Pleiotropic Effects of Metformin in Osteoarthritis
Abstract
1. Introduction
2. Metformin
3. Safety Profile of Metformin
4. Mechanism of Action of Metformin
5. Suggested Pleiotropic Effects of Metformin and Other Clinical Uses
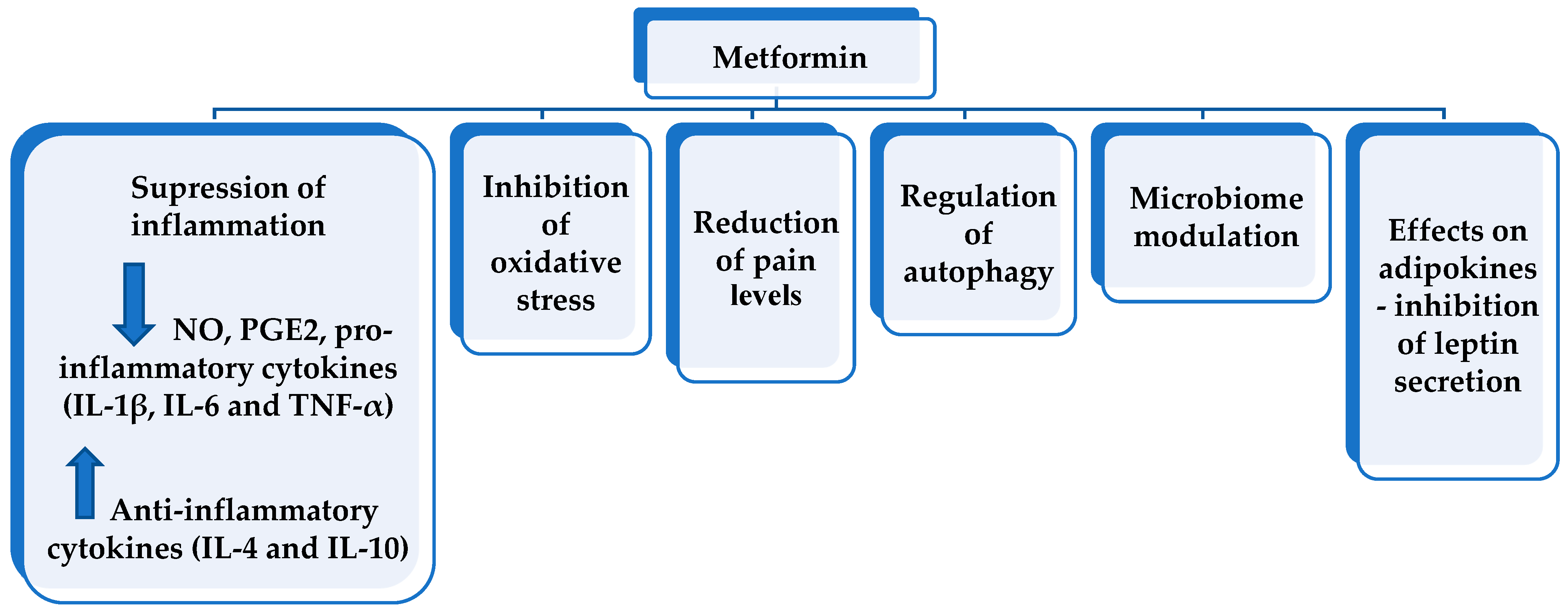
6. Pleiotropic Effects of Metformin in Osteoarthritis
6.1. Anti-Inflammatory Effect
6.2. Effects on Oxidative Stress
6.3. Effects on Pain Level
6.4. Regulation of Autophagy
6.5. Microbiome Modulation
6.6. Decrease in Leptin Level
6.7. Disease-Modifying Potential of Metformin in Osteoarthritis—Evidence from Animal Models, In Vitro Experiments, and Clinical Trials in Humans
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Hussain, S.M.; Wluka, A.E.; Lim, Y.Z.; Abram, F.; Pelletier, J.P.; Martel-Pelletier, J.; Cicuttini, F.M. Association between metformin use and disease progression in obese people with knee osteoarthritis: Data from the Osteoarthritis Initiative—A prospective cohort study. Arthritis Res. Ther. 2019, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Postgrad. Med. J. 2014, 90, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—Looking beyond the ‘usual suspects. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Dumond, H.; Presle, N.; Terlain, B.; Mainard, D.; Loeuille, D.; Netter, P.; Pottie, P. Evidence for a Key Role of Leptin in Osteoarthritis. Arthritis Rheum. 2003, 48, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.M.; Arden, N.K.; Doherty, M.; Bannwarth, B.; Bijlsma, J.W.J.; Dieppe, P.; Gunther, K.; Hauselmann, H.; Herrero-Beaumont, G.; Kaklamanis, P.; et al. EULAR Recommendations 2003: An evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 2003, 62, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, N.; Dimitrova, P. Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine. Arthritis Res. Ther. 2011, 13, R44. [Google Scholar] [CrossRef]
- Gallagher, B.; Tjoumakaris, F.P.; Harwood, M.I.; Good, R.P.; Ciccotti, M.G.; Freedman, K.B. Chondroprotection and the prevention of osteoarthritis progression of the knee: A systematic review of treatment agents. Am. J. Sport. Med. 2015, 43, 734–744. [Google Scholar] [CrossRef]
- Tan, Q.; Jiang, A.; Li, W.; Song, C.; Leng, H. Metabolic syndrome and osteoarthritis: Possible mechanisms and management strategies. Med. Nov. Technol. Devices 2021, 9, 100052. [Google Scholar] [CrossRef]
- Lambova, S.N.; Batsalova, T.; Moten, D.; Stoyanova, S.; Georgieva, E.; Belenska-Todorova, L.; Kolchakova, D.; Dzhambazov, B. Serum leptin and resistin levels in knee osteoarthritis—Clinical and radiologic links: Towards precise definition of metabolic type knee osteoarthritis. Biomedicines 2021, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, D.Y.; Ericksen, A.; Robbins, R.C. Patellofemoral Pain Syndrome. Am. Fam. Physician 2019, 99, 88–94. [Google Scholar] [PubMed]
- Ruaro, B.; Cutolo, M.; Alessandri, E.; Zaottini, F.; Picasso, R.; Pistoia, F.; Ferrari, G.; Martinoli, C. Don’t forget the jumper’s knee in the young sportsman: Evaluation of patellar tendinopathy with a high frequency ultrasound probe B. Reumatismo 2019, 71, 160–162. [Google Scholar] [CrossRef]
