A Narrative Review of Point of Care Ultrasound Assessment of the Optic Nerve in Emergency Medicine
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
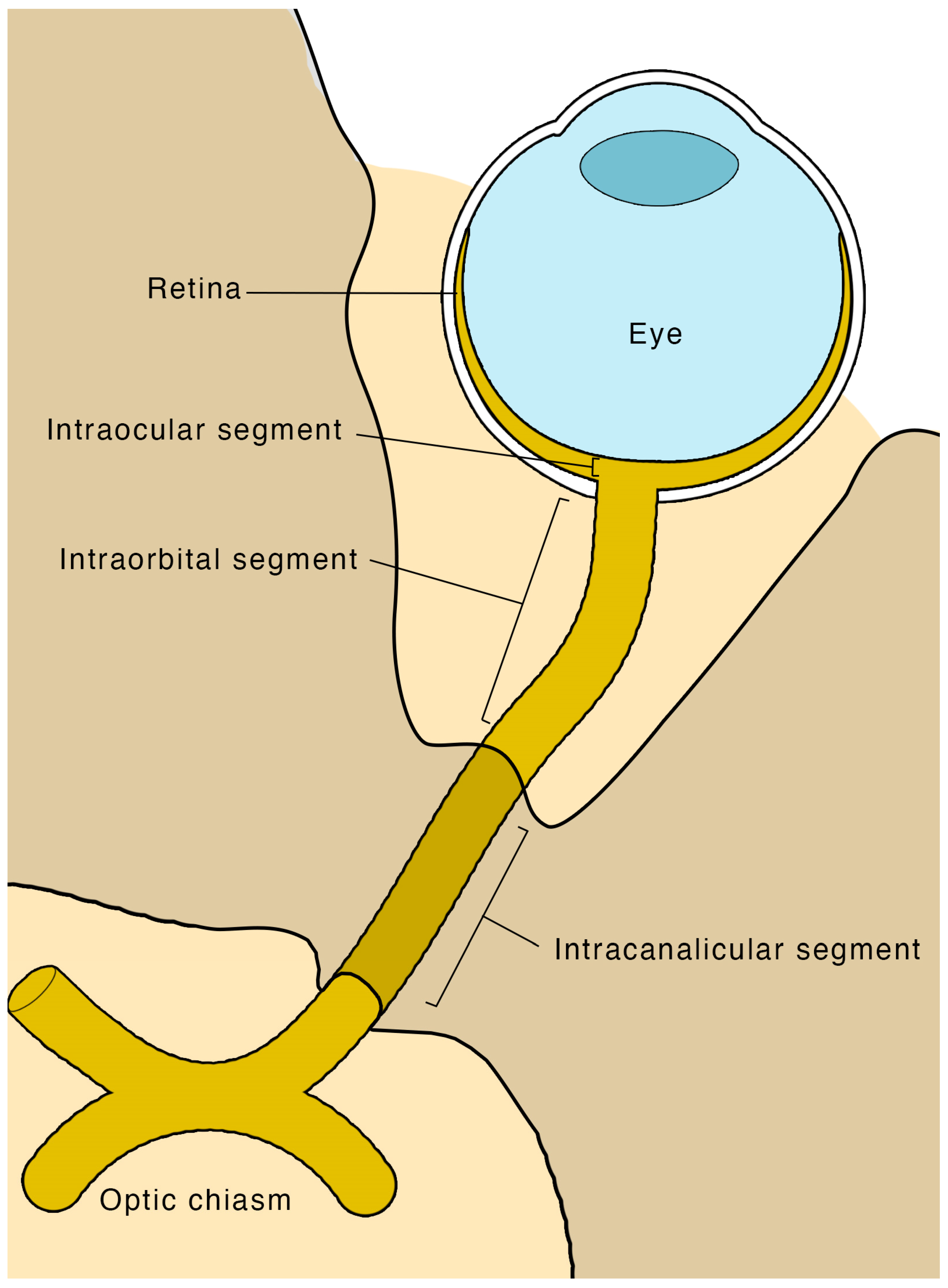
4.1. Anatomy and Physiology
4.2. Indications and Contraindications
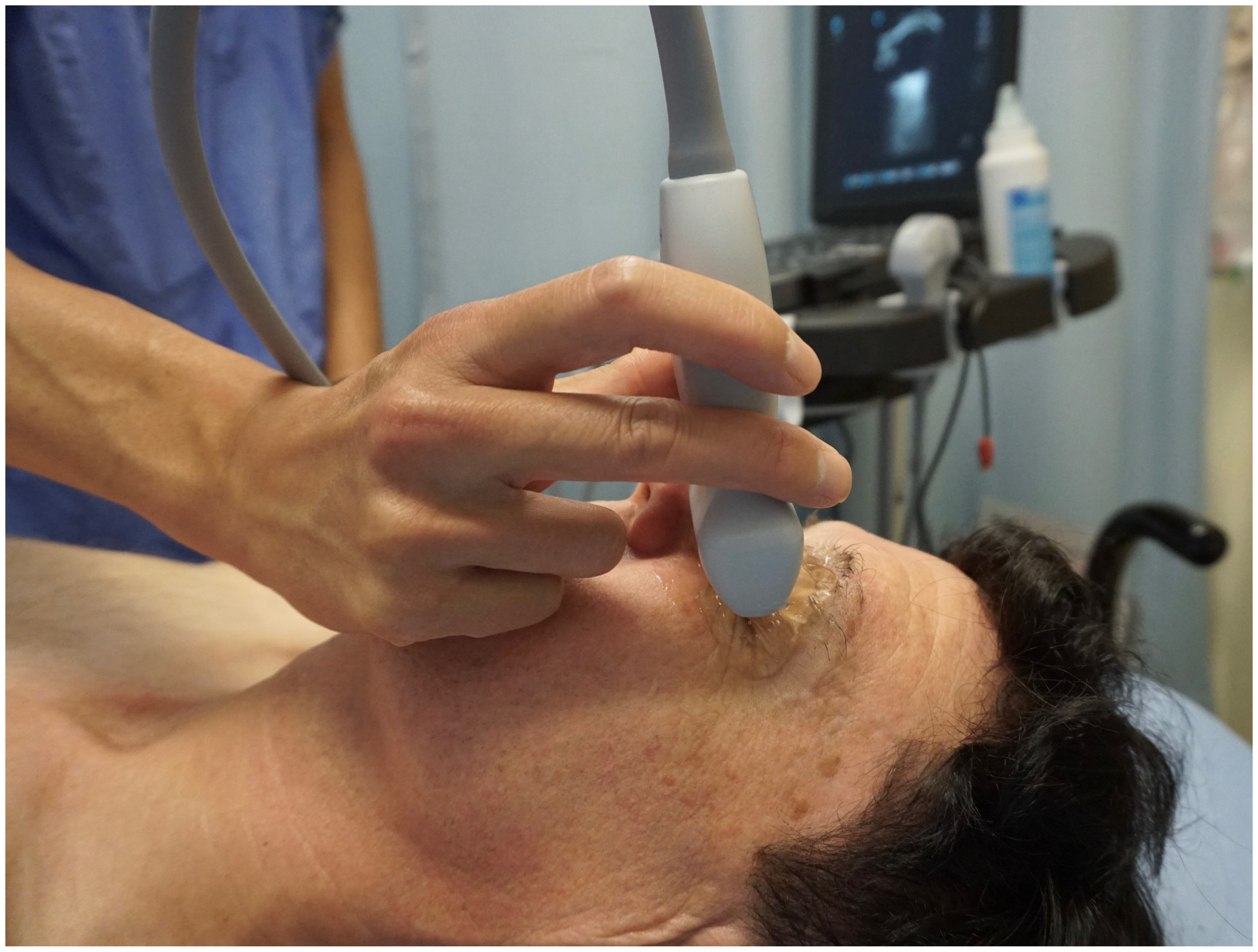
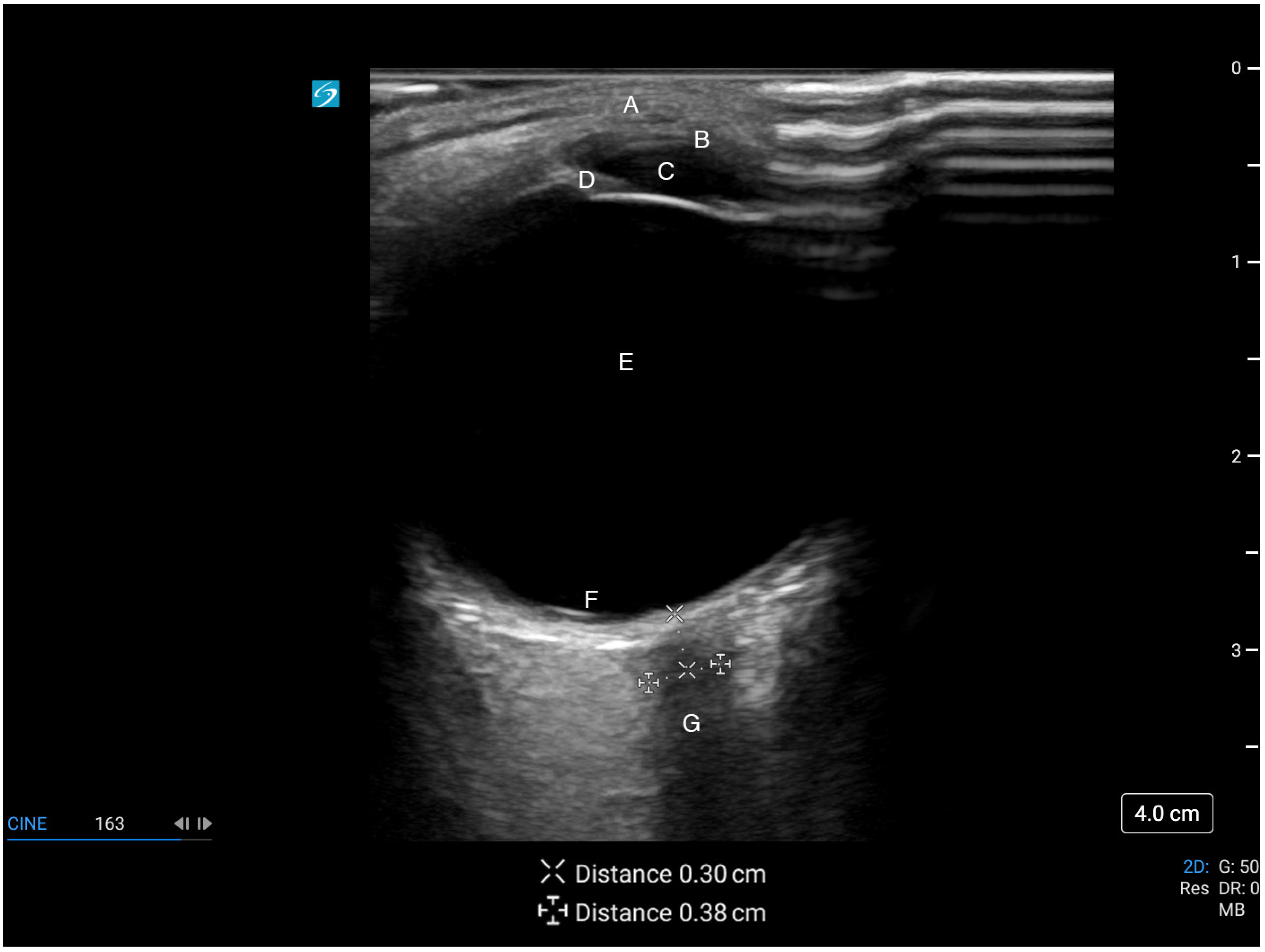
4.3. Scanning Technique and Sonographic Anatomy
4.4. Clinical Applications: Increased Intracranial Pressure
4.5. Clinical Applications: Idiopathic Intracranial Hypertension
4.6. Clinical Applications: Optic Neuritis
4.7. Clinical Applications: Acute Mountain Sickness
4.8. Clinical Applications: Pediatrics
4.9. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jimenez Restrepo, J.N.; León, O.J.; Quevedo Florez, L.A. Ocular ultrasonography: A useful instrument in patients with trauma brain injury in emergency service. Emerg. Med. Int. 2019, 2019, 9215853. [Google Scholar] [CrossRef] [PubMed]
- Lochner, P.; Czosnyka, M.; Naldi, A.; Lyros, E.; Pelosi, P.; Mathur, S.; Fassbender, K.; Robba, C. Optic nerve sheath diameter: Present and future perspectives for neurologists and critical care physicians. Neurol. Sci. 2019, 40, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Kilker, B.A.; Holst, J.M.; Hoffmann, B. Bedside ocular ultrasound in the emergency department. Eur. J. Emerg. Med. 2014, 21, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, S.Y.; Hong, D.Y.; Sung, B.Y.; Lee, S.; Paik, J.H.; Jung, H.M. Comparison of ultrasonography and computed tomography for measuring optic nerve sheath diameter for the detection of elevated intracranial pressure. Clin. Neurol. Neurosurg. 2021, 204, 106609. [Google Scholar] [CrossRef]
- Hanafi, M.G.; Verki, M.M.; Parei, S.N. Ultrasonic assessment of optic nerve sheath to detect increased intracranial pressure. J. Med. Ultrasound 2019, 27, 104–106. [Google Scholar] [CrossRef]
- Ohle, R.; McIsaac, S.M.; Woo, M.Y.; Perry, J.J. Sonography of the optic nerve sheath diameter for detection of raised intracranial pressure compared to computed tomography: A systematic review and meta-analysis. J. Ultrasound Med. 2015, 34, 1285–1294. [Google Scholar] [CrossRef]
- Robba, C.; Santori, G.; Czosnyka, M.; Corradi, F.; Bragazzi, N.; Padayachy, L.; Taccone, F.S.; Citerio, G. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2018, 44, 1284–1294. [Google Scholar] [CrossRef]
- Aletreby, W.; Alharthy, A.; Brindley, P.G.; Kutsogiannis, D.J.; Faqihi, F.; Alzayer, W.; Balhahmar, A.; Soliman, I.; Hamido, H.; Alqahtani, S.A.; et al. Optic nerve sheath diameter ultrasound for raised intracranial pressure: A literature review and meta-analysis of its diagnostic accuracy. J. Ultrasound Med. 2022, 41, 585–595. [Google Scholar] [CrossRef]
- Koziarz, A.; Sne, N.; Kegel, F.; Nath, S.; Badhiwala, J.H.; Nassiri, F.; Mansouri, A.; Yang, K.; Zhou, Q.; Rice, T.; et al. Bedside optic nerve ultrasonography for diagnosing increased intracranial pressure. Ann. Intern. Med. 2019, 171, 896–905. [Google Scholar] [CrossRef]
- Kim, S.E.; Hong, E.P.; Kim, H.C.; Lee, S.U.; Jeon, J.P. Ultrasonographic optic nerve sheath diameter to detect increased intracranial pressure in adults: A meta-analysis. Acta Radiol. 2019, 60, 221–229. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Rochwerg, B.; Taljaard, M.; Kyeremanteng, K.; English, S.W.; Sekhon, M.S.; Griesdale, D.E.G.; Dowlatshahi, D.; et al. Diagnosis of elevated intracranial pressure in critically ill adults: Systematic review and meta-analysis. BMJ 2019, 366, l4225. [Google Scholar] [CrossRef] [PubMed]
