Biosensors Based on Phenol Oxidases (Laccase, Tyrosinase, and Their Mixture) for Estimating the Total Phenolic Index in Food-Related Samples
Abstract
1. Introduction
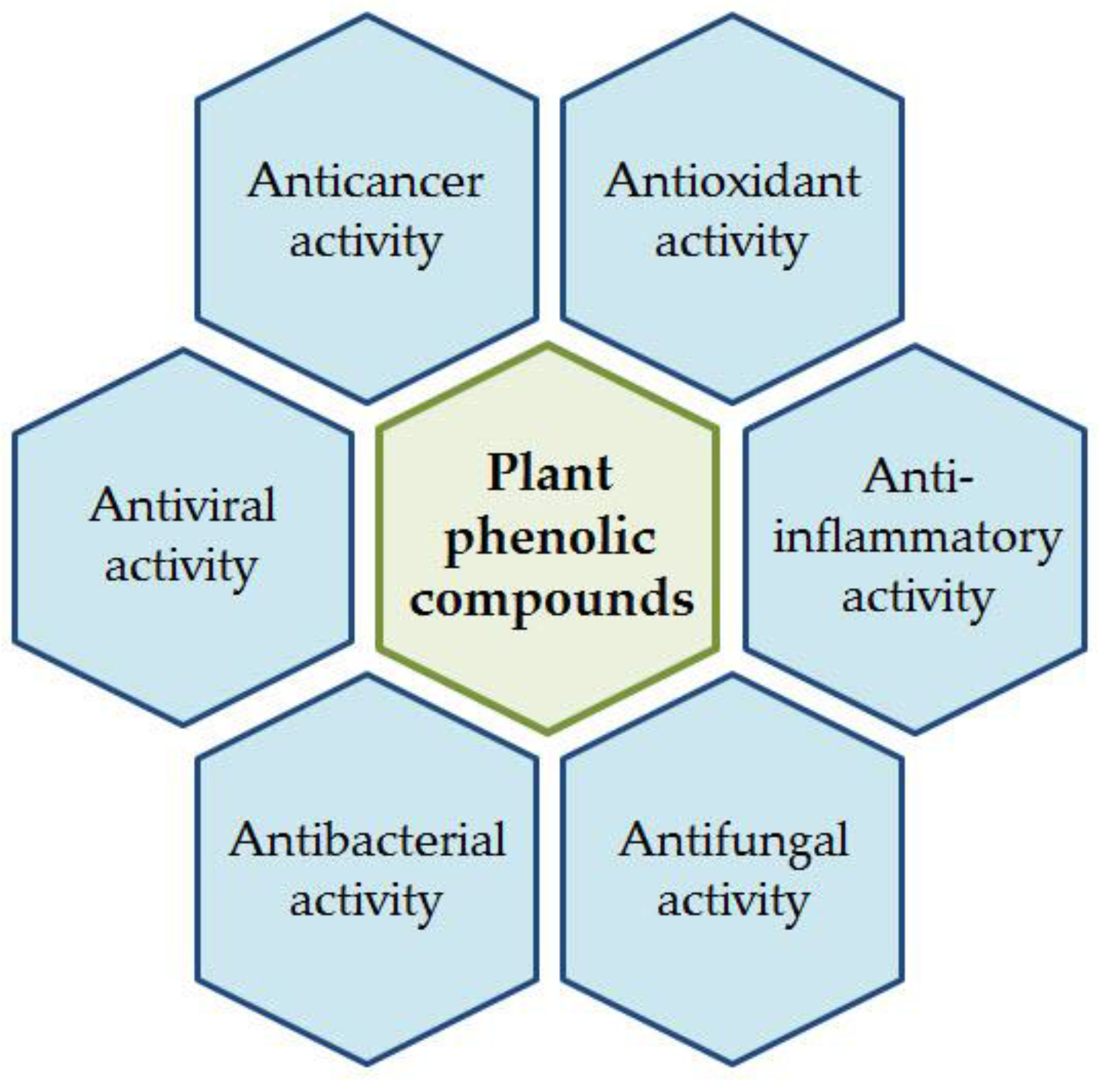
1.1. Diversity and Significance of Plant Phenolic Compounds
1.2. Estimation of Total Phenolic Content
2. Phenol Oxidases
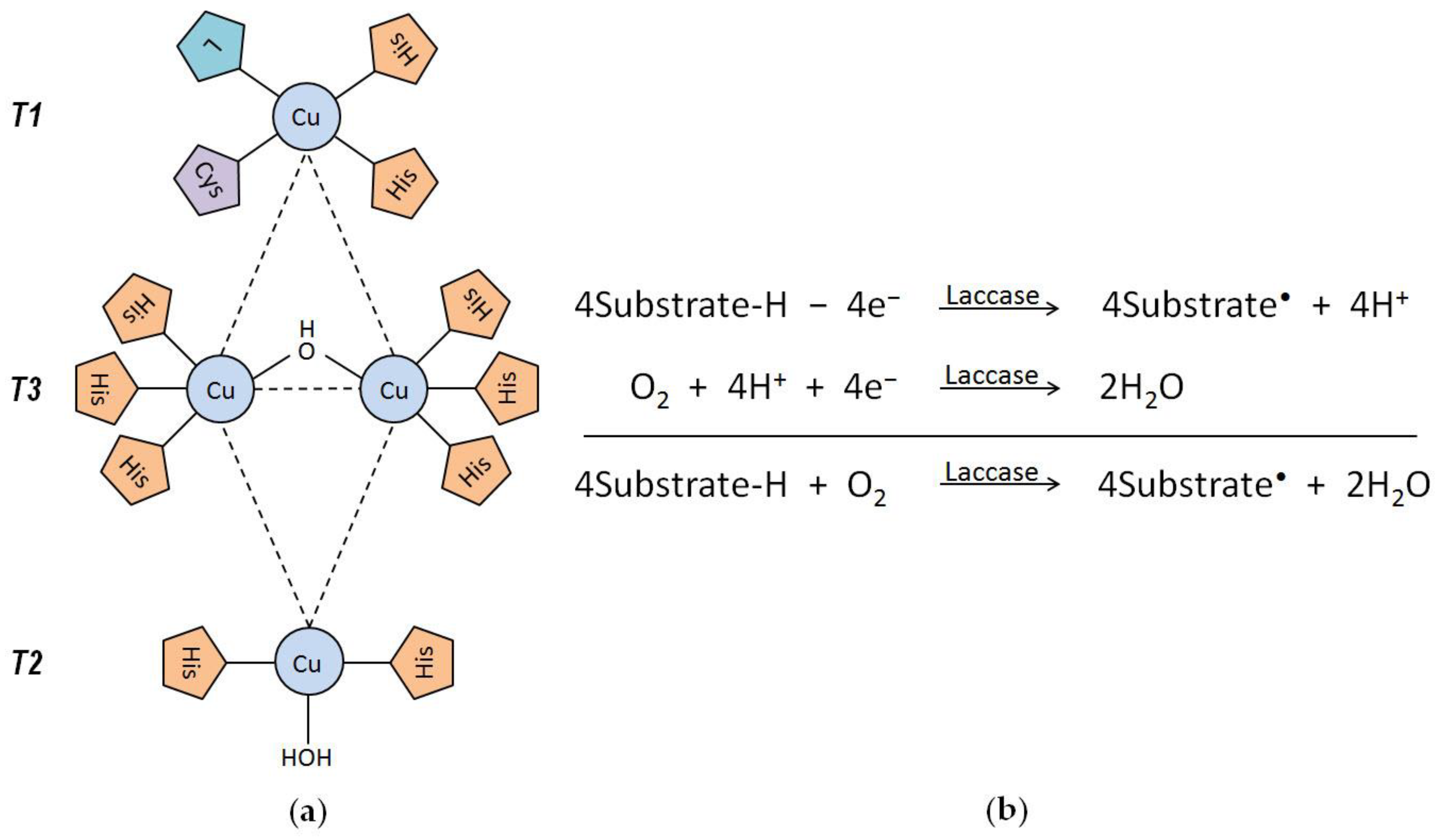
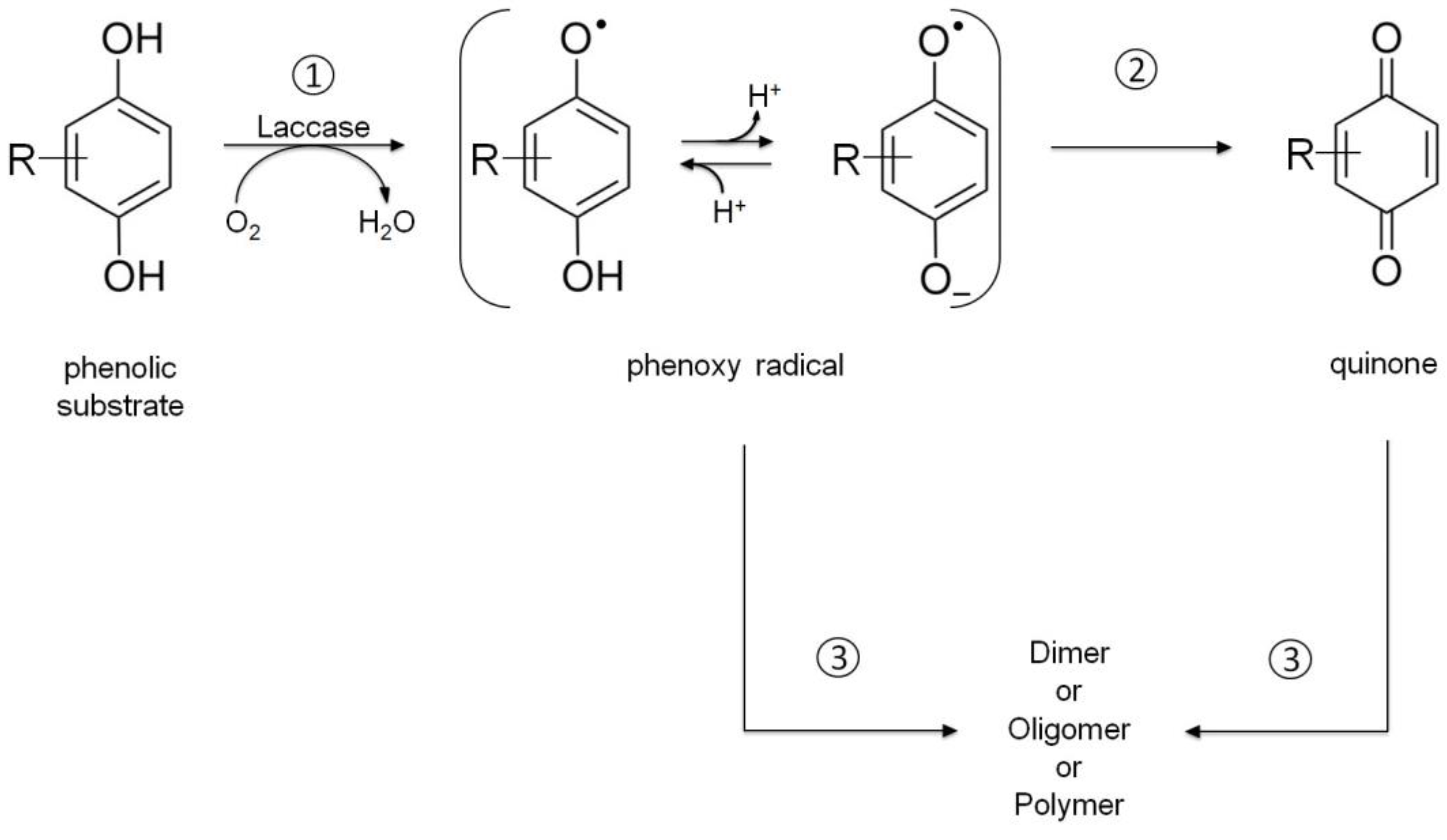
2.1. Laccase
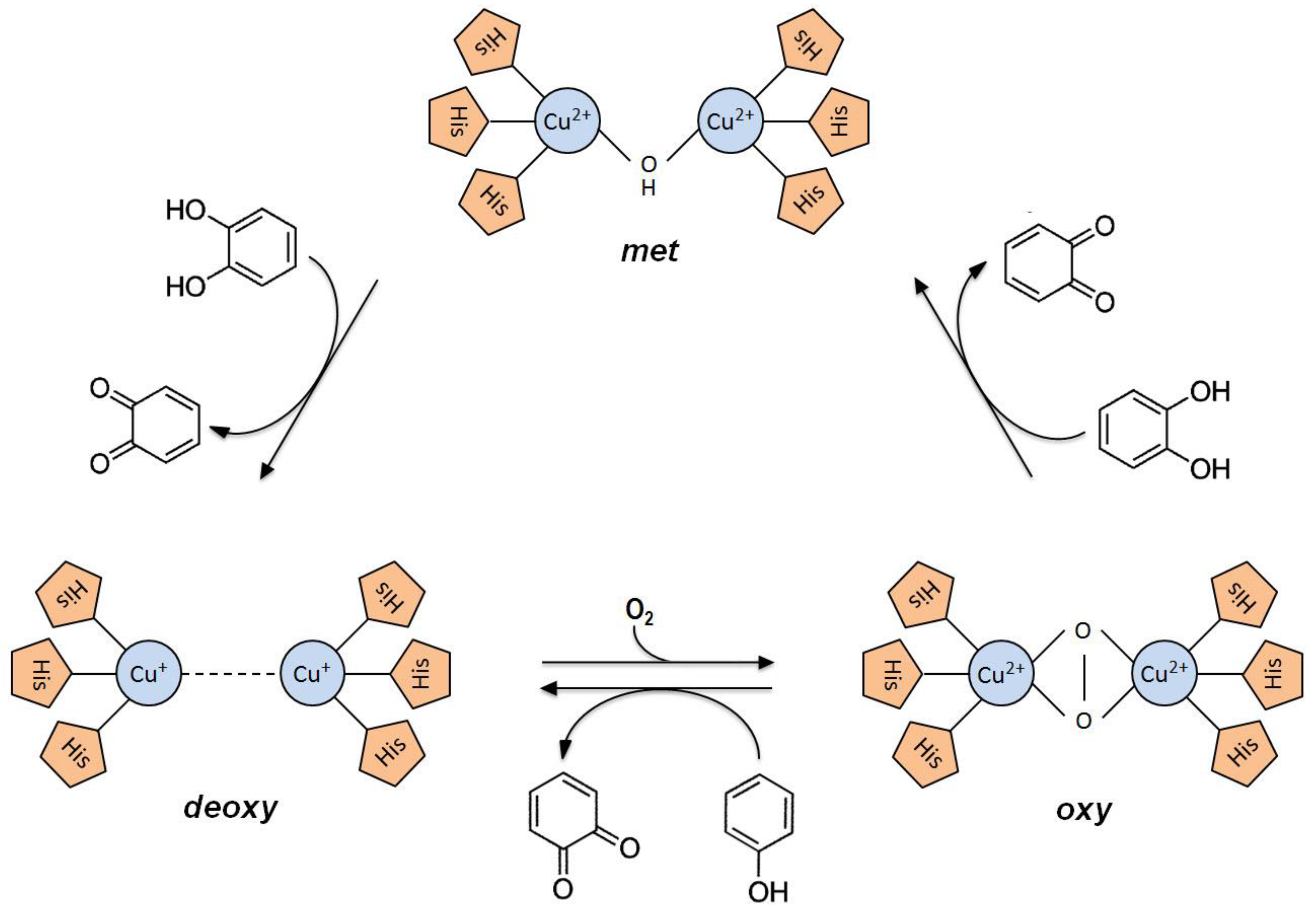
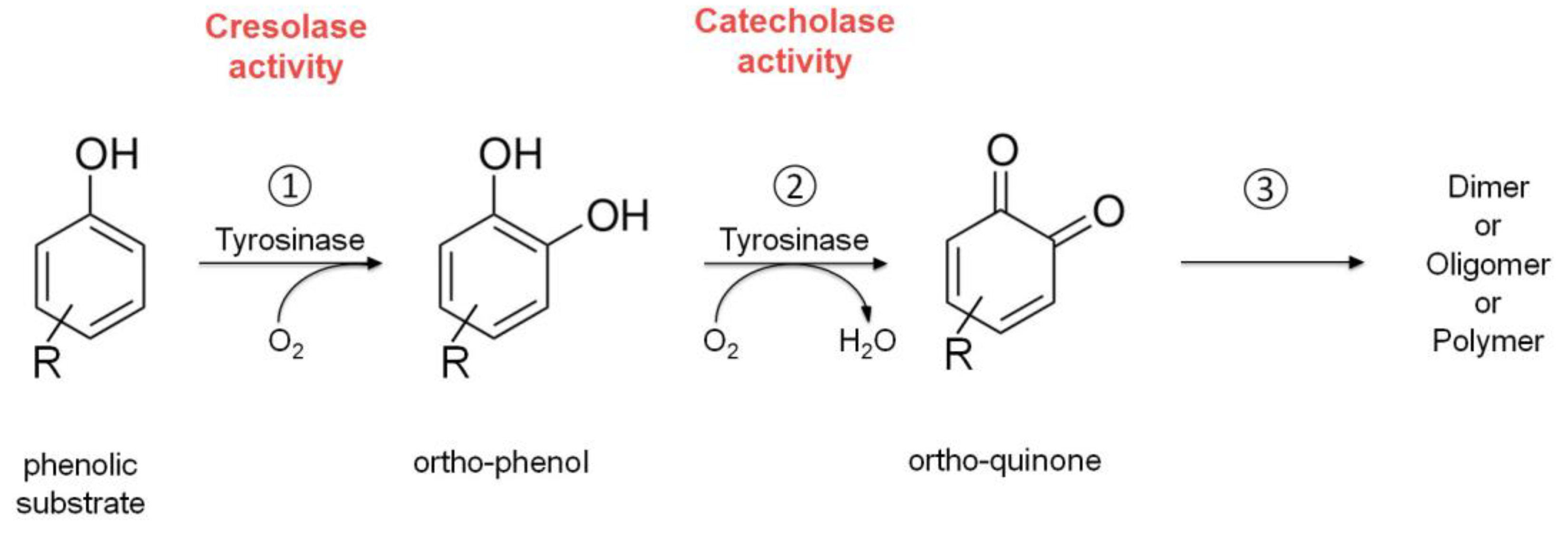
2.2. Tyrosinase
3. PO-Based Biosensors for Estimating TPI in FRSs
3.1. Definitions and Classification
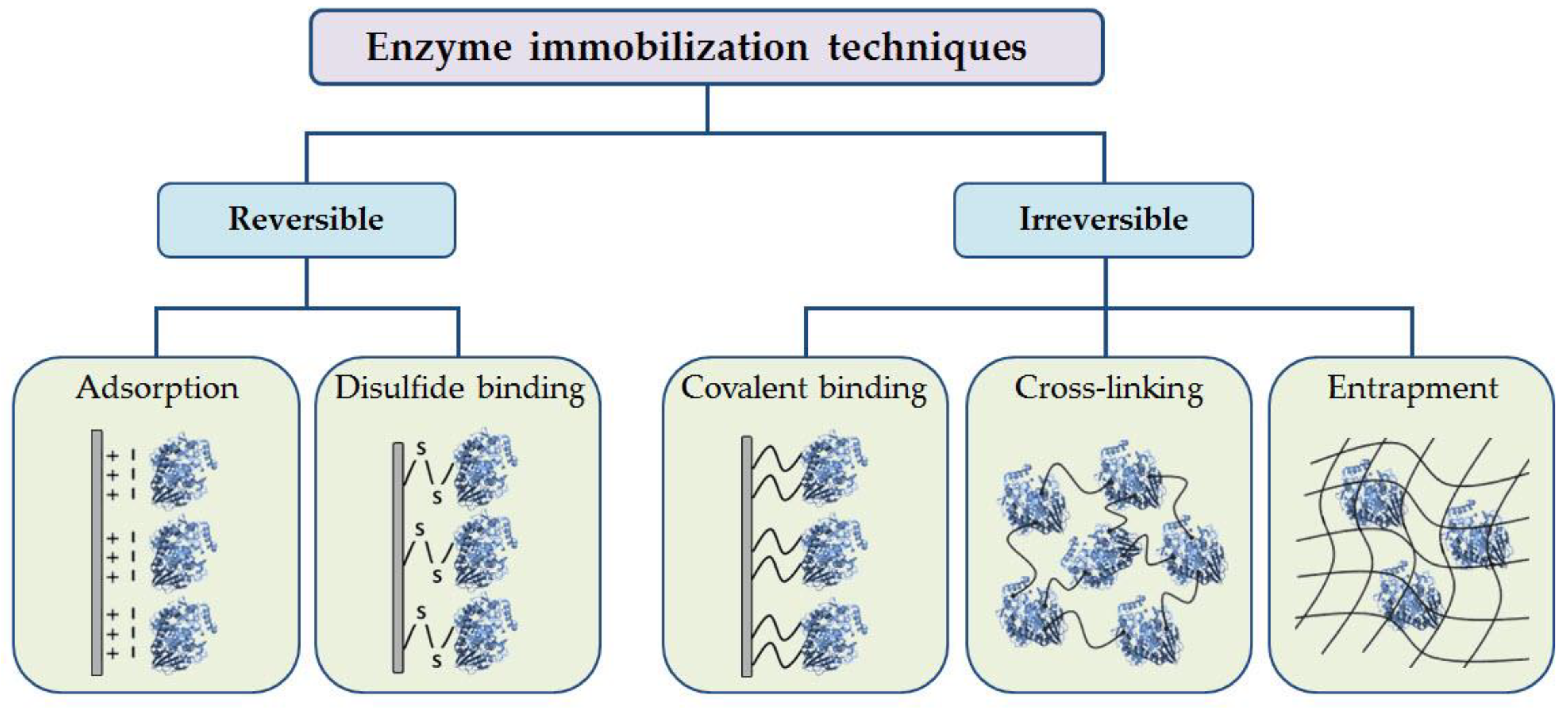
3.2. POs Immobilization
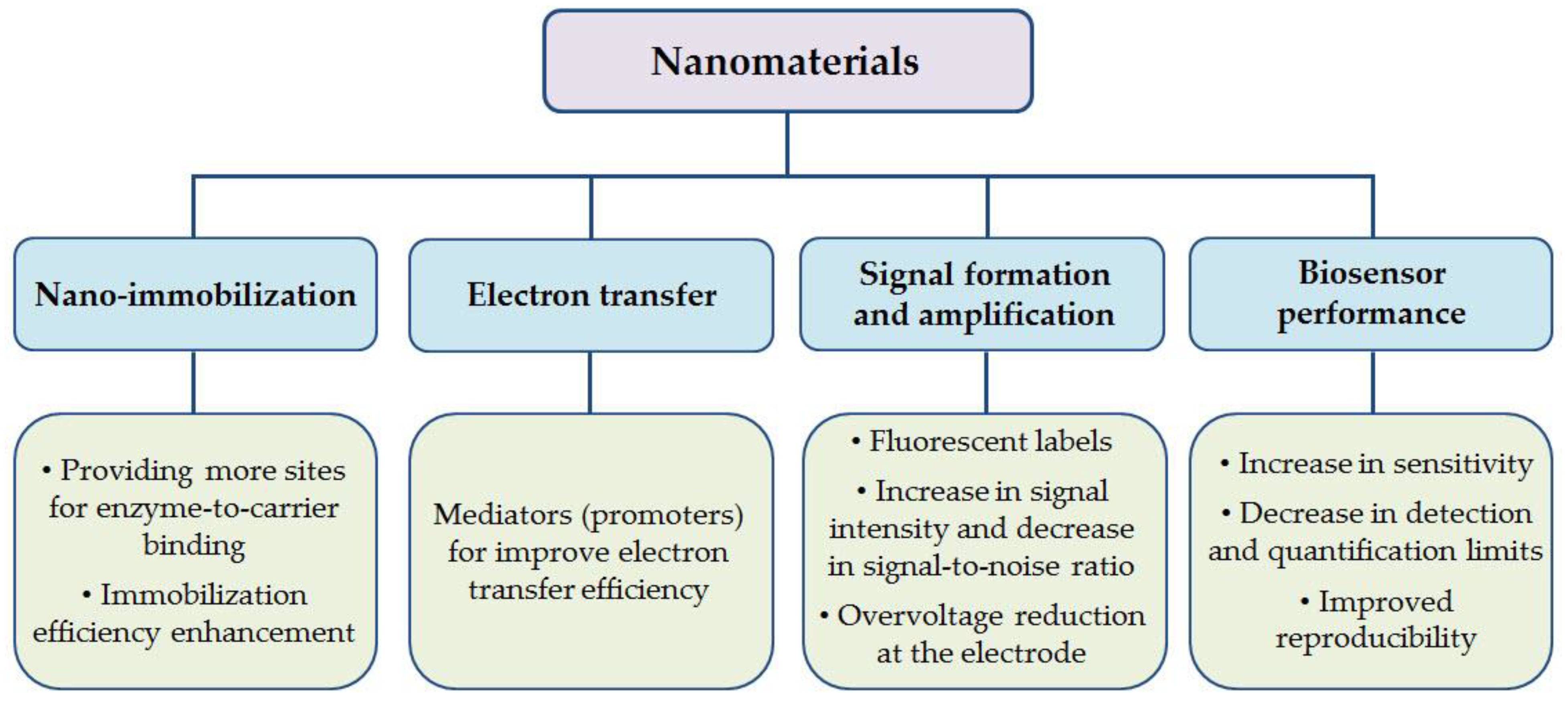
3.3. Functions of Nanomaterials
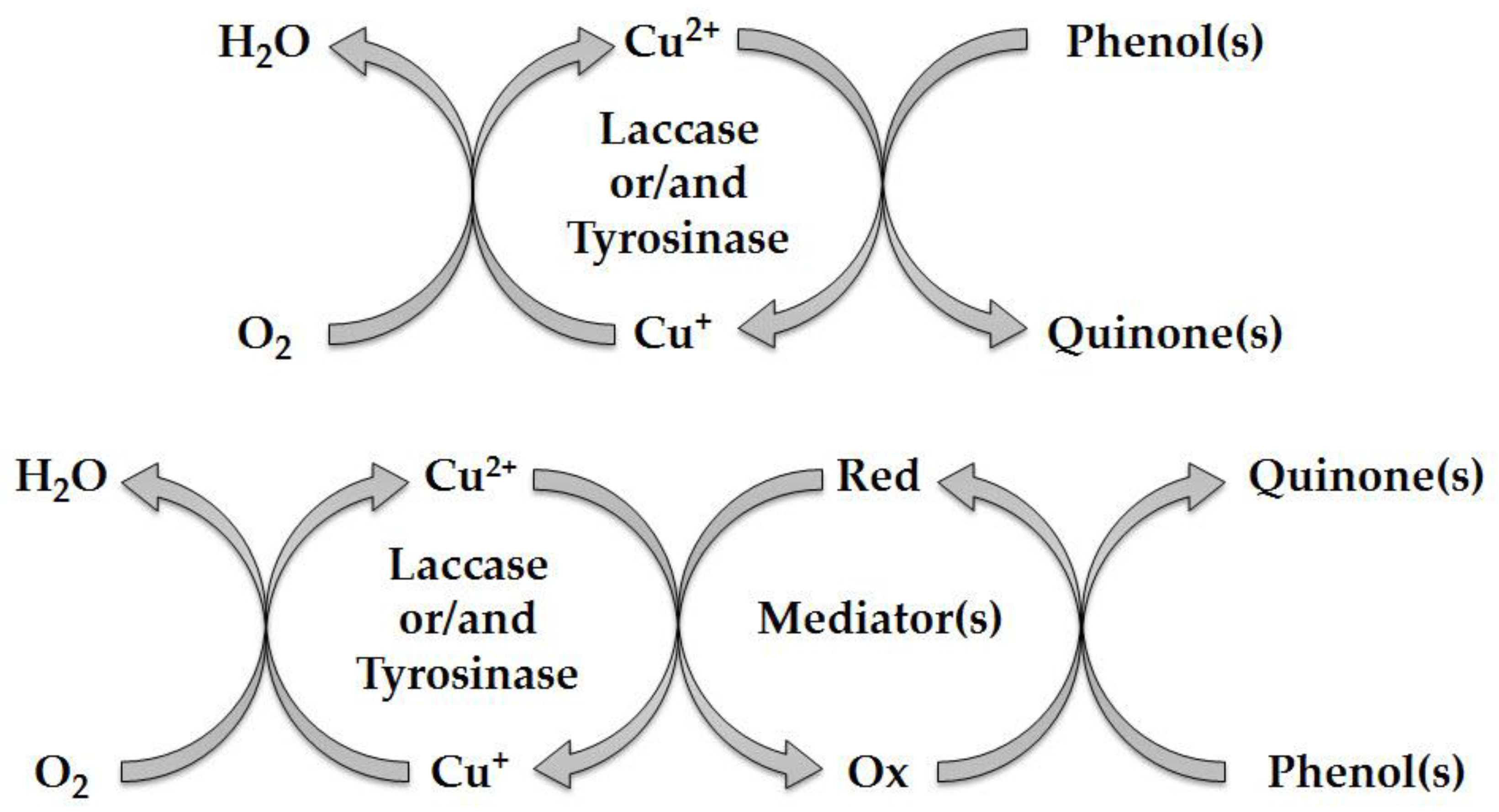
3.4. Catalytic Cycle
3.5. TPI Estimation
3.6. Interference
3.7. Validation
3.8. Optical Biosensors
3.8.1. Fluorescent Biosensors
3.8.2. Colorimetric Biosensors
3.9. Electrochemical Biosensors
3.9.1. Potentiometric Biosensors
3.9.2. Voltammetric Biosensors
3.9.3. Amperometric Biosensors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology—Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2022; Chapter 7; pp. 1–16. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- de Lourdes Reis Giada, M. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-González, J.A., Ed.; IntechOpen: London, UK, 2013; Chapter 4; pp. 87–112. [Google Scholar]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G. Antibacterial Activity of Plant Polyphenols. In Secondary Metabolites—Trends and Reviews; Vijayakumar, R., Raja, S., Eds.; IntechOpen: London, UK, 2022; Chapter 14; pp. 1–14. [Google Scholar]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Microbiology by numbers. Nat. Rev. Microbiol. 2011, 9, 628. [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Pallauf, K.; Duckstein, N.; Rimbach, G. A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 2017, 76, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef] [PubMed]
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Stark, A.H.; Madar, Z. Olive Oil as a Functional Food: Epidemiology and Nutritional Approaches. Nutr. Rev. 2002, 60, 170–176. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Ramli, M.E.; Salleh, R.M.; Tajarudin, H.A.; Zulkurnain, M. Influence of amylose content on phenolics fortification of different rice varieties with butterfly pea (Clitoria ternatea) flower extract through parboiling. LWT Food Sci. Technol. 2021, 147, 111493. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Nanda, P.K.; Chauhan, P.; Pradhan, S.R.; Biswas, S. Antioxidant Efficacy of Litchi (Litchi chinensis Sonn.) Pericarp Extract in Sheep Meat Nuggets. Antioxidants 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.R.; Marins, J.C.B.; de Oliveira, L.L.; Bastos, D.S.S.; Soares Júnior, D.T.; da Silva, C.D.; Fontes, E.A.F. Beverage based on whey permeate with phenolic extract of jabuticaba peel: A pilot study on effects on muscle and oxidative stress in trained individuals. J. Funct. Foods 2020, 65, 103749. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology—Volume 299—Oxidants and Antioxidants (Part A); Packer, L., Ed.; Academic Press: London, UK; New York, NY, USA, 1999; Chapter 14; pp. 152–178. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2018, 101, 1466–1472. [Google Scholar] [CrossRef]
- Medina, M.B. Simple and Rapid Method for the Analysis of Phenolic Compounds in Beverages and Grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- Kljusurić, J.G.; Mihalev, K.; Bečić, I.; Polović, I.; Georgieva, M.; Djaković, S.; Kurtanjek, Ž. Near-Infrared Spectroscopic Analysis of Total Phenolic Content and Antioxidant Activity of Berry Fruits. Food Technol. Biotechnol. 2016, 54, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ni, Y.; Kokot, S. A Novel Near-Infrared Spectroscopy and Chemometrics Method for Rapid Analysis of Several Chemical Components and Antioxidant Activity of Mint (Mentha haplocalyx Briq.) Samples. Appl. Spectrosc. 2014, 68, 245–254. [Google Scholar] [CrossRef]
- Schmutzler, M.; Huck, C.W. Automatic sample rotation for simultaneous determination of geographical origin and quality characteristics of apples based on near infrared spectroscopy (NIRS). Vib. Spectrosc. 2014, 72, 97–104. [Google Scholar] [CrossRef]
- Lu, X.; Ross, C.F.; Powers, J.R.; Aston, D.E.; Rasco, B.A. Determination of Total Phenolic Content and Antioxidant Activity of Garlic (Allium sativum) and Elephant Garlic (Allium ampeloprasum) by Attenuated Total Reflectance–Fourier Transformed Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Jantasee, A.; Thumanu, K.; Muangsan, N.; Leeanansaksiri, W.; Maensiri, D. Fourier Transform Infrared Spectroscopy for Antioxidant Capacity Determination in Colored Glutinous Rice. Food Anal. Methods 2014, 7, 389–399. [Google Scholar] [CrossRef]
- Schmutzler, M.; Huck, C.W. Simultaneous detection of total antioxidant capacity and total soluble solids content by Fourier transform near-infrared (FT-NIR) spectroscopy: A quick and sensitive method for on-site analyses of apples. Food Control 2016, 66, 27–37. [Google Scholar] [CrossRef]
