Abstract
Temporomandibular disorder (TMD) is a common condition disabling people and bringing up costs. The aim of this study was to investigate the effects of manual therapy on pain intensity, maximum mouth opening (MMO) and disability. Searches were conducted in six databases for randomised controlled trials (RCTs). Selection of trials, data extraction and methodological quality assessment were conducted by two reviewers with discrepancies resolved by a third reviewer. Estimates were presented as mean differences (MDs) or standardized mean differences (SMDs) with 95% confidence intervals (CIs). Quality of the evidence was assessed using the GRADE approach. Twenty trials met the eligibility criteria and were included. For pain intensity, high and moderate quality evidence demonstrated the additional effects of manual therapy at short- (95% CI −2.12 to −0.82 points) and long-term (95% CI −2.17 to −0.40 points) on the 0–10 points scale. For MMO, moderate to high quality evidence was found in favour of manual therapy alone (95% CI 0.01 to 7.30 mm) and its additional effects (95% CI 1.58 to 3.58 mm) at short- and long-term (95% CI 1.22 to 8.40 mm). Moderate quality evidence demonstrated an additional effect of manual therapy for disability (95% CI = −0.87 to −0.14). Evidence supports manual therapy as effective for TMD.
1. Introduction
Temporomandibular disorders (TMD) can be defined as a group of pathologies of the temporomandibular joint and muscles involved [1]. TMD can be classified as myogenic (i.e., muscle and myofascial origin), arthrogenic, mixed and joint-related disorders (i.e., disc displacements with or without reduction, arthritis or subluxation) according to The Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) [1,2]. It is a common health condition worldwide with an estimated prevalence ranging from 11% to 31%, and is especially high in people with multiple sclerosis [3,4]. After a new episode of TMD, 27% of people persist with significant pain one year later [5,6,7], and recurrence is common [8]. Its related pain and disability bring direct (e.g., use of medication to alleviate symptoms) and indirect (e.g., productivity loss) costs [9,10,11,12,13]; therefore, effective management of the condition is important.
Management options for TMD include occlusal splints, cognitive behavioural therapy, acupuncture, manual therapy, therapeutic exercises, nonsteroidal anti-inflammatory drugs, surgical treatment and others [14,15,16,17,18,19]. Counselling and a conservative approach are generally advocated as a first management choice by health professionals for patients with disabling TMD [19]. Previous systematic review suggested that manual therapy may improve pain intensity, function, and oral health-related quality of life in this population [19]; however, their scope and methods adopted might have compromised the effect estimates presented. These consist of the inclusion of trials that did not adequately compare manual therapy to investigate its effectiveness and the inclusion of non-randomised controlled trials (RCTs) [19]. In addition, the evidence needs updating, as new trials have been published since then. Thus, a new systematic review of randomized controlled trials that methodologically isolate manual therapy to assess its isolate or additional effects is needed to inform the current state of the evidence on this topic.
The aim of this systematic review of RCTs was to investigate the efficacy of manual therapy approaches and whether they enhance effects when combined with other active intervention on pain intensity, maximum mouth opening (MMO) and disability in TMD. The quality of evidence was assessed using the Grading of Recommendations Assessment (GRADE) approach [20].
2. Methods
2.1. Study Design
This systematic review of RCTs followed the Cochrane recommendations [21] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [22] (Supplementary File S1: PRISMA checklist). Its protocol was prospectively registered in the International Prospective Registry of Systematic Reviews (PROSPERO) platform (CRD42022372298) and Open Science Framework (DOI: 10.17605/OSF.IO/XSN42).
2.2. Search Strategy and Study Selection
Searches were conducted on MEDLINE, COCHRANE, EMBASE, AMED, PSYCINFO and PEDRO without language or date restrictions up to 3 October 2022. Search terms were related to “randomised controlled trials” and “temporomandibular disorders”. A detailed search strategy is in the Supplementary File S2: Search Strategy. In addition, we hand searched identified systematic reviews published in the field for potentially relevant full texts that do not identify in the optimized searches. After searches, the retrieved references were exported to an Endnote® file and duplicates were removed. Then, two independent reviewers (JPM and LAS) screened titles and abstracts and assessed potential full texts. Those trials fulfilling our eligibility criteria were included. The between-reviewer discrepancies were resolved by a third reviewer (VCO).
2.3. Inclusion and Exclusion Criteria
We included RCTs investigating people of both sexes, regardless of age, diagnosed with TMD of any duration or type/classification, i.e., myogenic, arthrogenic, mixed and joint-related disorders. The intervention of interest was any manual therapy approach, i.e., any clinician-applied movement of the joints and other structures such as joint mobilization or manipulation (thrust), massage, myofascial release techniques/soft-tissue mobilization, muscle energy techniques, passive stretching and others, as investigated previously [17], using the hands and/or any assisting device. We compared the intervention of interest with control (i.e., placebo, no intervention, waiting list or sham) to investigate the potential specific effects of manual therapy. To investigate whether manual therapy approaches enhance the estimated effects of other active intervention, we also considered comparisons between manual therapy approaches combined with any other active intervention and the other active intervention standing alone. Our outcomes of interest were pain intensity, maximum mouth opening/MMO (i.e., maximum distance between the edge of the upper incisors and the edge of the lower incisors with or without pain) and oral disability. We considered any valid instrument such as Visual Analog Scale—VAS or Numerical Rating Scales—NRS [23] for pain intensity, ruler and caliper for maximum mouth opening [24], and Jaw Functional Limitation Scale (JFLS) [25] and Mandibular Function Impairment Questionnaire (MFIQ) [26] for disability.
2.4. Data Extraction
Two independent reviewers (JPM and LAS) extracted characteristics and outcome data from included trials. Between-reviewer disagreements were resolved by a third reviewer (VCO). The extracted data include study type; the participants; details about the interventions and comparator; outcomes and time-points for the purpose of this review. For our outcomes of interest, we extracted post-intervention means (first option) or within-group mean changes over time, standard deviations (SDs) and sample sizes for each of our groups of interest to investigate the effects at immediate- short- and long-term. Immediate effects were considered as the point of measure right after a single session of manual therapy. We considered short-term effects follow-ups from one to 12 weeks after randomization and long-term effects as follow-ups over 12 weeks after randomization. If more than one time-point was available within the same follow-up period, the one closer to the end of the intervention was considered. When outcome data were not reported, at first, the authors were contacted. If we received no answer, we imputed when possible following the recommendations [21]. When authors did not respond and imputation was not possible, trials were excluded from the quantitative analysis.
2.5. Risk of Bias Assessment
Two independent reviewers (JPM and LAS) assessed the risk of bias of included trials using the 0–10 PEDRO scale [27]. According to this scale, higher scores represent a higher methodological quality. Discrepancies were resolved by a third reviewer (VCO). When available, we used scores already on the PEDRO database (https://pedro.org.au/, accessed on 10 November 2022).
2.6. Data Analysis
When possible, data were converted to a common scale and meta-analysis was conducted using random-effects models (DerSimonian and Laird method). Mean differences (MDs) and 95% CIs were reported in forest-plots. When it was not possible to convert data to a common scale, estimates were presented as standardized mean differences (SMDs). The clinical importance of the interventions of interest was interpreted by comparing the estimated effect sizes and 95% CI in association with the minimum clinically important difference (MCID) of the outcome of interest, or Minimal Detectable Change (MDC) when MCID was not available. MCID considered for pain intensity was 2 points on the 0–10 points scale [28]; MDC of 5 mm for MMO [29]; MDC of 8 points on the 0–68 on Migraine Functional Impact Questionnaire (MFIQ) or 7 points on the 0–63 on Craniofacial Pain and Disability Inventory (CF-PDI) [29] for disability. We used the Hedges’ g effect size measure when estimates were presented as SMD, considering the cut-off points of 0.20, 0.50 and 0.80 for small, medium and large effects, respectively. All analyses were performed in the Comprehensive Meta-analysis software, version 2.2.04 (Biostat, Englewood, NJ, USA). Heterogeneity was assessed using I². We planned to perform subgroup and sensitivity analyses to assess the impact of potential sources of heterogeneity and risk of bias on the estimates. All procedures followed the recommended methods [21].