- Dağdelen, K.; Ekici, M. Measuring optic nerve sheath diameter using ultrasonography in patients with idiopathic intracranial hypertension. Arq. Neuropsiquiatr. 2022, 80, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Kishk, N.A.; Ebraheim, A.M.; Ashour, A.S.; Badr, N.M.; Eshra, M.A. Optic nerve sonographic examination to predict raised intracranial pressure in idiopathic intracranial hypertension: The cut-off points. Neuroradiol. J. 2018, 31, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Del Saz-Saucedo, P.; Redondo-González, O.; Mateu-Mateu, Á.; Huertas-Arroyo, R.; García-Ruiz, R.; Botia-Paniagua, E. Sonographic assessment of the optic nerve sheath diameter in the diagnosis of idiopathic intracranial hypertension. J. Neurol. Sci. 2016, 361, 122–127. [Google Scholar] [CrossRef]
- Ebraheim, A.M.; Mourad, H.S.; Kishk, N.A.; Badr Eldin, N.; Saad, A.A. Sonographic assessment of optic nerve and ophthalmic vessels in patients with idiopathic intracranial hypertension. Neurol. Res. 2018, 40, 728–735. [Google Scholar] [CrossRef]
- Jeub, M.; Schlapakow, E.; Ratz, M.; Kindler, C.; Schievelkamp, A.H.; Wabbels, B.; Kornblum, C. Sonographic assessment of the optic nerve and the central retinal artery in idiopathic intracranial hypertension. J. Clin. Neurosci. 2020, 72, 292–297. [Google Scholar] [CrossRef]
- Lochner, P.; Cantello, R.; Brigo, F.; Coppo, L.; Nardone, R.; Tezzon, F.; Raymkulova, O.; Strigaro, G.; Comi, C.; Leone, M.A. Transorbital sonography in acute optic neuritis: A case-control study. Am. J. Neuroradiol. 2014, 35, 2371–2375. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kim, Y.H.; Baek, S.H.; Son, M.H.; Lee, J.H.; Kim, B.J. Transorbital ultrasonography in acute optic neuritis: Can it be a supportive diagnostic tool? Mult. Scler. Relat. Disord. 2019, 31, 54–58. [Google Scholar] [CrossRef]
- Fagenholz, P.J.; Gutman, J.A.; Murray, A.F.; Noble, V.E.; Camargo, C.A.; Harris, N.S. Optic nerve sheath diameter correlates with the presence and severity of acute mountain sickness: Evidence for increased intracranial pressure. J. Appl. Physiol. 2009, 106, 1207–1211. [Google Scholar] [CrossRef]
- Sutherland, A.I.; Morris, D.S.; Owen, C.G.; Bron, A.J.; Roach, R.C. Optic nerve sheath diameter, intracranial pressure and acute mountain sickness on Mount Everest: A longitudinal cohort study. Br. J. Sport. Med. 2008, 42, 183–188. [Google Scholar] [CrossRef]
- Lawley, J.S.; Oliver, S.J.; Mullins, P.; Morris, D.; Junglee, N.A.; Jelleyman, C.; Macdonald, J.H. Optic nerve sheath diameter is not related to high altitude headache: A randomized controlled trial. High Alt. Med. Biol. 2012, 13, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Keyes, L.E.; Paterson, R.; Boatright, D.; Browne, V.; Leadbetter, G.; Hackett, P. Optic nerve sheath diameter and acute mountain sickness. Wilderness Environ. Med. 2013, 24, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Strapazzon, G.; Brugger, H.; Cappello, T.D.; Procter, E.; Hofer, G.; Lochner, P. Factors associated with optic nerve sheath diameter during exposure to hypobaric hypoxia. Neurology 2014, 82, 1914–1918. [Google Scholar] [CrossRef]