- Papoti, V.T.; Tsimidou, M.Z. Looking through the qualities of a fluorimetric assay for the total phenol content estimation in virgin olive oil, olive fruit or leaf polar extract. Food Chem. 2009, 112, 246–252. [Google Scholar] [CrossRef]
- Benítez-Martínez, S.; Valcárcel, M. Graphene quantum dots as sensor for phenols in olive oil. Sens. Actuators B Chem. 2014, 197, 350–357. [Google Scholar] [CrossRef]
- Squeo, G.; Caponio, F.; Paradiso, V.M.; Summo, C.; Pasqualone, A.; Khmelinskii, I.; Sikorska, E. Evaluation of total phenolic content in virgin olive oil using fluorescence excitation–emission spectroscopy coupled with chemometrics. J. Sci. Food Agric. 2019, 99, 2513–2520. [Google Scholar] [CrossRef]
- Hallmann, E.; Rozpara, E.; Słowianek, M.; Leszczyńska, J. The effect of organic and conventional farm management on the allergenic potency and bioactive compounds status of apricots (Prunus armeniaca L.). Food Chem. 2019, 279, 171–178. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Ozdemir Olgun, F.A.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin-Ciocalteu Antioxidant Capacity Assay for Measuring Lipophilic Antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef]
- Marko-Varga, G.; Emnéus, J.; Gorton, L.; Ruzgas, T. Development of enzyme-based amperometric sensors for the determination of phenolic compounds. Trends Anal. Chem. 1995, 14, 319–328. [Google Scholar] [CrossRef]
- Sołoducho, J.; Cabaj, J. Phenolic Compounds Hybrid Detectors. J. Biomater. Nanobiotechnol. 2013, 4, 17–27. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Gul, I.; Ahmad, M.S.; Naqvi, S.M.S.; Hussain, A.; Wali, R.; Farooqi, A.A.; Ahmed, I. Polyphenol oxidase (PPO) based biosensors for detection of phenolic compounds: A Review. J. App. Biol. Biotech. 2017, 5, 72–85. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Ribovski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino-Junior, L.H.; Banks, C.E.; Oliveira, O.N.; et al. Polyphenol oxidase-based electrochemical biosensors: A review. Anal. Chim. Acta 2020, 1139, 198–221. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Laccase and Tyrosinase Biosensors Used in the Determination of Hydroxycinnamic Acids. Int. J. Mol. Sci. 2021, 22, 4811. [Google Scholar] [CrossRef]
- Kadam, A.A.; Saratale, G.D.; Ghodake, G.S.; Saratale, R.G.; Shahzad, A.; Magotra, V.K.; Kumar, M.; Palem, R.R.; Sung, J.-S. Recent Advances in the Development of Laccase-Based Biosensors via Nano-Immobilization Techniques. Chemosensors 2022, 10, 58. [Google Scholar] [CrossRef]