- Sanchis-Alfonso, V.; Dye, S.F. How to Deal With Anterior Knee Pain in the Active Young Patient. Sport. Health 2017, 9, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Poonpet, T.; Honsawek, S. Adipokines: Biomarkers for osteoarthritis? World J. Orthop. 2014, 5, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Cicuttini, F.M.; Baker, J.R.; Spector, S.T. The association of obesity with osteoarthritis of the hand and knee in women: A twin study. J. Rheumatol. 1996, 23, 1221–1226. [Google Scholar] [PubMed]
- Zhao, C.W.; Gao, Y.H.; Song, W.X.; Liu, B.; Ding, L.; Dong, N.; Qi, X. An update on the emerging role of resistin on the pathogenesis of osteoarthritis. Mediat. Inflamm. 2019, 2019, 1532164. [Google Scholar] [CrossRef]
- Bao, J.P.; Chen, W.P.; Feng, J.; Hu, P.F.; Shi, Z.L.; Wu, L.D. Leptin plays a catabolic role on articular cartilage. Mol. Biol. Rep. 2010, 37, 3265–3272. [Google Scholar] [CrossRef]
- Martin, L.J.; Piltonen, M.H.; Gauthier, J.; Convertino, M.; Acland, E.L.; Dokholyan, N.V.; Mogil, J.S.; Diatchenko, L.; Maixner, W. Differences in the Antinociceptive Effects and Binding Properties of Propranolol and Bupranolol Enantiomers. J. Pain 2015, 16, 1321–1333. [Google Scholar] [CrossRef]
- Valdes, A.M.; Mrcp, A.A.; Muir, K.; Zhang, W. Association of beta-blocker use with less prevalent joint pain and lower opioid requirement in people with osteoarthritis. Arthritis Care Res. 2017, 69, 1076–1081. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, X.; Lu, X. Captopril, an angiotensin-converting enzyme inhibitor, possesses chondroprotective efficacy in a rat model of osteoarthritis through suppression local renin-angiotensin system. J. Clin. Exp. Med. 2015, 8, 12584–12592. [Google Scholar]
- Shirinsky, I.; Shirinsky, V. Does renin-angiotensin-aldosterone system blockade influence pain, function and radiographic progression in knee osteoarthritis? An analysis of Osteoarthritis Initiative data. Ann. Rheum. Dis. 2016, 75, 835. [Google Scholar] [CrossRef]
- Belenska-Todorova, L.; Lambova, S.N.; Stoyanova, S.; Georgieva, E.; Batsalova, T.; Moten, D.; Kolchakova, D.; Dzhambazov, B. Disease-modifying potential of metformin and alendronate in an experimental mouse model of osteoarthritis. Biomedicines 2021, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Tartagni, E.; Ertek, S. Metformin and its clinical use: New insights for an old drug in clinical practice. Arch. Med. Sci. 2012, 8, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin-Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; Giuliani, A.; Mensà, E.; Sabbatinelli, J.; De Nigris, V.; Rippo, M.R.; La Sala, L.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Pleiotropic effects of metformin: Shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res. Rev. 2018, 48, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hui, F.; Zhang, Y.; Ren, T.; Li, X.; Zhao, M.; Zhao, Q. Role of metformin in overweight and obese people without diabetes: A systematic review and network meta-analysis. Eur. J. Clin. Pharmacol. 2019, 75, 437–450. [Google Scholar] [CrossRef]
- Homburg, R.; Insler, V. Ovulation induction in perspective. Hum. Reprod. Update 2002, 8, 449–462. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, X.; Gao, P.; Shi, J.; Wu, Z.; Guo, Z.; Wang, Z.; Song, Y. The potential effect of metformin on cancer: An umbrella review. Front. Endocrinol. 2019, 10, 617. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Z.; Zhao, P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. Front. Pharmacol. 2022, 13, 952560. [Google Scholar] [CrossRef]