- Kanaan, N.C.; Lipman, G.S.; Constance, B.B.; Holck, P.S.; Preuss, J.F.; Williams, S.R. Optic nerve sheath diameter increase on ascent to high altitude: Correlation with acute mountain sickness. J. Ultrasound Med. 2015, 34, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, V.; Tawfik, D.; Tan, Y.J.; Dunbar, T.; Haileselassie, B.; Su, E. Ultrasonographic optic nerve sheath diameter measurement to detect intracranial hypertension in children with neurological injury: A systematic review. Pediatr. Crit. Care Med. 2020, 21, E858–E868. [Google Scholar] [CrossRef] [PubMed]
- Şık, N.; Ulusoy, E.; Çitlenbik, H.; Öztürk, A.; Er, A.; Yılmaz, D.; Duman, M. The role of sonographic optic nerve sheath diameter measurements in pediatric head trauma. J. Ultrasound 2022, 25, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Padayachy, L.C.; Padayachy, V.; Galal, U.; Pollock, T.; Fieggen, A.G. The relationship between transorbital ultrasound measurement of the optic nerve sheath diameter (ONSD) and invasively measured ICP in children.: Part II: Age-related ONSD cut-off values and patency of the anterior fontanelle. Child’s Nerv. Syst. 2016, 32, 1779–1785. [Google Scholar] [CrossRef]
- Lin, S.D.; Kahne, K.R.; El Sherif, A.; Mennitt, K.; Kessler, D.; Ward, M.J.; Platt, S.L. The use of ultrasound-measured optic nerve sheath diameter to predict ventriculoperitoneal shunt failure in children. Pediatr. Emerg. Care 2019, 35, 268–272. [Google Scholar] [CrossRef]
- Hall, M.K.; Spiro, D.M.; Sabbaj, A.; Moore, C.L.; Hopkins, K.L.; Meckler, G.D. Bedside optic nerve sheath diameter ultrasound for the evaluation of suspected pediatric ventriculoperitoneal shunt failure in the emergency department. Child’s Nerv. Syst. 2013, 29, 2275–2280. [Google Scholar] [CrossRef]
- Bowling, B. Kanski’s Clinical Ophthalmology, 8th ed.; Saunders Ltd.: London, UK, 2015. [Google Scholar]
- Soldatos, T.; Chatzimichail, K.; Papathanasiou, M.; Gouliamos, A. Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg. Med. J. 2009, 26, 630–634. [Google Scholar] [CrossRef]
- Stevens, R.R.F.; Gommer, E.D.; Aries, M.J.H.; Ertl, M.; Mess, W.H.; Huberts, W.; Delhaas, T. Optic nerve sheath diameter assessment by neurosonology: A review of methodologic discrepancies. J. Neuroimaging 2021, 31, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Cannata, G.; Pezzato, S.; Esposito, S.; Moscatelli, A. Optic nerve sheath diameter ultrasound: A non-invasive approach to evaluate increased intracranial pressure in critically ill pediatric patients. Diagnostics 2022, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.; Fauci, A.; Kasper, D.; Hauser, S.; Jameson, J.; Loscalzo, J. Harrisons Manual of Medicine, 18th ed.; McGraw-Hill Professional: New York, NY, USA, 2012. [Google Scholar]
- Liu, D.; Kahn, M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am. J. Ophthalmol. 1993, 116, 548–556. [Google Scholar] [CrossRef]
- Hansen, H.; Helmke, K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg. Radiol. Anat. 1996, 18, 323–328. [Google Scholar] [CrossRef]
- Hansen, H.C.; Helmke, K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J. Neurosurg. 1997, 87, 34–40. [Google Scholar] [CrossRef]
- Jennings, J.B.; Oliva, C.; Joyce, M.; Vitto, M.J.; Tozer, J.; Taylor, L.A.; Evans, D.P. Inter-rater reliability of optic nerve sheath diameter measurement using real-time ultrasonography. Ultrasound J. 2022, 14, 4–6. [Google Scholar] [CrossRef]