- Peter, M.G.; Wollenberger, U. Phenol-oxidizing enzymes: Mechanisms and applications in biosensors. In Frontiers in Biosensorics I. Fundamental Aspects; Scheller, F.W., Schubert, F., Fedrowitz, J., Eds.; Birkhäuser: Basel, Switzerland, 1997; Chapter 5; pp. 63–82. [Google Scholar] [CrossRef]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Florescu, M.; Bala, C. Biosensors for Antioxidants Detection: Trends and Perspectives. Biosensors 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Assessing Antioxidant Activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef]
- Ibarra-Escutia, P.; Gómez, J.J.; Calas-Blanchard, C.; Marty, J.L.; Ramírez-Silva, M.T. Amperometric biosensor based on a high resolution photopolymer deposited onto a screen-printed electrode for phenolic compounds monitoring in tea infusions. Talanta 2010, 81, 1636–1642. [Google Scholar] [CrossRef]
- Vlamidis, Y.; Gualandi, I.; Tonelli, D. Amperometric biosensors based on reduced GO and MWCNTs composite for polyphenols detection in fruit juices. J. Electroanal. Chem. 2017, 799, 285–292. [Google Scholar] [CrossRef]
- Chawla, S.; Rawal, R.; Pundir, C.S. Fabrication of polyphenol biosensor based on laccase immobilized on copper nanoparticles/chitosan/multiwalled carbon nanotubes/polyaniline-modified gold electrode. J. Biotechnol. 2011, 156, 39–45. [Google Scholar] [CrossRef]
- Gamella, M.; Campuzano, S.; Reviejo, A.J.; Pingarrón, J.M. Electrochemical Estimation of the Polyphenol Index in Wines Using a Laccase Biosensor. J. Agric. Food Chem. 2006, 54, 7960–7967. [Google Scholar] [CrossRef]
- Di Fusco, M.; Tortolini, C.; Deriu, D.; Mazzei, F. Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 2010, 81, 235–240. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Lete, C.; Lupu, S.; Palacios-Santander, J.M.; Bellido-Milla, D. Assessment of the Polyphenol Indices and Antioxidant Capacity for Beers and Wines Using a Tyrosinase-Based Biosensor Prepared by Sinusoidal Current Method. Sensors 2019, 19, 66. [Google Scholar] [CrossRef]
- ElKaoutit, M.; Naranjo-Rodriguez, I.; Temsamani, K.R.; Hernández-Artiga, M.P.; Bellido-Milla, D.; Hidalgo-Hidalgo de Cisneros, J.L. A comparison of three amperometric phenoloxidase–Sonogel–Carbon based biosensors for determination of polyphenols in beers. Food Chem. 2008, 110, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Carralero Sanz, V.; Mena, M.L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes: Application to the measurement of a bioelectrochemical polyphenols index in wines. Anal. Chim. Acta 2005, 528, 1–8. [Google Scholar] [CrossRef]