- He, M.; Lu, B.; Opoku, M.; Zhang, L.; Xie, W.; Jin, H.; Chen, S.; Li, Y.; Deng, Z. Metformin Prevents or Delays the Development and Progression of Osteoarthritis: New Insight and Mechanism of Action. Cells 2022, 11, 3012. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Tokunaga, C.; Dalal, S.; Richardson, C.; Yoshino, K.I.; Hara, K.; Kemp, B.E.; Witters, L.A.; Mimura, O.; Yonezawa, K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 2003, 8, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Yang, B.; Lotz, M.; Liu-Bryan, R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum. 2011, 63, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Y.; Zhang, Y.; Liu, J.; Yao, Z.; Zhang, C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends 2018, 12, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Huan, Z.; Wang, Y.; Xu, J. Metformin protects chondrocytes against IL-1β induced injury by regulation of the AMPK/NF-κB signaling pathway. Pharmazie 2020, 75, 632–636. [Google Scholar] [PubMed]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Bahrambeigi, S.; Yousefi, B.; Rahimi, M.; Shafiei-Irannejad, V. Metformin; an old antidiabetic drug with new potentials in bone disorders. Biomed. Pharmacother. 2019, 109, 1593–1601. [Google Scholar] [CrossRef]
- Shamim, H.; Jean, M.; Umair, M.; Muddaloor, P.; Farinango, M.; Ansary, A.; Dakka, A.; Nazir, Z.; White, C.T.; Habbal, A.B.; et al. Role of metformin in the management of polycystic ovarian syndrome-associated acne: A systematic review. Cureus 2022, 14, e28462. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner, G.; Brand, K.; Bailey, C.J. Metformin and the heart: Update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metabolism 2022, 130, 155160. [Google Scholar] [CrossRef] [PubMed]
- Kinaan, M.; Ding, H.; Triggle, C.R. Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med. Princ. Pract. 2015, 24, 401–415. [Google Scholar] [CrossRef]
- Standeven, K.F.; Ariëns, R.A.S.; Whitaker, P.; Ashcroft, A.E.; Weisel, J.W.; Grant, P.J. The effect of dimethylbiguanide on thrombin activity, FXIII activation, fibrin polymerization, and fibrin clot formation. Diabetes 2002, 51, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, A.M.; Sedlinsky, C.; McCarthy, A.D.; Blanco, A.; Schurman, L. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur. J. Pharmacol. 2006, 536, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Mai, Q.G.; Zhang, Z.M.; Xu, S.; Lu, M.; Zhou, R.P.; Zhao, L.; Jia, C.H.; Wen, Z.H.; Jin, D.D.; Bai, X.C. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J. Cell. Biochem. 2011, 112, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J.; Leibson, C.L.; Achenbach, S.J.; Therneau, T.M.; Khosla, S. Fracture risk in type 2 diabetes: Update of a population-based study. J. Bone Miner. Res. 2008, 23, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; He, Z.; Zhou, X.; Xian, L.; Yuan, T.; Jia, X.; Hong, J.; He, L.; Liu, J. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int. J. Oncol. 2013, 43, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Pandini, G.; Sciacca, L.; Pezzino, V.; Squatrito, S.; Belfiore, A.; Vigneri, R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch. Physiol. Biochem. 