- Abramowicz, J.S.; Adhikari, S.; Dickman, E.; Estroff, J.A.; Harris, G.R.; Nomura, J.; Silverman, R.H.; Taylor, L.A.; Barr, R.G. Ocular ultrasound: Review of bioeffects and safety, including fetal and point-of-care perspective. J. Ultrasound Med. 2022, 41, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Yildizdas, D.; Aslan, N. Is ocular sonography a reliable method for the assessment of elevated intracranial pressure in children? J. Pediatr. Intensive Care 2021, 10, 14–22. [Google Scholar] [CrossRef]
- Lin, J.-J.; Chen, A.E.; Lin, E.E.; Hsia, S.-H.; Chiang, M.-C.; Lin, K.-L. Point-of-care ultrasound of optic nerve sheath diameter to detect intracranial pressure in neurocritically ill children—A narrative review. Biomed. J. 2020, 43, 231–239. [Google Scholar] [CrossRef]
- Romagnuolo, L.; Tayal, V.; Tomaszewski, C.; Saunders, T.; Norton, H.J. Optic nerve sheath diameter does not change with patient position. Am. J. Emerg. Med. 2005, 23, 686–688. [Google Scholar] [CrossRef]
- Hirzallah, M.I.; Lochner, P.; Hafeez, M.U.; Lee, A.G.; Krogias, C.; Dongarwar, D.; Manchanda, R.; Ouellette, L.; Hartman, N.D.; Ertl, M.; et al. Quality assessment of optic nerve sheath diameter ultrasonography: Scoping literature review and Delphi protocol. J. Neuroimaging 2022, 32, 808–824. [Google Scholar] [CrossRef]
- Aspide, R.; Bertolini, G.; Albini Riccioli, L.; Mazzatenta, D.; Palandri, G.; Biasucci, D.G. A proposal for a new protocol for sonographic assessment of the optic nerve sheath diameter: The CLOSED protocol. Neurocrit. Care 2020, 32, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Raio, C.; Klein, L.; Felicetta, M. Posterior globe depth for optic nerve sheath diameter measurement in ocular ultrasound in healthy volunteers. J. Ultrasound Med. 2022, 41, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Sitanaya, S.N.; Kamayanti, F.; Nugroho, H.A.; Prabowo, B. Comparing ultrasonographic optic nerve sheath diameter to head computed tomography scan to predict intracranial pressure elevation. SAGE Open Med. 2022, 10, 205031212210778. [Google Scholar] [CrossRef] [PubMed]
- Pansell, J.; Bell, M.; Rudberg, P.; Friman, O.; Cooray, C. Optic nerve sheath diameter measurement by ultrasound: Evaluation of a standardized protocol. J. Neuroimaging 2022, 32, 104–110. [Google Scholar] [CrossRef]
- Betcher, J.; Becker, T.K.; Stoyanoff, P.; Cranford, J.; Theyyunni, N. Military trainees can accurately measure optic nerve sheath diameter after a brief training session. Mil. Med. Res. 2018, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Hassen, G.W.; Bruck, I.; Donahue, J.; Mason, B.; Sweeney, B.; Saab, W.; Weedon, J.; Patel, N.; Perry, K.; Matari, H.; et al. Accuracy of optic nerve sheath diameter measurement by emergency physicians using bedside ultrasound. J. Emerg. Med. 2015, 48, 450–457. [Google Scholar] [CrossRef]
- Oberfoell, S.; Murphy, D.; French, A.; Trent, S.; Richards, D. Inter-rater reliability of sonographic optic nerve sheath diameter measurements by emergency medicine physicians. J. Ultrasound Med. 2017, 36, 1579–1584. [Google Scholar] [CrossRef]
- Bruce, B.B.; Lamirel, C.; Wright, D.W.; Ward, A.; Heilpern, K.L.; Biousse, V.; Newman, N.J. Nonmydriatic ocular fundus photography in the emergency department. N. Engl. J. Med. 2011, 364, 387–389. [Google Scholar] [CrossRef]
- Teismann, N.; Lenaghan, P.; Nolan, R.; Stein, J.; Green, A. Point-of-care ocular ultrasound to detect optic disc swelling. Acad. Emerg. Med. 2013, 20, 920–925. [Google Scholar] [CrossRef]