- Gil, D.M.A.; Rebelo, M.J.F. Evaluating the antioxidant capacity of wines: A laccase-based biosensor approach. Eur. Food Res. Technol. 2010, 231, 303–308. [Google Scholar] [CrossRef]
- ElKaoutit, M.; Naranjo-Rodriguez, I.; Temsamani, K.R.; de la Vega, M.D.; Hidalgo-Hidalgo de Cisneros, J.L. Dual Laccase–Tyrosinase Based Sonogel–Carbon Biosensor for Monitoring Polyphenols in Beers. J. Agric. Food Chem. 2007, 55, 8011–8018. [Google Scholar] [CrossRef]
- Gonzalez-Anton, R.; Osipova, M.M.; Garcia-Hernandez, C.; Dubinina, T.V.; Tomilova, L.G.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Subphthalocyanines as electron mediators in biosensors based on phenol oxidases: Application to the analysis of red wines. Electrochim. Acta 2017, 255, 239–247. [Google Scholar] [CrossRef]
- Zrinski, I.; Pungjunun, K.; Martinez, S.; Zavašnik, J.; Stanković, D.; Kalcher, K.; Mehmeti, E. Evaluation of phenolic antioxidant capacity in beverages based on laccase immobilized on screen-printed carbon electrode modified with graphene nanoplatelets and gold nanoparticles. Microchem. J. 2020, 152, 104282. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol Oxidases in Crops: Biochemical, Physiological and Genetic Aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R.; Abadjian, M.-C.Z. Natural Monophenols as Therapeutics, Antioxidants and Toxins; Electron Transfer, Radicals and Oxidative Stress. Nat. Prod. J. 2015, 5, 142–151. [Google Scholar] [CrossRef]
- Dinçkaya, E.; Akyilmaz, E.; Akgöl, S.; Tatar Önal, S.; Zihnioğlu, F.; Telefoncu, A. A Novel Catechol Oxidase Enzyme Electrode for the Specific Determination of Catechol. Biosci. Biotechnol. Biochem. 1998, 62, 2098–2100. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Baysal, Ş.H.; Dinçkaya, E. Investigation of metal activation of a partially purified polyphenol oxidase enzyme electrode. Int. J. Environ. Anal. Chem. 2007, 87, 755–761. [Google Scholar] [CrossRef]
- Güell, M.; Siegbahn, P.E.M. Theoretical study of the catalytic mechanism of catechol oxidase. J. Biol. Inorg. Chem. 2007, 12, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Prexler, S.M.; Frassek, M.; Moerschbacher, B.M.; Dirks-Hofmeister, M.E. Catechol Oxidase versus Tyrosinase Classification Revisited by Site-Directed Mutagenesis Studies. Angew. Chem. Int. Ed. 2019, 58, 8757–8761. [Google Scholar] [CrossRef] [PubMed]
- Creative Enzymes. Available online: https://www.creative-enzymes.com/ (accessed on 10 December 2022).