Two independent reviewers (JPM and LAS) assessed the quality of the current evidence using the GRADE system (Classification of Recommendations, Evaluation, Development and Evaluations) [30,31]. Any disagreement was resolved by consensus or a third reviewer (VCO). According to the four-level GRADE system, the evidence may range from high to very low quality, with low levels indicating that future high-quality trials are likely to change estimated effects. In the current review, evidence began from high quality and was downgraded for each of the following issues: serious imprecision when analysed sample less than 400 [32]; serious risk of bias when more than 25% of the analysed participants are from trials with a high risk of bias (i.e., PEDRO scores less than 7 out of 10) [33]; and serious inconsistency when I2 > 50%, visual inspection of forest plots or when pooling was not possible [21]. We evaluated the publication bias using visual inspection of funnel plots and the Egger’s test adopting an α = 0.1 when data from at least ten trials were pooled in the same meta-analysis [20,34].
3. Results
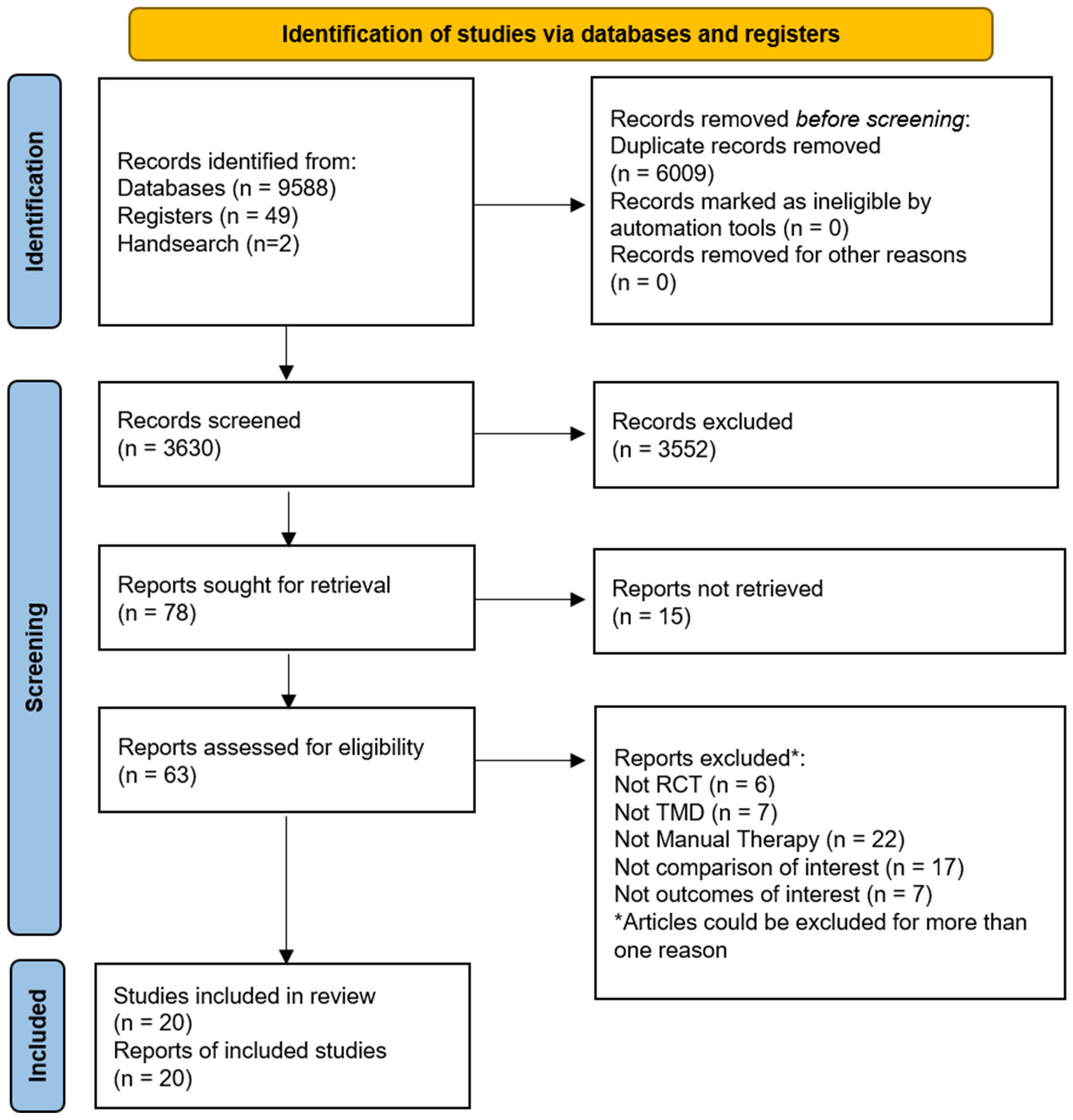
A total of 9639 records were retrieved from our searches, 6009 duplicates were removed, and the remaining 3630 titles and abstracts were screened. Then, 63 potential full texts were assessed for eligibility and 20 trials were included [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The study selection flow diagram is available in Figure 1.
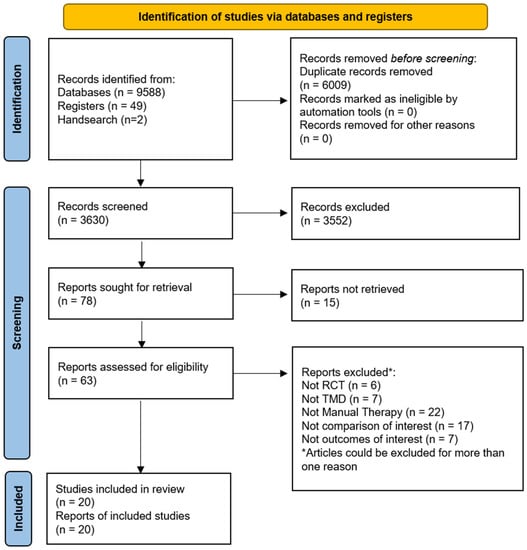
Figure 1.
Flow of studies through the literature search and screening. RCT = randomised controlled trial; TMD = Temporomandibular disorders.
3.1. Characteristics of Included Trials and Assessment of Risk of Bias
The included trials were published between 2005 and 2022, conducted in Spain (five trials), Brazil (four trials), Japan (two trials), USA (two trials), Turkey (two trials), Australia (one trial), Iran (one trial), Portugal (one trial), Thailand (one trial) and Croatia (one trial). Most of them (85%) used some version of the Diagnostic Criteria for TMD. Five trials investigated the effects of manual therapy versus control (sham or wait list) and fifteen trials investigated the additional effects of manual therapy on pain intensity (16 trials), MMO (16 trials) and/or disability (7 trials).