- Lochner, P.; Leone, M.A.; Coppo, L.; Nardone, R.; Zedde, M.L.; Cantello, R.; Brigo, F. B-mode transorbital iltrasononography for the diagnosis of acute optic neuritis: A systematic review. Clin. Neurophysiol. 2016, 127, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Wayman, D.; Carmody, K.A. Optic neuritis diagnosed by bedside emergency physician-performed ultrasound: A case report. J. Emerg. Med. 2014, 47, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Saigh, M.P.; Plauché, H.M.; Butts, C.; Karam, A.K.; Suau, S.J.; Moreno-Walton, L. Acute optic neuritis diagnosed by bedside ultrasound in an emergency department. J. Emerg. Med. 2019, 57, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.P.; Kashani, S.; Mailhot, T.; Omer, T. More than meets the eye: Point-of-care ultrasound diagnosis of acute optic neuritis in the emergency department. Am. J. Emerg. Med. 2019, 37, 177.e1–177.e4. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, H.N.; Honigman, B.; Theis, K.; Nicholas, D. High-altitude pulmonary edema at a ski resort. West. J. Med. 1996, 164, 222–227. [Google Scholar]
- Lochner, P.; Falla, M.; Brigo, F.; Pohl, M.; Strapazzon, G. Ultrasonography of the optic nerve sheath diameter for diagnosis and monitoring of acute mountain sickness: A systematic review. High Alt. Med. Biol. 2015, 16, 195–203. [Google Scholar] [CrossRef]
- Ballantyne, J.; Hollman, A.S.; Hamilton, R.; Bradnam, M.S.; Carachi, R.; Young, D.G.; Dutton, G.N. Transorbital optic nerve sheath ultrasonography in normal children. Clin. Radiol. 1999, 54, 740–742. [Google Scholar] [CrossRef]
- Godshall, A. 436: USEEICP: Ultrasound screening evaluation in the eyes for intracranial pressure. Crit. Care Med. 2013, 41, A105–A106. [Google Scholar] [CrossRef]
- Kerscher, S.R.; Schöni, D.; Neunhoeffer, F.; Wolff, M.; Haas-Lude, K.; Bevot, A.; Schuhmann, M.U. The relation of optic nerve sheath diameter (ONSD) and intracranial pressure (ICP) in pediatric neurosurgery practice—Part II: Influence of wakefulness, method of ICP measurement, intra-individual ONSD-ICP correlation and changes after therapy. Child’s Nerv. Syst. 2020, 36, 107–115. [Google Scholar] [CrossRef]
- Jayanth, A.; Benabbas, R.; Chao, J.; Sinert, R. Diagnostic modalities to determine ventriculoperitoneal shunt malfunction: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 39, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Gauthey, M.; Tessaro, M.O.; Breitbart, S.; Kulkarni, A.V.; Davis, A.L. Reliability and feasibility of optic nerve point-of-care ultrasound in pediatric patients with ventricular shunts. Child’s Nerv. Syst. 2022, 38, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.R.F.; Huberts, W.; Gommer, E.D.; Ertl, M.; Aries, M.; Mess, W.H.; Delhaas, T. An automated algorithm for optic nerve sheath diameter assessment from B-mode ultrasound images. J. Neuroimaging 2021, 31, 724–732. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Study Design | Study Population | Intervention | Comparator | Results |
|---|---|---|---|---|---|
| Kim et al., 2021 [4] | Prospective observational study | 199 adults with suspected raised ICP | Sonographic ONSD | CT scan | Median sonographic ONSD wider in raised ICP patients (5.7 mm vs. 4.3 mm) |
| Ideal cut point of 5.3 mm Sensitivity 75% Specificity 91% | |||||
| Hanafi et al., 2019 [5] | Prospective observational study | 112 adults with traumatic headache vs. controls | Sonographic ONSD | CT scan | Mean sonographic ONSD wider in patients (6.06 mm vs. 3.04 mm) |