- Merck. Available online: https://www.sigmaaldrich.com/ (accessed on 10 December 2022).
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure–function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011, 68, 117–128. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A green catalyst for the biosynthesis of poly-phenols. Crit. Rev. Biotechnol. 2018, 38, 294–307. [Google Scholar] [CrossRef]
- Yaropolov, A.I.; Skorobogat’ko, O.V.; Vartanov, S.S.; Varfolomeyev, S.D. Laccase. Appl. Biochem. Biotechnol. 1994, 49, 257–280. [Google Scholar] [CrossRef]
- Minussi, R.C.; Pastore, G.M.; Durán, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Molčanov, K.; Kojić-Prodić, B.; Roboz, M. Structural characterization of p-benzosemiquinone radical in a solid state: The radical stabilization by a low-barrier hydrogen bond. Acta. Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2006, 62, 1051–1060. [Google Scholar] [CrossRef]
- van Gelder, C.W.G.; Flurkey, W.H.; Wichers, H.J. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 1997, 45, 1309–1323. [Google Scholar] [CrossRef]
- Rolff, M.; Schottenheim, J.; Decker, H.; Tuczek, F. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: Molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011, 40, 4077–4098. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, Á.; Rodríguez-López, J.N.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1995, 1247, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nagel, B.; Dellweg, H.; Gierasch, L.M. Glossary for chemists of terms used in biotechnology (IUPAC Recommendations 1992). Pure Appl. Chem. 1992, 64, 143–168. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Tóth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification (Technical Report). Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Andreu-Navarro, A.; Fernández-Romero, J.M.; Gómez-Hens, A. Determination of polyphenolic content in beverages using laccase, gold nanoparticles and long wavelength fluorimetry. Anal. Chim. Acta 2012, 713, 1–6. [Google Scholar] [CrossRef]
- Mediavilla, M.; Revenga-Parra, M.; Gutiérrez-Sánchez, C.; Hernández-Apaolaza, L.; Pariente, F.; Lorenzo, E. Fluorescent enzymatic assay for direct total polyphenol determination in food-related samples. Talanta 2022, 247, 123576. [Google Scholar] [CrossRef]
- Chawla, S.; Rawal, R.; Kumar, D.; Pundir, C.S. Amperometric determination of total phenolic content in wine by laccase immobilized onto silver nanoparticles/zinc oxide nanoparticles modified gold electrode. Anal. Biochem. 2012, 430, 16–23. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Devender; Pundir, C.S. An amperometric biosensor based on laccase immobilized onto Fe3O4NPs/cMWCNT/PANI/Au electrode for determination of phenolic content in tea leaves extract. Enzyme Microb. Technol. 2012, 51, 179–185. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Pundir, C.S. Polyphenol biosensor based on laccase immobilized onto silver nanoparticles/multiwalled carbon nanotube/polyaniline gold electrode. Anal. Biochem. 2011, 419, 196–204. [Google Scholar] [CrossRef]
- Chawla, S.; Rawal, R.; Sharma, S.; Pundir, C.S. An amperometric biosensor based on laccase immobilized onto nickel nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode for determination of phenolic content in fruit juices. Biochem. Eng. J. 2012, 68, 76–84. [Google Scholar] [CrossRef]
- Boubezari, I.; Bessueille, F.; Bonhomme, A.; Raimondi, G.; Zazoua, A.; Errachid, A.; Jaffrezic-Renault, N. Laccase-Based Biosensor Encapsulated in a Galactomannan-Chitosan Composite for the Evaluation of Phenolic Compounds. Biosensors 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- de Macêdo, I.Y.L.; Garcia, L.F.; Oliveira Neto, J.R.; de Siqueira Leite, K.C.; Ferreira, V.S.; Ghedini, P.C.; de Souza Gil, E. Electroanalytical tools for antioxidant evaluation of red fruits dry extracts. Food Chem. 2017, 217, 326–331. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Neto, J.R.; Rezende, S.G.; Lobón, G.S.; Garcia, T.A.; Macedo, I.Y.L.; Garcia, L.F.; Alves, V.F.; Torres, I.M.S.; Santiago, M.F.; Schmidt, F.; et al. Electroanalysis and laccase-based biosensor on the determination of phenolic content and antioxidant power of honey samples. Food Chem. 2017, 237, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.F.; Benjamin, S.R.; Marreto, R.N.; Lopes, F.M.; Golveia, J.C.S.; Fernandes, N.C.; Santiago, M.F.; de Souza Gil, E. Laccase Carbon Paste Based Biosensors for Antioxidant Capacity. The Effect of Different Modifiers. Int. J. Electrochem. Sci. 2015, 10, 5650–5660. [Google Scholar]
- Mohtar, L.G.; Aranda, P.; Messina, G.A.; Nazareno, M.A.; Pereira, S.V.; Raba, J.; Bertolino, F.A. Amperometric biosensor based on laccase immobilized onto a nanostructured screen-printed electrode for determination of polyphenols in propolis. Microchem. J. 2019, 144, 13–18. [Google Scholar] [CrossRef]
- Verrastro, M.; Cicco, N.; Crispo, F.; Morone, A.; Dinescu, M.; Dumitru, M.; Favati, F.; Centonze, D. Amperometric biosensor based on Laccase immobilized onto a screen-printed electrode by Matrix Assisted Pulsed Laser Evaporation. Talanta 2016, 154, 438–445. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Vasilescu, I.; Radoi, A.; Litescu, S.C.; Radu, G.L. Disposable biosensor based on platinum nanoparticles-reduced graphene oxide-laccase biocomposite for the determination of total polyphenolic content. Talanta 2013, 110, 164–170. [Google Scholar] [CrossRef]
- Vasilescu, I.; Eremia, S.A.V.; Kusko, M.; Radoi, A.; Vasile, E.; Radu, G.L. Molybdenum disulphide and graphene quantum dots as electrode modifiers for laccase biosensor. Biosens. Bioelectron. 2016, 75, 232–237. [Google Scholar] [CrossRef]
- Boujakhrout, A.; Jimenez-Falcao, S.; Martínez-Ruiz, P.; Sánchez, A.; Díez, P.; Pingarrón, J.M.; Villalonga, R. Novel reduced graphene oxide–glycol chitosan nanohybrid for the assembly of an amperometric enzyme biosensor for phenols. Analyst 2016, 141, 4162–4169. [Google Scholar] [CrossRef]
- Nadifiyine, S.; Haddam, M.; Mandli, J.; Chadel, S.; Blanchard, C.C.; Marty, J.L.; Amine, A. Amperometric Biosensor Based on Tyrosinase Immobilized on to a Carbon Black Paste Electrode for Phenol Determination in Olive Oil. Anal. Lett. 2013, 46, 2705–2726. [Google Scholar] [CrossRef]
- Draghi, P.F.; Fernandes, J.C.B. Label-free potentiometric biosensor based on solid-contact for determination of total phenols in honey and propolis. Talanta 2017, 164, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Kanjilal, B.; Sarkar, P. Electrochemical Sensor for Detection of Polyphenols in Tea and Wine with Differential Pulse Voltammetry and Electrochemical Impedance Spectroscopy Utilizing Tyrosinase and Gold Nanoparticles Decorated Biomembrane. J. Electrochem. Soc. 2017, 164, B118–B126. [Google Scholar] [CrossRef]