2008, 114, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Hyun, B.; Shin, S.; Lee, A.; Lee, S.; Song, Y.; Ha, N.-J.; Cho, K.-H.; Kim, K. Metformin Down-regulates TNF-α Secretion via Suppression of Scavenger Receptors in Macrophages. Immune Netw. 2013, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Ye, S. Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes. J. Inflamm. 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Esteghamati, A.; Eskandari, D.; Mirmiranpour, H.; Noshad, S.; Mousavizadeh, M.; Hedayati, M.; Nakhjavani, M. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Clin. Nutr. 2013, 32, 179–185. [Google Scholar] [CrossRef]
- Feng, X.; Pan, J.; Li, J.; Zeng, C.; Qi, W.; Shao, Y.; Liu, X.; Liu, L.; Xiao, G.; Zhang, H.; et al. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging 2020, 12, 1087–1103. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.E.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Westphal, S.; Kraus, D.; Meier, B.; Perwitz, N.; Ott, V.; Fasshauer, M.; Klein, H.H. Metformin inhibits leptin secretion via a mitogen-activated protein kinase signalling pathway in brown adipocytes. J. Endocrinol. 2004, 183, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.P.; Alemzadeh, R.; Langley, G.; D’Angelo, L.; Smith, P.; Holshouser, S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metab.-Clin. Exp. 2001, 50, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Flores, G.D.C.; Guzmán-Priego, C.G.; Parra-Flores, L.I.; Murbartián, J.; Torres-López, J.E.; Granados-Soto, V. Metformin: A Prospective Alternative for the Treatment of Chronic Pain. Front. Pharmacol. 2020, 11, 558474. [Google Scholar] [CrossRef]
- Xiang, H.-C.; Lin, L.-X.; Hu, X.-F.; Zhu, H.; Li, H.-P.; Zhang, R.-Y.; Hu, L.; Liu, W.T.; Zhao, Y.-L.; Shu, Y.; et al. AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J. Neuroinflamm. 2019, 16, 34. [Google Scholar] [CrossRef]
- Norsted Gregory, E.; Codeluppi, S.; Gregory, J.A.; Steinauer, J.; Svensson, C.I. Mammalian target of rapamycin in spinal cord neurons mediates hypersensitivity induced by peripheral inflammation. Neuroscience 2010, 169, 1392–1402. [Google Scholar] [CrossRef]
- Burton, M.D.; Tillu, D.V.; Mazhar, K.; Mejia, G.L.; Asiedu, M.N.; Inyang, K.; Hughes, T.; Lian, B.; Dussor, G.; Price, T.J. Pharmacological activation of AMPK inhibits incision-evoked mechanical hypersensitivity and the development of hyperalgesic priming in mice. Neuroscience 2017, 359, 119–129. [Google Scholar] [CrossRef]
- Garza Carbajal, A.; Ebersberger, A.; Thiel, A.; Ferrari, L.; Acuna, J.; Brosig, S.; Isensee, J.; Moeller, K.; Siobal, M.; Rose-John, S.; et al. Oncostatin M induces hyperalgesic priming and amplifies signaling of cAMP to ERK by RapGEF2 and PKA. J. Neurochem. 2021, 157, 1821–1837. [Google Scholar] [CrossRef]
- Reggiori, F.; Komatsu, M.; Finley, K.; Simonsen, A. Autophagy: More than a nonselective pathway. Int. J. Cell Biol. 2012, 2012, 219625. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Korotkyi, O.; Kyriachenko, Y.; Kobyliak, N.; Falalyeyeva, T.; Ostapchenko, L. Crosstalk between gut microbiota and osteoarthritis: A critical view. J. Funct. Foods 2020, 68, 103904. [Google Scholar] [CrossRef]