| Ideal cut point of 5.3 mm yielding: Sensitivity 96% Specificity 71% | |||||
| Ohle et al., 2015 [6] | Meta-analysis | 478 adults across 12 studies with suspected raised ICP | Sonographic ONSD | CT scan | Sensitivity 96% Specificity 92% +LR 12.5 −LR 0.05 |
| Robba et al., 2018 [7] | Meta-analysis | 320 adults across 7 studies with suspected raised ICP | Sonographic ONSD | Invasive ICP measurement | DOR 68 +LR 5.4 −LR 0.09 |
| Aletreby et al., 2022 [8] | Meta-analysis | 619 adults across 16 studies with suspected raised ICP | Sonographic ONSD | Invasive ICP measurement | Sensitivity 90% Specificity 85% +LR 6.1 −LR 0.11 DOR 46.7 |
| Koziarz et al., 2019 [9] | Meta-analysis | 4551 patients of any age across 71 studies with suspected raised ICP | Sonographic ONSD | Any reference standard (CT or invasive ICP measurement) | Traumatic brain injury: Sensitivity 97% Specificity 86% +LR 6.9 −LR 0.04 |
| Non-traumatic injury Sensitivity 92% Specificity 86% +LR 6.9 −LR 0.09 | |||||
| Optimal cut point of 5.0 mm | |||||
| Kim et al., 2019 [10] | Meta-analysis | 352 adults across 6 studies with suspected raised ICP | Sonographic ONSD with a cut point of 5.0 mm in all included studies | CT scan | Sensitivity 99% Specificity 73% +LR 4.6 −LR 0.05 DOR 178 |
| Fernando et al., 2019 [11] | Meta-analysis | 5123 adults across 40 studies with suspected raised ICP | Physical exam, sonographic ONSD, or CT imaging | Invasive ICP measurement or craniotomy with operative diagnosis of raised ICP | Pupillary dilation: Sensitivity 28% Specificity 86% |
| Motor posturing: Sensitivity 54% Specificity 64% | |||||
| Decreased level of consciousness: Sensitivity 76% Specificity 40% | |||||
| Sonographic ONSD: AUROC 0.94 | |||||
| CT absence or compression of basal cisterns: Sensitivity 86% Specificity 61% | |||||
| CT any midline shift: Sensitivity 81% Specificity 43% | |||||
| CT severe midline shift: Sensitivity 21% Specificity 89% |
| Author and Year | Study Design | Study Population | Intervention | Comparator | Results |
|---|---|---|---|---|---|
| Dağdelen et al., 2022 [12] | Case-control study | 47 adults with IIH | Sonographic ONSD | 50 healthy controls | Mean sonographic ONSD wider in cases (6.4 mm vs. 4.9 mm) |
| Ideal cut point of 5.7 mm yielding: Sensitivity 100% Specificity 98% | |||||
| Kishk et al., 2018 [13] | Case-control study | 99 females with IIH (90 definite IIH, 9 probable IIH) | Sonographic ONSD | 35 age-matched healthy controls | Mean sonographic ONSD wider in cases (6.57 mm vs. 5.50 mm) |
| Ideal cut point of 6.05 mm yielding: Sensitivity 73% Specificity 91% | |||||
| del Saz-Saucedo et al., 2016 [14] | Prospective observational | 30 patients with suspected IIH | Sonographic ONSD | Lumbar puncture with CSF opening pressure of ≥25 cmH2O | Mean sonographic ONSD wider in IIH patients (6.8 mm vs. 5.7 mm) |
| Idea cut point of 6.3 mm yielding: Sensitivity 95% Specificity 91% +LR 10.4 −LR 0.06 | |||||
| Ebraheim et al., 2018 [15] | Case-control study | 24 adults with IIH (20 definite IIH, 4 probable IIH). All cases received acetazolamide treatment. | Sonographic ONSD | 30 controls | Mean sonographic ONSD higher in IIH patients (6.7 mm vs. 5.5 mm) |
| ONSD was not correlated with CSF opening pressure | |||||
| 1 week post-LP (10–15 mL withdrawn), mean ONSD not significantly different from baseline (6.6 mm vs. 6.7 mm) | |||||
| 4 weeks post-LP and after treatment with acetazolamide, mean ONSD decreased from baseline (6.4 mm vs. 6.8 mm) | |||||