The modalities of manual therapy used were manual pressure release techniques (six trials), joint manipulation (four trials), joint mobilization (one trial), soft-tissue mobilization (one trial), stretching (one trial), instrumental-assisted techniques (two trials), massage (one trial), multimodalities (i.e., combination of two or more modalities of manual therapy) (five trials) and not specified (one trial). When outcome data was not adequately provided, we contacted the authors but received no answer, so we reported the findings that were available. Findings from trials with skewed data were reported separately. Further information regards the characteristics of the included trials are presented in Table 1.

Table 1.
Characteristics of the included trials (n = 20).
The PEDRO scores of the included trials ranged from 1 to 9 points out of 10 (median = 7 points). Fourteen trials (70%) were classified as low risk of bias (i.e., scores ≥ 7 points). The main reasons for increasing risk of bias were not blinding therapists (20 trials [100%]), not blinding participants (14 trials [70%]), not performing concealed allocation (7 trials [35%]) and not blinding assessors and not performing an intention-to-treat analysis (5 trials [25%]). Detailed risk of bias assessment is presented in Table 2.

Table 2.
Risk of bias assessment—Pedro scale (n = 20).
3.2. Effects of Manual Therapy on Pain Intensity in People with Temporomandibular Disorders
Four trials [39,42,44,45] investigated the effects of manual therapy when compared with control (sham or waiting list) and twelve trials investigated the additional effects of manual therapy when combined with other active intervention on pain intensity [35,36,38,41,43,46,47,49,50,51,52,53]. Seven trials used the 0–10 NRS [39,42,46,47,49,50,52], five trials used the 0–10 VAS [36,38,41,43,51] and four trials used the 0–100 VAS [35,44,45,53]. For pooling, outcome data were converted to a common 0–10 points scale.
3.2.1. Manual Therapy versus Control on Pain Intensity
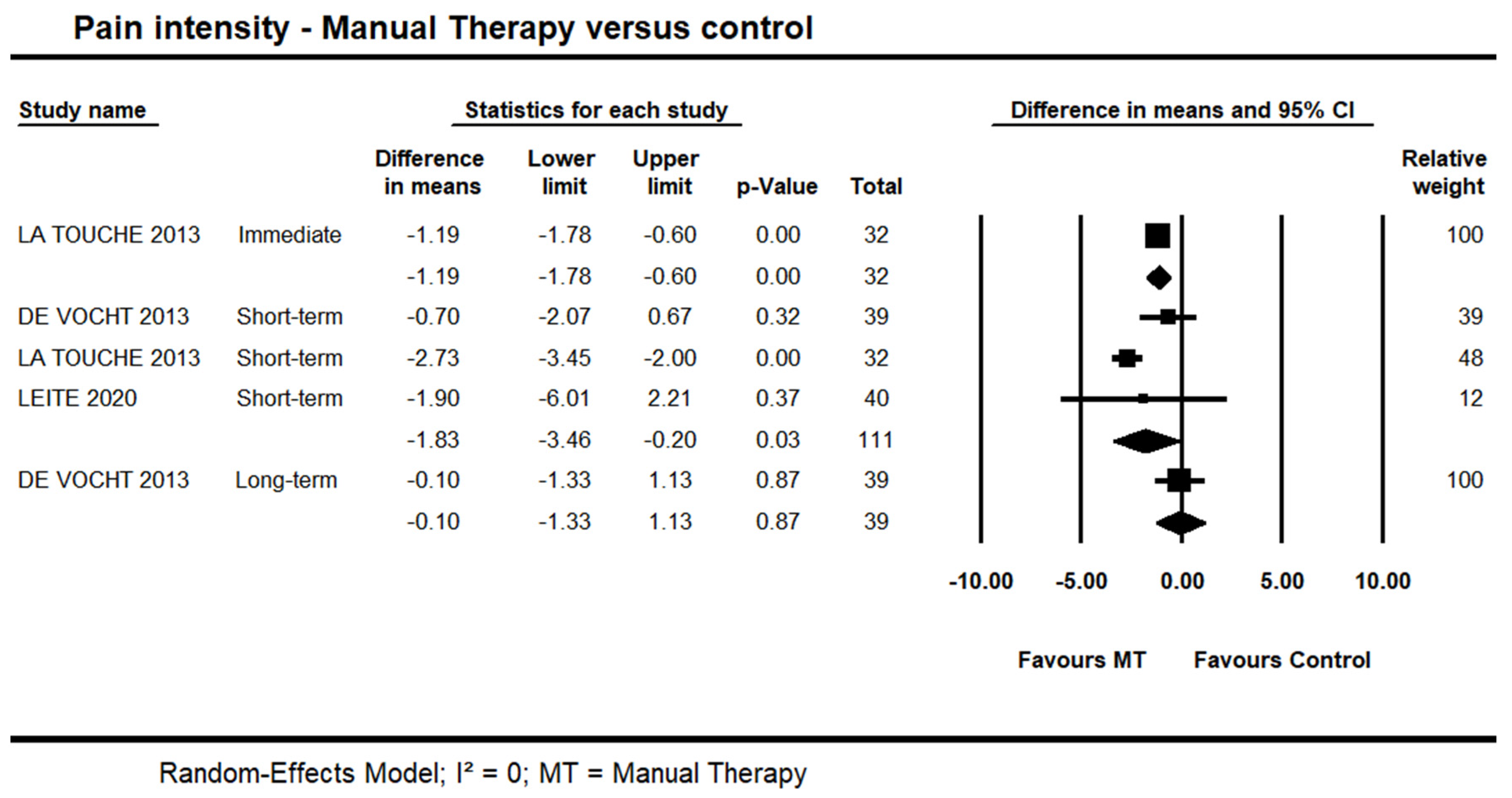
One trial [44] provided low quality evidence of an immediate effect of manual therapy on pain intensity (MD = −0.88 points on the 0–10 points scale, 95% CI −1.57 to −0.19; n = 32). Data from three trials [39,44,45] also provided low quality evidence of a potential short-term effect for manual therapy on pain intensity (95% CI −3.46 to −0.20; I² = 0.0; n = 111). Long-term, one trial [39] provided very-low quality evidence of no difference (95% CI −1.33 to 1.13; n = 39) (Figure 2). It was not possible to include one trial in the pooling due to skewed data [42]. In this trial, the author reported a statistically significant difference in favour of manual therapy versus control at short- and long-terms; however, no detailed information in regards to the between-group difference and variability was reported.
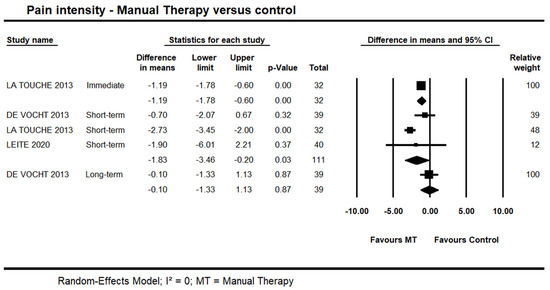
Figure 2.
Forest plot of manual therapy versus control on pain intensity at immediate-, short- and long-term. Studies included were: Devocht et al., 2013 [39]; La Touche et al., 2013 [44]; Leite et al., 2020 [45].