- Hammami, A.; Kuliček, J.; Raouafi, N. A naphthoquinone/SAM-mediated biosensor for olive oil polyphenol content. Food Chem. 2016, 209, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Bonanni, A.; Finotti, E.; Tomassetti, M. Biosensors for determination of total and natural antioxidant capacity of red and white wines: Comparison with other spectrophotometric and fluorimetric methods. Biosens. Bioelectron. 2004, 19, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Montereali, M.R.; Vastarella, W.; Della Seta, L.; Pilloton, R. Tyrosinase biosensor based on modified screen printed electrodes: Measurements of total phenol content. Int. J. Environ. Anal. Chem. 2005, 85, 795–806. [Google Scholar] [CrossRef]
- Freire, R.S.; Thongngamdee, S.; Durán, N.; Wang, J.; Kubota, L.T. Mixed enzyme (laccase/tyrosinase)-based remote electrochemical biosensor for monitoring phenolic compounds. Analyst 2002, 127, 258–261. [Google Scholar] [CrossRef]
- Kochana, J.; Nowak, P.; Jarosz-Wilkołazka, A.; Bieroń, M. Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds. Microchem. J. 2008, 89, 171–174. [Google Scholar] [CrossRef]
- Montereali, M.R.; Della Seta, L.; Vastarella, W.; Pilloton, R. A disposable Laccase–Tyrosinase based biosensor for amperometric detection of phenolic compounds in must and wine. J. Mol. Catal. B Enzym. 2010, 64, 189–194. [Google Scholar] [CrossRef]
- Guisan, J.M. Immobilization of Enzymes and Cells, 3rd ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 1–377. [Google Scholar] [CrossRef]
- Habeeb, A.F.S.A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2018, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, S.; Wardak, C.; Jaroszyńska-Wolińska, J.; Herbert, P.A.F.; Panek, R. Cold Plasma as an Innovative Construction Method of Voltammetric Biosensor Based on Laccase. Sensors 2018, 18, 4086. [Google Scholar] [CrossRef] [PubMed]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Cartoni, A.; Calandra, P.; Tempesta, E.; Giardi, M.T.; Antonacci, A.; Arduini, F.; et al. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Biosens. Bioelectron. 2020, 163, 112299. [Google Scholar] [CrossRef]
- Touloupakis, E.; Chatzipetrou, M.; Boutopoulos, C.; Gkouzou, A.; Zergioti, I. A polyphenol biosensor realized by laser printing technology. Sens. Actuators B Chem. 2014, 193, 301–305. [Google Scholar] [CrossRef]
- Biswas, T.T.; Yu, J.; Nierstrasz, V.A. Piezoelectric inkjet printing of tyrosinase (polyphenol oxidase) enzyme on atmospheric plasma treated polyamide fabric. Sci. Rep. 2022, 12, 6828. [Google Scholar] [CrossRef]
- Gil, D.M.A.; Rebelo, M.J.F. Gallic acid interference on polyphenolic amperometric biosensing using Trametes versicolor laccase. J. Mol. Catal. B Enzym. 2011, 72, 193–198. [Google Scholar] [CrossRef]
- Phenol-Explorer. Database on Polyphenol Content in Foods. Available online: http://phenol-explorer.eu/ (accessed on 10 December 2022).
- Danzer, K.; Currie, L.A. Guidelines for calibration in analytical chemistry. Part I. Fundamentals and single component calibration (IUPAC Recommendations 1998). Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
- Lorenzo, M.; Moldes, D.; Rodríguez Couto, S.; Sanromán, M.A. Inhibition of laccase activity from Trametes versicolor by heavy metals and organic compounds. Chemosphere 2005, 60, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara Reddy, M.; Kanwal, H.K. Influence of carbon, nitrogen sources, inducers, and substrates on lignocellulolytic enzyme activities of Morchella spongiola. J. Agric. Food Res. 2022, 7, 100271. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Danial, E.N. Isolation, Identification and Medium Optimization for Tyrosinase Production by a Newly Isolated Bacillus subtilis NA2 Strain. J. App. Pharm. Sci. 2018, 8, 93–101. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Analysis of Potent Odour-Active Volatile Thiols in Foods and Beverages with a Focus on Wine. Molecules 2019, 24, 2472. [Google Scholar] [CrossRef] [PubMed]
- Bonnaffoux, H.; Roland, A.; Schneider, R.; Cavelier, F. Spotlight on release mechanisms of volatile thiols in beverages. Food Chem. 2021, 339, 127628. [Google Scholar] [CrossRef] [PubMed]
- Ros, J.R.; Rodríguez-López, J.N.; García-Cánovas, F. Effect of L-ascorbic acid on the monophenolase activity of tyrosinase. Biochem. J. 1993, 295, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-T.; Liang, Y.-Q.; Chai, W.-M.; Wie, Q.-M.; Yu, Z.-Y.; Wang, L.-J. Effect of ascorbic acid on tyrosinase and its anti-browning activity in fresh-cut Fuji apple. J. Food Biochem. 2021, 45, e13995. [Google Scholar] [CrossRef]
- Jain, H. The medicinal value and the numerous sources of vitamin c—A review. J. Nutr. Health Food Eng. 2015, 2, 124–134. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Mataix, E.; Luque de Castro, M.D. Simultaneous (or sequential) determination of the total polyphenol index (or I280) and density in wines by flow injection. Analyst 2001, 126, 251–255. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. In Methods in Enzymology—Volume 335—Flavonoids and Other Polyphenols; Packer, L., Ed.; Academic Press: London, UK; New York, NY, USA, 2001; Chapter 17; pp. 190–203. [Google Scholar] [CrossRef]
- Yurtsever, M.; Şengil, İ.A. Biosorption of Pb(II) ions by modified quebracho tannin resin. J. Hazard. Mater. 2009, 163, 58–64. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Alliu, Y.B.; Ofomaja, A.E.; Unuabonah, I.E. Synchronous attenuation of metal ions and colour in aqua stream using tannin–alum synergy. Desalination 2011, 271, 34–40. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Naczk, M.; Oickle, D.; Pink, D.; Shahidi, F. Protein Precipitating Capacity of Crude Canola Tannins: Effect of pH, Tannin, and Protein Concentrations. J. Agric. Food Chem. 1996, 44, 2144–2148. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Khan, A.; Hashem, A.; Abd Allah, E.F.; Al-Harrasi, A. The molecular mass and isoelectric point of plant proteomes. BMC Genomics 2019, 20, 631. [Google Scholar] [CrossRef] [PubMed]
- Xu, F. Effects of Redox Potential and Hydroxide Inhibition on the pH Activity Profile of Fungal Laccases. J. Biol. Chem. 1997, 272, 924–928. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Yin, Q.; Zhou, G.; Peng, C.; Zhang, Y.; Kües, U.; Liu, J.; Xiao, Y.; Fang, Z. The first fungal laccase with an alkaline pH optimum obtained by directed evolution and its application in indigo dye decolorization. AMB Express 2019, 9, 151. [Google Scholar] [CrossRef]
- Tarasov, A.V.; Khamzina, E.I.; Bukharinova, M.A.; Stozhko, N.Y. Flexible Potentiometric Sensor System for Non-Invasive Determination of Antioxidant Activity of Human Skin: Application for Evaluating the Effectiveness of Phytocosmetic Products. Chemosensors 2021, 9, 76. [Google Scholar] [CrossRef]