| Ideal cut point of 6.2 mm yielding: Sensitivity 88% Specificity 100% | |||||
| Jeub et al., 2020 [16] | Case-control study | 19 adults with IIH (15 definite IIH, 4 probable IIH) | Sonographic ONSD | 20 healthy controls | Mean sonographic ONSD higher in IIH patients (values not provided) |
| 24 h after LP (30 mL withdrawn), mean ONSD decreased (mean reduction right eye 0.4 mm, left eye 0.5 mm) | |||||
| Ideal cut point of 5.8 mm yielding: Sensitivity 81% Specificity 80% |
| Author and Year | Study Design | Study Population | Intervention | Comparator | Results |
|---|---|---|---|---|---|
| Lochner et al., 2014 [17] | Case-control study | 21 adults with unilateral first episode optic neuritis | Sonographic ONSD | Unaffected eye and 21 healthy age- and sex-matched controls | Median ONSD higher in affected eye than the unaffected eye (6.3 mm vs. 5.5 mm) and controls (6.3 mm vs. 5.2 mm) |
| Kwon et al., 2019 [18] | Prospective observational study | 17 adults with unilateral first episode optic neuritis | Sonographic ONSD | Unaffected eye | Median ONSD higher in affected eye than in unaffected eye (5.5 mm vs. 5.0 mm) |
| Author and Year | Study Design | Study Population | Intervention | Comparator | Results |
|---|---|---|---|---|---|
| Fagenholz et al., 2009 [19] | Prospective observational study | 69 adults with AMS traveling through Pheriche, Nepal (4240 m) | Sonographic ONSD | 217 adults without AMS | Mean ONSD higher in patients with AMS (5.3 mm vs. 4.5 mm) |
| ONSD positively correlated with total LLS | |||||
| ONSD associated with AMS (OR 6.3) | |||||
| Sutherland et al., 2008 [20] | Prospective cohort study | 13 adult mountaineers ascending Mount Everest | Sonographic ONSD | N/A | An increase in 1000 m is associated with ONSD increase of 0.1 mm |
| ONSD positively correlated with LLS | |||||
| Lawley et al., 2012 [21] | Prospective cohort study | 23 adults at sea level passively transported to 3777 m via cable car | Sonographic ONSD | N/A | Mean ONSD higher in patients at high-altitude than at sea level (5.6 mm vs. 5.2 mm) |
| ONSD did not change when headache resolved with acclimatization | |||||
| Keyes et al., 2013 [22] | Prospective cohort study | 57 adults at 1400 m passively transported to 4300 m via car | Sonographic ONSD | N/A | Mean ONSD higher in AMS patients at altitude when compared with their baseline altitude (4.3 mm vs. 4.0 mm). |
| Mean ONSD decreased in AMS patients at altitude after oxygen therapy (4.3 mm vs. 4.1 mm) | |||||
| Mean ONSD higher in AMS patients at altitude compared with non-AMS patients (4.3 mm vs. 4.0 mm) | |||||
| Strapazzon et al., 2014 [23] | Prospective cohort study | 19 adults ascending from 262 m to 3830 m passively via helicopter | Sonographic ONSD | N/A | Mean ONSD higher at altitude than at baseline (6.4 mm vs. 5.5 mm). ONSD increased in a parabola, peaking at 24 h and remained elevated at 8 days. |
| ONSD associated with diagnosis of AMS | |||||
| Kanaan et al., 2015 [24] | Prospective cohort study | 86 adults ascending from 1240 m to 3545 m passively via car, then hiked and slept at 3810 m | Sonographic ONSD | N/A | Mean ONSD higher at altitude than at baseline (6.1 mm vs. 5.6 mm). This increase was larger in subjects with AMS than those without (mean 0.57 mm vs. 0.21 mm increase) |
| Change in ONSD associated with diagnosis of AMS (OR 1.99) |
| Author and Year | Study Design | Study Population | Intervention | Comparator | Results |