3.2.2. Additional Effects of Manual Therapy on Pain Intensity
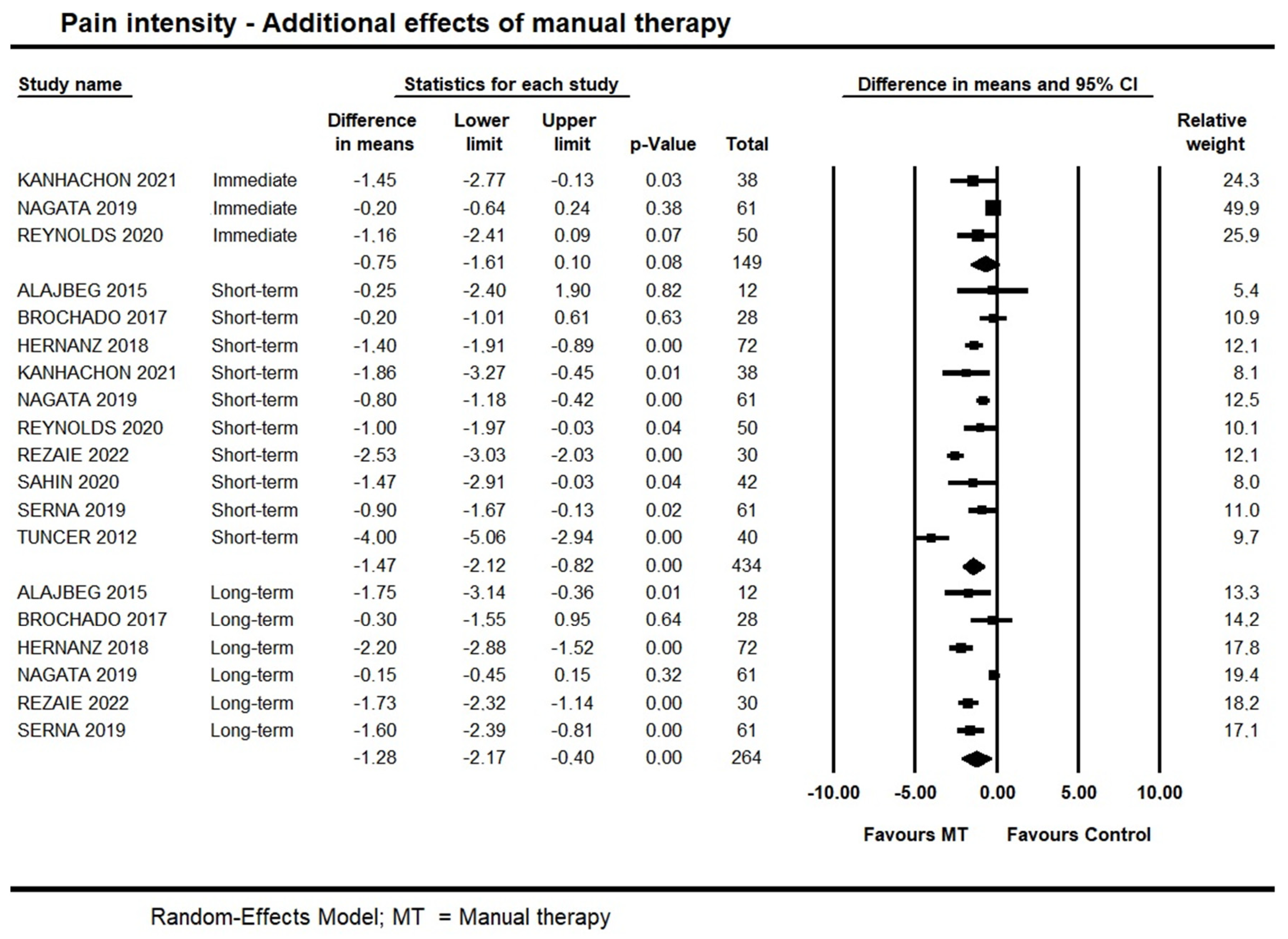
Five trials [36,43,46,47,49] investigated the immediate additional effects of manual therapy on pain intensity, and two of them were not pooled due to skewed data [36] and lack of standard deviation measures [46]. Pooled data provided moderate quality evidence for no immediate additional effect of manual therapy on pain intensity (95% CI −1.61 to 0.10; I² = 0.0; n = 149). One of the two trials not pooled [36] also showed no immediate additional effect for manual therapy (p = 0.53); however, the other trial [46] suggested an additional effect of manual therapy combined with exercise when compared with exercises alone (−2.9 points out of 10).
Short-term, high-quality evidence from 10 trials showed an additional effect of manual therapy on pain intensity (95% CI −2.12 to −0.82; I² = 10; n = 434). Publication bias was not found (Supplementary File S3: Funnel plot), and sensitivity analysis removing trials with a high risk of bias [35,38] did not detect any potential impact on the estimates (95% CI −2.41 to −1.00; 8 trials, n = 394). We also conducted a subgroup analysis exploring whether different modalities of manual therapy impacted on the estimates; no impact was found. Findings are available on Supplementary File S4: Manual therapy modalities subgroup analysis on pain intensity.
At long-term, moderate quality evidence from six trials supported an additional effect of manual therapy (95% CI −2.17 to −0.40; I² = 0.0; n = 342). Forest plots with estimates in the different time-points are shown in Figure 3.
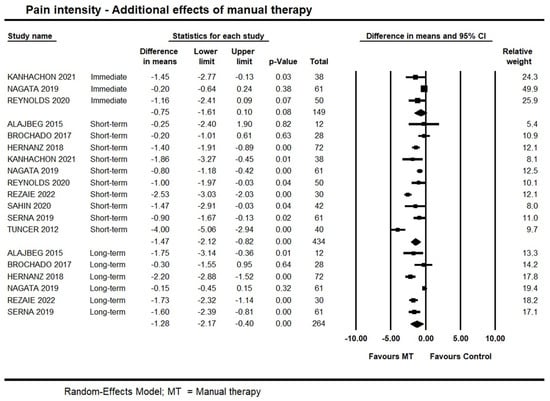
Figure 3.
Forest plot of the additional effects of manual therapy on pain intensity at immediate-, short- and long-term. Studies included were: Alajbeg et al., 2015 [35]; Brochado et al., 2017 [38]; Hernanz et al., 2018 [41]; Kanhachon et al., 2021 [43]; Nagata et al., 2019 [47]; Reynolds et al., 2020 [49]; Rezaie et al., 2022 [50]; Sahin et al., 2020 [51]; Serna et al., 2019 [52]; Tuncer et al., 2012.
3.3. Effects of Manual Therapy on Maximum Mouth Opening in People with Temporomandibular Disorders
Three trials [42,45,48] investigated the effects of manual therapy when compared with the control (sham or waiting list) and 13 trials investigated its additional effects on MMO [35,36,37,41,43,46,47,49,50,51,52,53,54]. MMO was assessed with Caliper in six trials [36,37,45,47,48,50], with Ruler in three trials [51,52,53], with other measurement tools in two trials [42,49] and four trials did not report the instrument used [35,41,46,54].