- Dunn, C.M.; Velasco, C.; Rivas, A.; Andrews, M.; Garman, C.; Jacob, P.B.; Jeffries, M.A. Identification of Cartilage Microbial DNA Signatures and Associations with Knee and Hip Osteoarthritis. Arthritis Rheumatol. 2020, 72, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Paul, H.A.; Reimer, R.A.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhang, J.; Yang, H.; Sun, Y. The role of leptin in osteoarthritis. Medicine 2018, 97, e0257. [Google Scholar] [CrossRef]
- Vuolteenaho, K.; Koskinen, A.; Moilanen, E. Leptin—A link between obesity and osteoarthritis. Applications for prevention and treatment. Basic Clin. Pharmacol. Toxicol. 2014, 114, 103–108. [Google Scholar] [CrossRef]
- Park, M.-J.; Moon, S.-J.; Baek, J.-A.; Lee, E.-J.; Jung, K.-A.; Kim, E.-K.; Kim, D.-S.; Lee, J.-H.; Kwok, S.-K.; Min, J.-K.; et al. Metformin Augments Anti-Inflammatory and Chondroprotective Properties of Mesenchymal Stem Cells in Experimental Osteoarthritis. J. Immunol. 2019, 203, 127–136. [Google Scholar] [CrossRef]
- Wang, C.; Yao, Z.; Zhang, Y.; Yang, Y.; Liu, J.; Shi, Y.; Zhang, C. Metformin Mitigates Cartilage Degradation by Activating AMPK/SIRT1-Mediated Autophagy in a Mouse Osteoarthritis Model. Front. Pharmacol. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Na, H.S.; Kwon, J.Y.; Lee, S.Y.; Lee, S.H.; Lee, A.R.; Woo, J.S.; Jung, K.; Cho, K.-H.; Choi, J.-W.; Lee, D.H.; et al. Metformin attenuates monosodium-iodoacetate-induced osteoarthritis via regulation of pain mediators and the autophagy–lysosomal pathway. Cells 2021, 10, 681. [Google Scholar] [CrossRef]
- Schadler, P.; Lohberger, B.; Stündl, N.; Stradner, M.H.; Glänzer, D.; Sadoghi, P.; Leithner, A.; Steinecker-Frohnwieser, B. The Effect of Body Mass Index and Metformin on Matrix Gene Expression in Arthritic Primary Human Chondrocytes. Cartilage 2021, 13 (Suppl. 2), 1004S–1018S. [Google Scholar] [CrossRef]
- Mohammed, M.M.; Al-shamma, K.J.; Jassim, N.A. Evaluation of the Clinical use of Metformin or Pioglitazone in Combination with Meloxicam in Patients with Knee Osteoarthritis; using Knee Injury and Osteoarthritis outcome Score. Iraqi J. Pharm. Sci. 2014, 23, 13–23. [Google Scholar] [CrossRef]
- Lu, C.-H.; Chung, C.-H.; Lee, C.-H.; Hsieh, C.-H.; Hung, Y.-J.; Lin, F.-H.; Tsao, C.-H.; Hsieh, P.-S.; Chien, W.-C. Combination COX-2 inhibitor and metformin attenuate rate of joint replacement in osteoarthritis with diabetes: A nationwide, retrospective, matched-cohort study in Taiwan. PLoS ONE 2018, 13, e0191242. [Google Scholar] [CrossRef] [PubMed]
| Indication for Metformin Treatment | Benefits from Metformin Use Due to Its Pleiotropic Effects | References |
|---|---|---|
| Diabetes mellitus type II | Obesity Cardiovascular protection Polycystic ovarian syndrome d Musculoskeletal diseases—osteopenia, osteoporosis, osteoarthritis Cancer Aging | [27,29,37,38] |
| Study (Author’s Group, Year) | Study Design | Conclusions about Pleiotropic Effects of Metformin in OA |
|---|---|---|
| ||
| Park M-J et al. (2019) [67] | Rats with experimental OA were treated with adipose mesenchymal stem cells with or without metformin stimulation. | The antinociceptive and chondroprotective effect of mesenchymal stem cells treated with metformin was superior compared to that of unstimulated cells. |
