons, cords, and rosettes, are not prominent in the breast NET; histological and immunohistochemical features of breast NEC are sometimes difficult to distinguish from lung NEC features [
23]. CgA, Syn, NSE, and CD56 were neuroendocrine differentiation markers for breast carcinoma with neuroendocrine features [
4]. It has been reported that only 23% of patients were detected with Syn+ and CgA+ at the same time [
15]. The expression level of neuroendocrine markers in tumor tissues of patients in this study was also not high. There were significant differences in cytological characteristics between focal and diffuse neuroendocrine differentiated breast carcinomas [
24]. However, when breast cancer of no special type with focal neuroendocrine differentiation was regarded as a separate entity, focal neuroendocrine differentiation had no obvious significance for its prognosis [
15,
25]. In this study, there was no difference between NEN and IBC with neuroendocrine differentiation in five-year OS, five-year DSS, and five-year PFS.
SCNEC was classified into NEC in 2012 and LCNEC was brought into NEC in 2019. A study indicated that approximately half of these patients had triple-negative breast cancer, with a 61.6% five-year DSS rate and 47.7% five-year OS rate [
26]. Chemotherapy, surgery, and stage were predictive factors of prognosis. In this study, there was only one case of pure SCNEC and pure LCNEC, respectively, accounting for a relatively low proportion. The special type of breast cancer with neuroendocrine differentiation was rare. According to one study, half of the invasive solid papillary carcinomas were accompanied by neuroendocrine differentiation [
7]. In this study, there were four cases of a special type of breast cancer with neuroendocrine differentiation, including two cases of solid papillary carcinoma, one case of invasive papillary carcinoma, and one case of type B mucinous carcinoma.
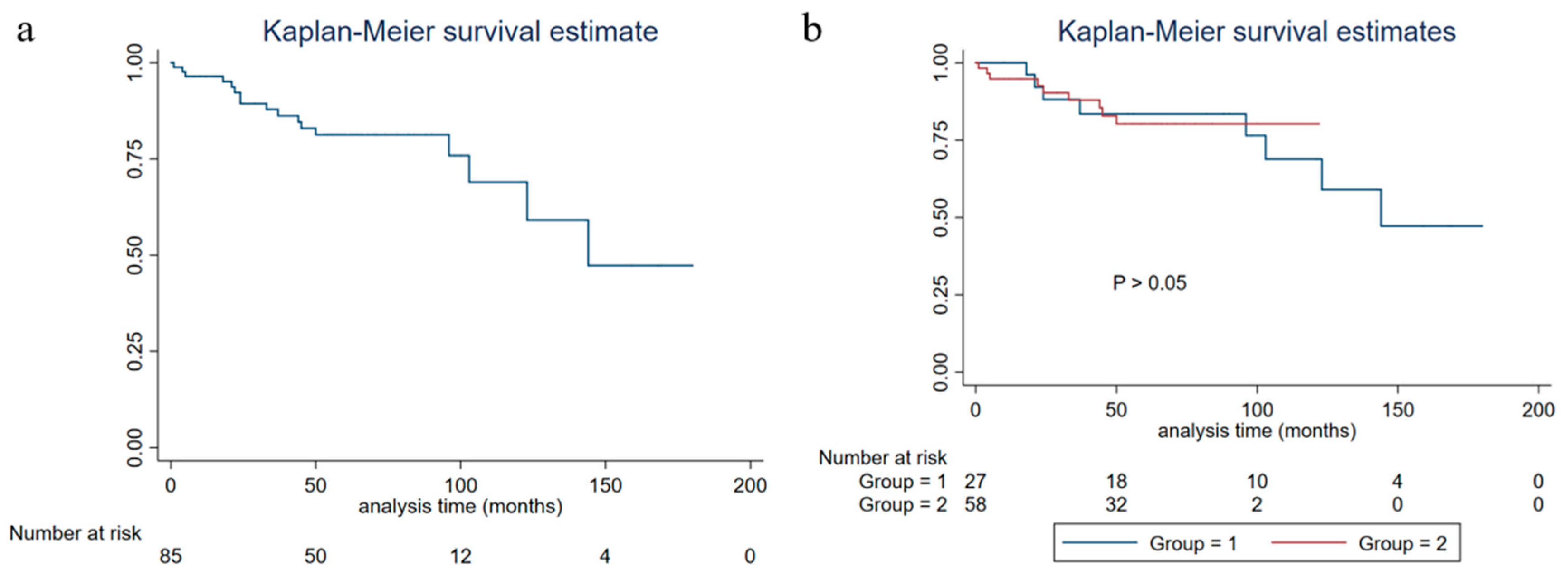
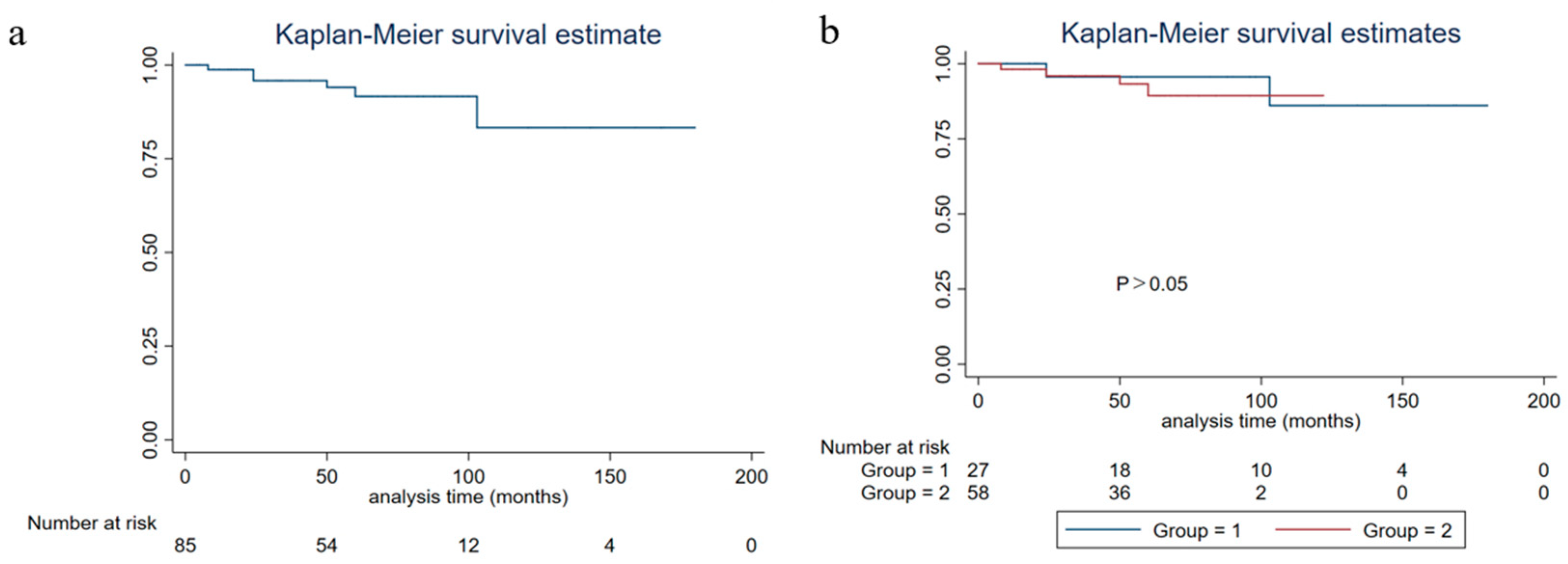
A study reported that the five-year OS and disease-free survival rates of HR positive/Her-2 negative breast cancer were 93.0% and 92.6%, respectively [