3.3.1. Manual Therapy versus Control on Maximum Mouth Opening
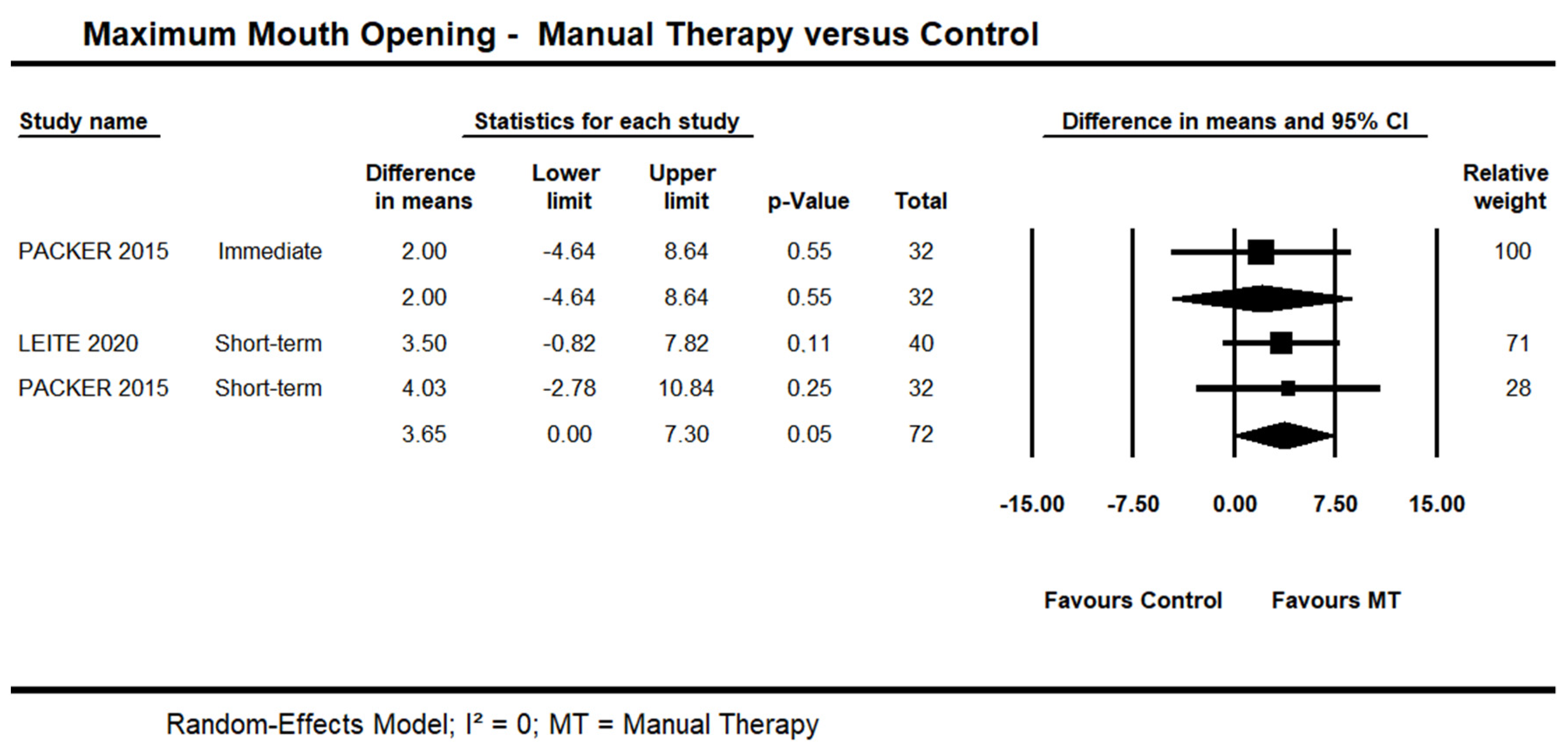
One trial [48] provided low quality evidence of no immediate effect of manual therapy on MMO (95% CI −4.64 to 8.64; n = 32). Short-term, moderate quality evidence from two trials [45,48] showed an effect on MMO (95% CI 0.01 to 7.30 mm; I² = 0.0; n = 72) (Figure 4). One trial [42] was not included in the pooling due to skewed data. A statistically significant difference was reported in favour of manual therapy versus control at the short- and long-term. No detailed information was available.
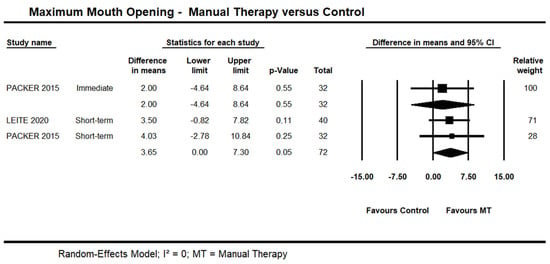
Figure 4.
Forest plot of manual therapy versus control on maximum mouth opening at immediate- and short-term. Studies included were: Leite et al., 2020 [45]; Packer et al., 2015.
3.3.2. Additional Effects of Manual Therapy on Maximum Mouth Opening
Seven trials [35,37,43,46,47,49,54] investigated the immediate additional effects of manual therapy on MMO. It was possible to pool data from five of them [35,37,43,47,49] due to a lack of standard deviations [46,54]. Moderate quality evidence demonstrated no between-group differences (95% CI −0.91 to 6.20 mm; I² = 0.0; n = 251). Contradictory findings were reported by the other two trials not included in the meta-analysis: Yoshida et al. [54] and Lucas et al. [46] found a between-group difference in favour of manual therapy on MMO.
Short-term, high-quality evidence from nine trials showed an additional effect of manual therapy on MMO (95% CI 1.58 to 3.58 mm; I² = 0; n = 494). Sensitivity analysis removing trials with high risk of bias [38] did not suggest an impact on the estimates (95% CI 1.43 to 3.88 mm; 8 trials, n = 394).
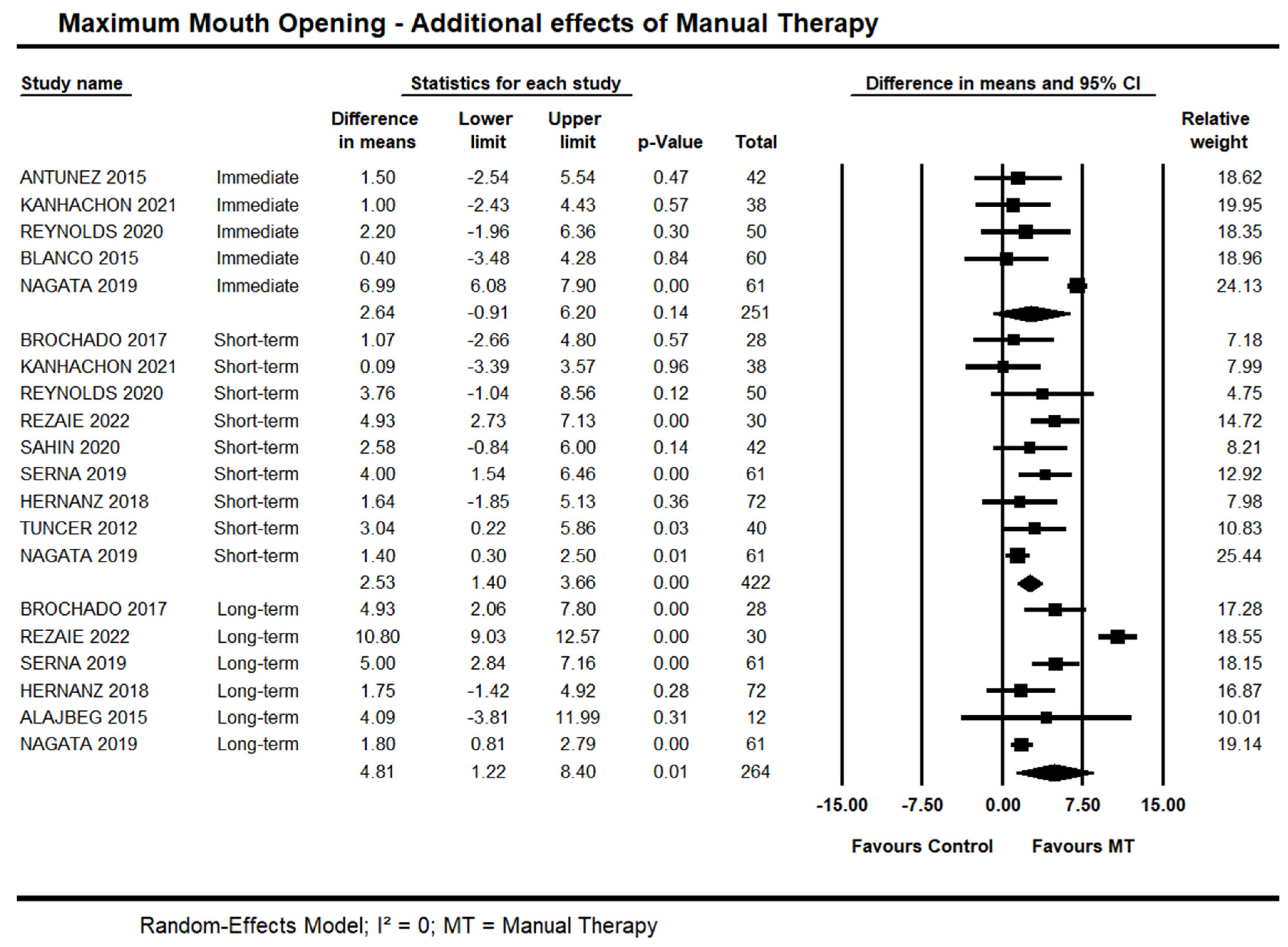
Long-term, moderate quality evidence from six trials showed an effect of manual therapy on MMO (95% CI 1.22 to 8.40 mm; I² = 0.0; n = 264). Sensitivity analysis removing trials with high risk of bias [35,38] did not suggest an impact on the estimates (95% CI 0.24 to 3.88; I² = 0.0; 4 trials, n = 224). Forest Plots with estimates at different time points are shown in Figure 5.