| Belenska-Todorova L et al. (2021) [23] | Mouse model of OA; metformin administered orally. | Decreased cartilage degeneration was confirmed histologically after treatment with metformin compared with controls. |
| Wang C et al. (2020) [68] | Mouse model of OA; metformin administered intraarticularly | Intraarticular administration of metformin led to improved histological findings, i.e., decreased cartilage damage, increased proteoglycan expression, and reduced the synovium thickness. |
| Na HS et al. (2021) [69] | OA in rats; metformin was administered orally. | Histological evidence of a decrease in cartilage destruction after treatment with metformin |
| ||
| Zhang M et al. (2020) [35] | Cell viability of ATDC5 cells was assessed after treatment with metformin. Effects of metformin on osteoarthritic changes induced in ATDC5 cells with IL-1β application. | Metformin promoted proliferation of ATDC5 cells; metformin reversed partly IL-1β-induced changes, i.e., the increase in the level of proteins of proinflammatory cytokines IL-6 and TNF-α. |
| Schadler P et al. (2021) [70] | Primary chondrocytes from 14 adult female patients undergoing knee arthroplasty for end-stage OA were cultivated and stimulated with metformin. Matrix gene expression was analyzed via polymerase chain reaction. | Metformin decreased the expression of catabolic genes ADAMTS5 and MMP-1. |
| Na HS et al. (2021) [69] | Human IL-1β-stimulated OA chondrocytes treated with metformin. | Metformin reduced catabolic factor gene expression (MMP-1, 3, 13), increased mRNA levels of tissue inhibitors of MMP-1 and 3 and increased expression of autophagolysosome markers. |
| ||
| Mohammed MM et al. (2014) [71] | 68 patients with symptomatic, radiologically confirmed knee OA; 12-week follow-up. | A greater reduction in serum levels of IL-1β, IL-8, and TNF-α was found in patients treated with metformin (2 × 500 mg daily) and meloxicam compared to those receiving meloxicam. |
| Lu CH et al. (2018) [72] | Patients with OA and diabetes mellitus type 2; 968 on metformin, 1936 without metformin; 10-year period of follow-up; the rate joint replacement surgery was assessed. | Significantly lower rate of joint replacement surgery was registered in metformin users at the end of follow-up compared with the control group (p = 0.003). |
| Wang Y et al. (2019) [1] | Osteoarthritis Initiative data; 56 patients on metformin; 762 patients were non-users of metformin. | Assessment of articular cartilage volume of the femur and tibia performed via MRI at baseline and at the fourth year has shown that the rate of medial cartilage volume loss was lower in patients taking metformin compared to metformin non-users (p = 0.02). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambova, S.N. Pleiotropic Effects of Metformin in Osteoarthritis. Life 2023, 13, 437. https://doi.org/10.3390/life13020437
Lambova SN. Pleiotropic Effects of Metformin in Osteoarthritis. Life. 2023; 13(2):437. https://doi.org/10.3390/life13020437
Chicago/Turabian StyleLambova, Sevdalina Nikolova. 2023. "Pleiotropic Effects of Metformin in Osteoarthritis" Life 13, no. 2: 437. https://doi.org/10.3390/life13020437
APA StyleLambova, S. N. (2023). Pleiotropic Effects of Metformin in Osteoarthritis. Life, 13(2), 437. https://doi.org/10.3390/life13020437