27]. The PFS of breast carcinoma with neuroendocrine features was lower than that of the same molecular typing of breast cancer of no special type [
28], which was consistent with the results of this study. The overall five-year PFS of patients in this study was 82.37% (95%CI: 0.7084–0.8966), five-year DSS was 91.53% (95%CI: 0.8022–0.9651), and five-year OS was 90.25% (95%CI: 0.7901–0.9564). The study of neoadjuvant therapy for this disease has been limited. In this study, there were seven patients receiving neoadjuvant chemotherapy, and none of them reached a pathological complete response. A study found that endocrine therapy or radiotherapy might improve the prognosis [
5]. It was suggested that HR positive NEN patients receive endocrine therapy, especially those with SCNEC with recurrence and metastasis [
29]. Endocrine therapy was also found to be effective for liver metastasis of breast cancer with neuroendocrine differentiation [
6]. However, surgery was still the main treatment method, and the effect of chemotherapy on prognosis was still uncertain [
16].
This study also analyzed the related factors of PFS. A study found that the prognostic factors of NEN of the breast were similar to those of gastrointestinal tract tumors [
30], among which lymph node metastasis was an adverse factor of OS [
5]. A previous study found that histological grade, pathological stage, ER status, and HER2 status were independent prognostic indicators of OS and disease-free survival [
31]. The results of this study showed that diabetes and stage IV disease were related to the PFS of breast carcinoma with neuroendocrine features patients. The influence of diabetes on the PFS of this disease may be related to higher BMI, and there is a lack of relevant research at present.
Limitations
This study was a retrospective study, and the sample size was relatively insufficient. We failed to compare this disease with other types of breast cancer because of lacking a control group. In addition, this study did not describe its imaging characteristics. Because of the rarity of this disease, there was a gap between the length of follow-up time of the patients.