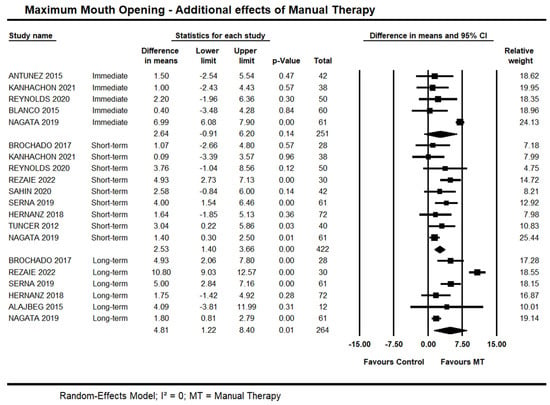
Figure 5.
Forest plot of the additional effects of manual therapy on maximum mouth opening at immediate-, short- and long-term. Studies included were: Alajbeg et al., 2015 [35]; Antunez et al., 2015 [36]; Blanco et al., 2015 [37]; Brochado et al., 2017 [38]; Hernanz et al., 2018 [41]; Kanhachon et al., 2021 [43]; Nagata et al., 2019 [47]; Reynolds et al., 2020 [49]; Rezaie et al., 2022 [50]; Sahin et al., 2020 [51]; Serna et al., 2019 [52]; Tuncer et al., 2012 [53].
3.4. Effects of Manual Therapy on Disability in People with Temporomandibular Disorders
For disability, one trial [45] investigated the effects of manual therapy when compared with control (sham or wait list) and four trials investigated the additional effects of manual therapy [40,49,51,52]. The outcome measures used were the 0–68 MFIQ [45], the 0–100 Fonseca Patient History Index (FPHI) [40], the 0–8 points JFLS [51], the 0–20 points JFLS [49] and the 0–63 points Craniofacial Pain and Disability Inventory (CF-PDI) [52]. Due to the heterogeneity of measures, we reported SMD.
3.4.1. Manual Therapy versus Control on Disability
Short-term, one trial [45] suggested an effect of manual therapy on disability when compared with control. Post-intervention disability differed 6.5% in favour of manual therapy (p = 0.01).
3.4.2. Additional Effects of Manual Therapy on Disability
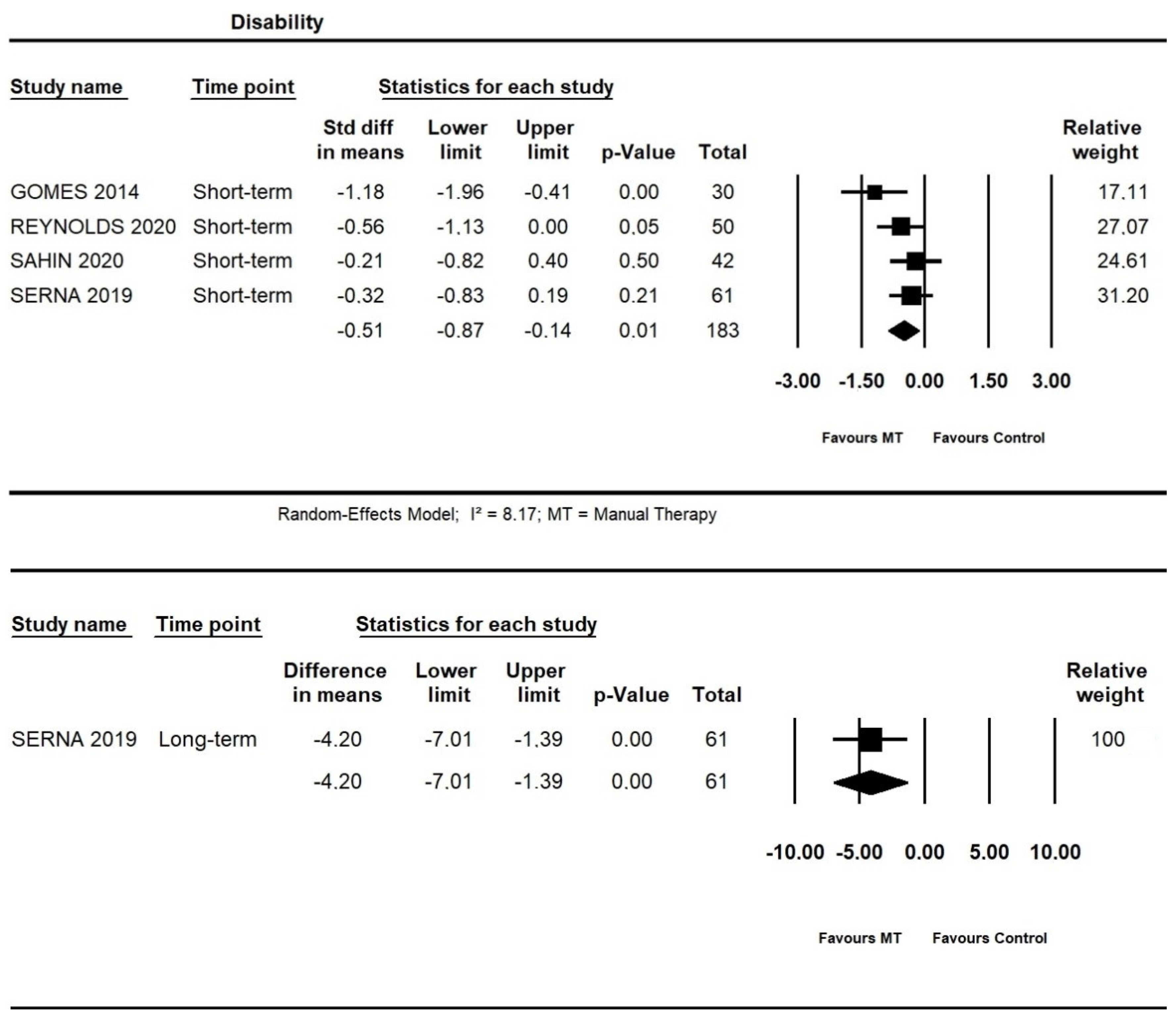
At short-term, moderate quality evidence from four trials showed an additional effect of manual therapy on disability (SMD = −0.51, 95% CI −0.87 to −0.14; n = 183). At long-term, one trial [52] provided low quality evidence for an additional effect of manual therapy on the disability (95% CI −7.01 to −1.39; I² = 8.17; n = 61) (Figure 6).