- Torrinha, Á.; Morais, S. Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. Trends Anal. Chem. 2021, 142, 116324. [Google Scholar] [CrossRef]

| Interferant | Standard: Interferant | Signal Change (%) | Detection Method | Source |
|---|---|---|---|---|
| Acetic acid | 1:1 | 14–20 | Cyclic voltammetry | [94,96] |
| Ascorbic acid | 1:0.1 1:1 1:0.05 NR 1:1 NR 1:10,000 | 7 10–15 9 NR 5 NR 17 | Fluorimetry Cyclic voltammetry Cyclic voltammetry Differential pulse voltammetry Amperometry Amperometry Amperometry | [93] [94,96] [97] [99] [71] [103] [106] |
| Caffeine | 1:1 | 23 | Amperometry | [71] |
| Citric acid | 1:0.1 1:1 | 8 5–11 | Fluorimetry Cyclic voltammetry | [93] [94,96,97] |
| Cysteine | 1:1 1:0.002 | 12–15 9 | Cyclic voltammetry Cyclic voltammetry | [94,96] [97] |
| Ethanol | 1:1 | 13 | Cyclic voltammetry | [96] |
| Fructose | 1:0.1 1:1 1:0.6 | 7 9–26 6 | Fluorimetry Cyclic voltammetry Cyclic voltammetry | [93] [94,96] [97] |
| Glucose | 1:0.1 1:1 1:0.22 1:1 | 5 10–24 10 5 | Fluorimetry Cyclic voltammetry Cyclic voltammetry Amperometry | [93] [94,96] [97] [71] |
| Sodium sulfite | 1:0.1 | 10 | Fluorimetry | [93] |
| Working Electrode 1 | PO Source 2 | Immobilization Technique 3 | Detection Method 4 | LOD (μM) | Storage Stability 5 | FRSs | Validation 6 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Laccase Biosensors | ||||||||
| Lac/ClSubPc/ITO | TV | Cross-linking using GA | CV | 0.169 | NR | Wine | NR | [70] |
| Lac/PhOSubPc/ITO | TV | Cross-linking using GA | CV | 0.155 | NR | Wine | NR | [70] |
| Lac/t-BuSubPc/ITO | TV | Cross-linking using GA | CV | 0.485 | NR | Wine | NR | [70] |
| Lac/CuNPs-CS/cMWCNTs-PANI/Au | GsR | Covalent binding with GA | CV | 0.156 | −20%/7 m | Tea and alcoholic beverages | FCA | [62] |
| Lac/CA/AgNPs/ZnONPs/Au | GsR | Covalent binding with GA | CV | 0.05 | −25%/5 m | Wine | FCA | [94] |
| Lac/Fe3O4NPs/cMWCNTs-PANI/Au | GsR | Covalent binding | CV | 0.03 | −25%/4 m | Tea | FCA | [95] |
| Lac/AgNPs/cMWCNTs-PANI/Au | GsR | Covalent binding | CV | 0.05 | −20%/4 m | Tea and alcoholic beverages | FCA | [96] |
| Lac/NiNPs/cMWCNTs-PANI/Au | GsR | Covalent binding | CV | 0.05 | −15%/4 m | Fruit juices | FCA | [97] |
| Lac-CS-GAL/4ATP/Au | TV | Entrapment in CS-GAL,cross-linking using GA | CV | 10–10 | 15 d | Olive oil | FCA | [98] |
| Lac-CPE | PS | Incorporation into the paste | DPV | 0.01 | NR | Fruits dry extracts | DPPH | [99] |
| Lac-CPE | PS | Incorporation into the paste | DPV | NR | NR | Honey | FCA | [100] |
| Lac-CPE | PS | Incorporation into the paste | DPV | 12 | 10 d | Roasted coffee | FCA, DPPH | [101] |
| Lac/CBPE | TV | Cross-linking using GA and BSA | Amp | 0.03 | NR | Olive oil | FCA | [107] |
| Lac-PESM/DM/Pt | TV | NR | Amp | NR | NR | Wine | FCA, ABTS | [68] |
| Lac/SPCE | TV | MAPLE | Amp | 1 | 100 d | Herbal extracts | FCA | [103] |
| Lac-PVA-AWP/SPCE | TV | Entrapment in PVA-AWP | Amp | 0.524 | −10%/2 m | Herbal tea | FCA | [60] |
| PPy/Lac/AuNPs/SPCE | NR | Entrapment in PPy | Amp | 0.83 | −15%/1 m | Propolis | FCA | [102] |
| Lac-nNafion/AuNPs/GNPLs-SPCE | RV | Entrapment in nNafion | Amp | 1.5 | 5 d | Wine and syrup | ABTS | [71] |
| nNafion/Lac/rGO/PtNPs/SPCE | TV | Entrapment in nNafion | Amp | 0.09 | 14 d | Herbal tea | FCA | [104] |
| Lac/GQDs/MoS2NFs/SPCE | TV | Adsorption | Amp | 0.32 | −15%/28 d | Wine | FCA | [105] |
| Lac-Nafion/SCE | TV | Cross-linking using GA, stabilization with Nafion | Amp | 0.06 | 7 d | Beer | FCA | [66] |
| Lac/rGO-MWCNTs/GCE | TV | Cross-linking using GA annd BSA | Amp | 0.3 | 15 d | Fruit juices | ABTS | [61] |
| Lac/rGO-GCS/GCE | TV | Cross-linking using GA | Amp | 0.076 | 15 d | Herbal tea | FCA | [106] |
| Lac/GCE | TV | Cross-linking using GA | Amp, FIA-Amp | 0.015, 0.235 | 15 d | Wine | FCA | [63] |
| Lac-PAP/MWCNTs/SPCE | TV | Entrapment in PAP | FIA-Amp | 0.588 | 10 d | Wine | FCA | [64] |
| Lac-PAP/MWCNTs/SPCE | TH | Entrapment in PAP | FIA-Amp | 1.763 | 10 d | Wine | FCA | [64] |
| Lac-PAP/SWCNTs/SPCE | TH | Entrapment in PAP | FIA-Amp | 3.527 | 10 d | Wine | FCA | [64] |
| Tyrosinase biosensors | ||||||||
| Tyr/cPVC-G/cPVC-G-KMnO4 | MA | Covalent binding with EDC | Potentiometry | 0.73 | 3 m | Honey and propolis | FCA | [108] |
| Tyr/ClSubPc/ITO | M | Cross-linking using GA | CV | 0.757 | NR | Wine | NR | [70] |
| Tyr/PhOSubPc/ITO | M | Cross-linking using GA | CV | 0.101 | NR | Wine | NR | [70] |
| Tyr/t-BulSubPc/ITO | M | Cross-linking using GA | CV | 0.21 | NR | Wine | NR | [70] |
| Tyr/AuNPs/ESM/GCE | M | Cross-linking using GA | DPV | 0.714 | −7%/1 m | Tea and wine | HPLC | [109] |
| Tyr-CS/rGO-MWCNTs/GCE | M | Entrapment in CS | Amp | 0.5 | 1 d | Fruit juices | ABTS | [61] |
| Tyr/AuNPs/GCE | M | Cross-linking using GA | Amp | 0.15 | 18 d | Wine | FCA | [67] |
| Tyr-CS/MPNQ/Au | M | Entrapment in CS | Amp | 0.019 | −6%/7 d | Olive oil | NR | [110] |
| DM/Tyr-KCG/TM/Pt | NR | Entrapment in KCG | Amp | NR | NR | Wine | FCA | [112] |
| Tyr-PEDOT/SCE | M | Entrapment in PEDOT | Amp | 4.33 | 10 d | Beer and wine | ABTS | [65] |
| Tyr/CBPE | AB | Cross-linking using GA and BSA | Amp | 0.006 | 21 d | Olive oil | FCA | [107] |
| Tyr/AuNPs-SPCE | M | Cross-linking using GA | Amp | 1.2 | NR | Beer | FCA | [111] |
| Tyr/APTES/Fc-SPCE | M | Cross-linking using GA and BSA | FIA-Amp | 4 | 30 d | Wine | NR | [113] |
| Laccase–tyrosinase biosensors | ||||||||
| Lac-Tyr-Nafion/SCE | TV, M | Cross-linking using GA, stabilization with Nafion | Amp | 0.026 | −6%/2 d, −17%/15 d | Beer | FCA | [69] |
| Lac-Tyr-DGS/APTES/Fc/SPCE | TV, M | Entrapment in DGS | FIA-Amp | 2 | −9%/2 d, −43%/5 d | Must and wine | FCA, I280 | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasov, A.; Stozhko, N.; Bukharinova, M.; Khamzina, E. Biosensors Based on Phenol Oxidases (Laccase, Tyrosinase, and Their Mixture) for Estimating the Total Phenolic Index in Food-Related Samples. Life 2023, 13, 291. https://doi.org/10.3390/life13020291
Tarasov A, Stozhko N, Bukharinova M, Khamzina E. Biosensors Based on Phenol Oxidases (Laccase, Tyrosinase, and Their Mixture) for Estimating the Total Phenolic Index in Food-Related Samples. Life. 2023; 13(2):291. https://doi.org/10.3390/life13020291
Chicago/Turabian StyleTarasov, Aleksey, Natalia Stozhko, Maria Bukharinova, and Ekaterina Khamzina. 2023. "Biosensors Based on Phenol Oxidases (Laccase, Tyrosinase, and Their Mixture) for Estimating the Total Phenolic Index in Food-Related Samples" Life 13, no. 2: 291. https://doi.org/10.3390/life13020291
APA StyleTarasov, A., Stozhko, N., Bukharinova, M., & Khamzina, E. (2023). Biosensors Based on Phenol Oxidases (Laccase, Tyrosinase, and Their Mixture) for Estimating the Total Phenolic Index in Food-Related Samples. Life, 13(2), 291. https://doi.org/10.3390/life13020291