|---|---|---|---|---|---|
| Bhargava et al., 2020 [25] | Meta-analysis | 543 children aged <18 years across 11 studies with suspected raised ICP | Sonographic ONSD | CT, MRI, invasive ICP measurement, or operative diagnosis | With any reference standard: Sensitivity 93% Specificity 74% +LR 3.6 −LR 0.1 0DOR 39 |
| With an invasive reference standard: Sensitivity 89% Specificity 74% +LR 3.4 −LR 0.15 DOR 22 | |||||
| Şik et al., 2022 [26] | Prospective observational study | 147 children aged 1 month to 18 years with suspected raised ICP after head trauma | Sonographic ONSD | CT scan | Subjects with abnormal CT scans: ONSD associated with reduced level of consciousness and elevated ICP on CT report Ideal cut point of 5.1 mm yielding: Sensitivity 93% Specificity 94% |
| Subjects without elevated ICP and with a space occupying lesion on CT Ideal cut point of 4 mm yielding: Sensitivity 75% Specificity 63% | |||||
| Padayachy et al., 2016 [27] | Prospective observational study | 56 children aged <1 year and 118 children between 1 and 14 years undergoing invasive ICP measurement under general anesthesia | Sonographic ONSD | Invasive ICP measurement | Subjects <1 year old, ideal cut point of 5.16 mm yielding: Sensitivity 80% Specificity 76% PPV 42% NPV 95% DOR 12.7 |
| Subjects 1–14 years old, ideal cut point of 5.75 mm yielding: Sensitivity 86% Specificity 70% PPV 78% NPV 81% DOR 14.5 | |||||
| Subjects with an open anterior fontanelle, ideal cut point of 5.16 mm yielding: Sensitivity 86% Specificity 75% PPV 50% NPV 95% DOR 18 | |||||
| Lin et al., 2019 [28] | Prospective observational study | 32 patients aged <21 years with suspected VP shunt failure | Sonographic ONSD with pre-determined cut point of >4 mm in infants ≤1 year and 4.5 mm in children >1 year | CT, MRI, or neurosurgical impression of VP shunt failure | Compared to neuroimaging (CT/MRI): Sensitivity 60% Specificity 67% +LR 1.8 −LR 0.6 PPV 25% NPV 90% |
| Compared to neurosurgical impression: Sensitivity 75% Specificity 68% +LR 2.3 −LR 0.37 PPV 25% NPV 95% | |||||
| Hall et al., 2013 [29] | Prospective observational study | 39 encounters with children aged 6 months to 18 years with suspected VP shunt failure | Sonographic ONSD with pre-determined cut points of >4 mm in infants ≤1 year and 4.5 mm in children >1 year | Neurosurgical decision to operate on VP shunt failure | Mean ONSD lower in those with VP shunt failure (4.5 mm vs. 5.0 mm) |
| Sensitivity 61% Specificity 22% PPV 44% NPV 36% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, T.; Ahn, J.S.; Manji, R.; Kim, D.J. A Narrative Review of Point of Care Ultrasound Assessment of the Optic Nerve in Emergency Medicine. Life 2023, 13, 531. https://doi.org/10.3390/life13020531
Lau T, Ahn JS, Manji R, Kim DJ. A Narrative Review of Point of Care Ultrasound Assessment of the Optic Nerve in Emergency Medicine. Life. 2023; 13(2):531. https://doi.org/10.3390/life13020531
Chicago/Turabian StyleLau, Torey, Justin S. Ahn, Rahim Manji, and Daniel J. Kim. 2023. "A Narrative Review of Point of Care Ultrasound Assessment of the Optic Nerve in Emergency Medicine" Life 13, no. 2: 531. https://doi.org/10.3390/life13020531
APA StyleLau, T., Ahn, J. S., Manji, R., & Kim, D. J. (2023). A Narrative Review of Point of Care Ultrasound Assessment of the Optic Nerve in Emergency Medicine. Life, 13(2), 531. https://doi.org/10.3390/life13020531