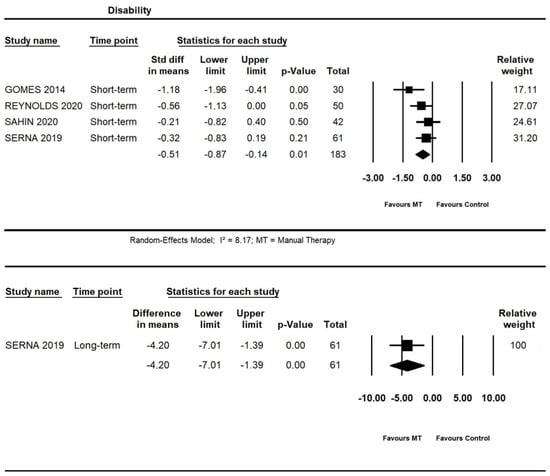
Figure 6.
Forest plot of the additional effects of manual therapy on disability short- and long-term. The studies included were: Gomes et al., 2014 [40]; Reynolds et al., 2020 [49]; Sahin et al., 2020 [51]; Serna et al., 2019 [52].
The overall quality of evidence in the systematic review ranged from very low to high. The summary of findings with the GRADE assessment is reported in Table 3.

Table 3.
Summary of findings with grade assessment (n = 20).
4. Discussion
This systematic review and meta-analysis found that manual therapy may have positive effects in the management of pain intensity, MMO and disability related to TMD; however, the effects’ sizes are small and may not be clinically relevant. Current quality of evidence ranged from very low to high, so future high quality RCTs are likely to change the estimates. Moderate quality of evidence supports joint manipulation, manual pressure, stretching and the combination of two or more manual therapies as additional therapies, with a similar small effect size. Therefore, the choice of the manual therapy technique should rely on the expertise of the health professional and preferences of the patient.
Our results corroborate with previous systematic reviews [17,19,55,56,57,58,59], which also found some positive effects in favour of manual therapy and other conservative interventions for pain intensity, MMO and disability, although the quality of the evidence has increased due to the inclusion of new trials. Among these, two recent systematic reviews [17,59] investigate the effects of conservative approaches in arthrogenic [17] and myogenic-related TMD [59]; however, manual therapy was considered as a general physical therapy approach and analysed together with other interventions such as exercises modalities, education and others. For that reason, our systematic review provides the most up-to-date evidence of manual therapy approaches for the management of TMD.
There are important issues to be addressed in order to improve the current state of the literature on this topic. The main reason to downgrade the level of evidence in our review was imprecision due to the sample size. Moreover, most of the included trials have a poor reporting quality and did not present data appropriately. Future high-quality RCTs should focus on recruit larger sample sizes and use the reporting checklist. Moreover, it is important to include economic evaluation and investigation of adverse events as outcomes to improve the decision-making process.
This systematic review was conducted with strong methodological rigor following recommendations. It updates and synthesizes all available evidence on the efficacy of manual therapy for pain intensity, MMO and disability in people with TMD. Estimating the effect sizes on critical outcomes for patients, assessing the certainty of evidence for each effect estimate and discussing the clinical relevance of the effect sizes across therapies informs patients and clinicians in their decision making. However, this review has some potential limitations. It included RCTs with patients with any diagnosis or type/classification (myogenic, arthrogenic or mixed) and also joint disorders. Subgroup analysis for the different classification of TMD was not possible due the limited number of trials including specifics types of TMD. Future trials should explore the effects of manual therapy in the different TMD diagnosis. In addition, our investigation was restricted to three clinical outcomes. It could be valuable to investigate other important clinical outcomes such as a health-related quality of life, pain pressure threshold and most importantly, the costs and adverse effects of the intervention.
5. Conclusions
We found moderate to high quality evidence of the positive effects of manual therapy modalities for pain intensity, maximum mouth opening and disability in temporomandibular disorders. However, the effect sizes are small and may not be clinically important. Future high-quality RCTs with larger sample sizes should explore the effects of manual therapy in the different TMD diagnosis, clarify adverse effects and include an economic evaluation for a better decision-making process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13020292/s1, Supplementary File S1: PRISMA Checklist; Supplementary File S2: Search Strategy; Supplementary File S3: Funnel plot; Supplementary File S4: Manual therapy modalities subgroup analysis on pain intensity.
Author Contributions
Conceptualization, V.C.O., J.P.M., L.S.V., P.R.M.P., F.S. and M.A.A.; Methodology, J.P.M., V.C.O. and L.A.S.; Analysis, J.P.M. and V.C.O.; Investigation, J.P.M., L.A.S. and V.C.O.; Resources, L.S.V. and F.S.; Writing—Original Draft Preparation, V.C.O., J.P.M., L.S.V., P.R.M.P., F.S. and M.A.A.; Writing—Review and Editing, V.C.O., J.P.M., L.S.V., P.R.M.P., F.S. and M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank the Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM) for institutional support and the CNPq, CAPES (Finance Code 001), and FAPEMIG for support and scholarships.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Abbreviation | Definition |
| CF-PDI | Craniofacial pain and disability inventory |
| CIs | Confidence intervals |
| DC/TMD | The Diagnostic Criteria for Temporomandibular Disorders |
| FPHI | Fonseca Patient History Index |
| GRADE | Grading of Recommendations Assessment |
| JFLS | Jaw Functional Limitation Scale |
| MCID | Minimal clinical important difference |
| MDC | Minimal Detectable Change |
| MDs | Mean differences |
| MFIQ | Mandibular Function Impairment Questionnaire |
| MFIQ | Migraine Functional Impact Questionnaire |
| MMO | Maximum mouth opening |
| NRS | Numerical Rating Scale |
| PRISMA | Prospective Reporting Items for Systematic Review and Meta-Analyses |
| PROSPERO | Prospective Registry of Systematic Reviews |
| RCTs | Randomised controlled trials |
| SDs | Standard deviations |
| SMDs | Standardized mean differences |
| TMD | Temporomandibular disorders |
| VAS | Visual Analog Scale |
References
- Manfredini, D.; Chiappe, G.; Bosco, M. Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) axis I diagnoses in an Italian patient population. J. Oral Rehabil. 2006, 33, 551–558. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef]
- Minervini, G.; Mariani, P.; Fiorillo, L.; Cervino, G.; Cicciù, M.; Laino, L. Prevalence of temporomandibular disorders in people with multiple sclerosis: A systematic review and meta-analysis. Cranio 2022, 1–9. [Google Scholar] [CrossRef]
- Forssell, H.; Kauko, T.; Kotiranta, U.; Suvinen, T. Predictors for future clinically significant pain in patients with temporomandibular disorder: A prospective cohort study. Eur. J. Pain 2017, 21, 188–197. [Google Scholar] [CrossRef]
- Ohrbach, R.; Dworkin, S.F. Five-year outcomes in TMD: Relationship of changes in pain to changes in physical and psychological variables. Pain 1998, 74, 315–326. [Google Scholar] [CrossRef]
- Velly, A.M.; Elsaraj, S.M.; Botros, J.; Samim, F.; der Khatchadourian, Z.; Gornitsky, M. The contribution of pain and disability on the transition from acute to chronic pain-related TMD: A 3-month prospective cohort study. Front. Pain Res. 2022, 3, 956117. [Google Scholar] [CrossRef]
- Nilsson, I.-M.; List, T. Does adolescent self-reported TMD pain persist into early adulthood? A longitudinal study. Acta Odontol. Scand. 2020, 78, 377–383. [Google Scholar] [CrossRef]
- Barry, F.; Chai, F.; Chijcheapaza-Flores, H.; Garcia-Fernandez, M.J.; Blanchemain, N.; Nicot, R. Systematic review of studies on drug-delivery systems for management of temporomandibular-joint osteoarthritis. J. Stomatol. Oral Maxillofac. Surg. 2021, 123, e336–e341. [Google Scholar] [CrossRef]
- Seo, H.; Jung, B.; Yeo, J.; Kim, K.-W.; Cho, J.-H.; Lee, Y.J.; Ha, I.-H. Healthcare utilisation and costs for temporomandibular disorders: A descriptive, cross-sectional study. BMJ Open 2020, 10, e036768. [Google Scholar] [CrossRef]
- Riley, P.; Glenny, A.-M.; Worthington, H.V.; Jacobsen, E.; Robertson, C.; Durham, J.; Davies, S.; Petersen, H.; Boyers, D. Oral splints for patients with temporomandibular disorders or bruxism: A systematic review and economic evaluation. Health Technol. Assess. 2020, 24, 1–224. [Google Scholar] [CrossRef]
- Prodoehl, J.; Kraus, S.; Stein, A.B. Predicting the number of physical therapy visits and patient satisfaction in individuals with temporomandibular disorder: A cohort study. J. Oral Rehabil. 2022, 49, 22–36. [Google Scholar] [CrossRef]
- Kothari, K.; Jayakumar, N.; Razzaque, A. Multidisciplinary management of temporomandibular joint ankylosis in an adult: Journey from arthroplasty to oral rehabilitation. BMJ Case Rep. 2021, 14, e245120. [Google Scholar] [CrossRef]
- Penlington, C.; Bowes, C.; Taylor, G.; Otemade, A.A.; Waterhouse, P.; Durham, J.; Ohrbach, R. Psychological therapies for temporomandibular disorders (TMDs). Cochrane Database Syst. Rev. 2022, 2022, CD013515. [Google Scholar] [CrossRef]
- de Souza, R.F.; da Silva, C.H.L.; Nasser, M.; Fedorowicz, Z.; A Al-Muharraqi, M. Interventions for managing temporomandibular joint osteoarthritis. Cochrane Database Syst. Rev. 2012, 2018, CD007261. [Google Scholar] [CrossRef]
- Mujakperuo, H.R.; Watson, M.; Morrison, R.; Macfarlane, T.V. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database Syst. Rev. 2010, 10, CD004715. [Google Scholar] [CrossRef]
- Ferrillo, M.; Nucci, L.; Giudice, A.; Calafiore, D.; Marotta, N.; Minervini, G.; D’Apuzzo, F.; Ammendolia, A.; Perillo, L.; de Sire, A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. Cranio 2022, 1–17. [Google Scholar] [CrossRef]
- Minervini, G.; Fiorillo, L.; Russo, D.; Lanza, A.; D’Amico, C.; Cervino, G.; Meto, A.; Di Francesco, F. Prosthodontic Treatment in Patients with Temporomandibular Disorders and Orofacial Pain and/or Bruxism: A Review of the Literature. Prosthesis 2022, 4, 253–262. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schuenemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions. 2021. Available online: https://training.cochrane.org/cochrane-handbook-systematic-reviews-interventions (accessed on 7 December 2020).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Katz, J.; Melzack, R. Measurement of Pain. Surg. Clin. North Am. 1999, 79, 231–252. [Google Scholar] [CrossRef]
- Wood, G.D.; A Branco, J. A comparison of three methods of measuring maximal opening of the mouth. J. Oral Surg. (Am. Dent. Assoc. 1965) 1979, 37, 175–177. [Google Scholar]
- Ohrbach, R.; Larsson, P.; List, T. The jaw functional limitation scale: Development, reliability, and validity of 8-item and 20-item versions. J. Orofac. Pain 2008, 22, 219–230. [Google Scholar]
- Stegenga, B.; De Bont, L.G.; De Leeuw, R.; Boering, G. Assessment of mandibular function impairment associated with temporomandibular joint osteoarthrosis and internal derangement. J. Orofac. Pain 1993, 7, 183–195. [Google Scholar]
- Macedo, L.G.; Elkins, M.R.; Maher, C.G.; Moseley, A.M.; Herbert, R.D.; Sherrington, C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J. Clin. Epidemiol. 2010, 63, 920–925. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Kropmans, T.; Dijkstra, P.; Stegenga, B.; Stewart, R.; De Bont, L. Smallest detectable difference in outcome variables related to painful restriction of the temporomandibular joint. J. Dent. Res. 1999, 78, 784–789. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Mueller, P.S.; Montori, V.; Bassler, D.; Koenig, B.A.; Guyatt, G.H. Ethical Issues in Stopping Randomized Trials Early Because of Apparent Benefit. Ann. Intern. Med. 2007, 146, 878–881. [Google Scholar] [CrossRef]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke Rehabilitation Evidence-Based Review: Methodology. Top. Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Trikalinos, T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. Can. Med. Assoc. J. 2007, 176, 1091–1096. [Google Scholar] [CrossRef]
- Alajbeg, I.; Gikić, M.; Peruzović, M.V. Mandibular Range of Movement and Pain Intensity in Patients with Anterior Disc Displacement without Reduction. Acta Stomatol. Croat. 2015, 49, 119–127. [Google Scholar] [CrossRef]
- Espejo-Antúnez, L.; Castro-Valenzuela, E.; Ribeiro, F.; Albornoz-Cabello, M.; Silva, A.; Rodríguez-Mansilla, J. Immediate effects of hamstring stretching alone or combined with ischemic compression of the masseter muscle on hamstrings extensibility, active mouth opening and pain in athletes with temporomandibular dysfunction. J. Bodyw. Mov. Ther. 2016, 20, 579–587. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, C.; Cocera-Morata, F.M.; Heredia-Rizo, A.M.; Ricard, F.; Almazán-Campos, G.; Oliva-Pascual-Vaca, Á. Immediate Effects of Combining Local Techniques in the Craniomandibular Area and Hamstring Muscle Stretching in Subjects with Temporomandibular Disorders: A Randomized Controlled Study. J. Altern. Complement. Med. 2015, 21, 451–459. [Google Scholar] [CrossRef]
- Brochado, F.T.; De Jesus, L.H.; Carrard, V.C.; Freddo, A.L.; Chaves, K.D.; Martins, M.D. Comparative effectiveness of photobiomodulation and manual therapy alone or combined in TMD patients: A randomized clinical trial. Braz. Oral Res. 2018, 32, e50. [Google Scholar] [CrossRef]
- DeVocht, J.W.; Goertz, C.M.; Hondras, M.A.; Long, C.R.; Schaeffer, W.; Thomann, L.; Spector, M.; Stanford, C.M. A pilot study of a chiropractic intervention for management of chronic myofascial temporomandibular disorder. J. Am. Dent. Assoc. 2013, 144, 1154–1163. [Google Scholar] [CrossRef]
- Gomes, C.A.F.D.P.; El Hage, Y.; Amaral, A.P.; Politti, F.; Biasotto-Gonzalez, D.A. Effects of massage therapy and occlusal splint therapy on electromyographic activity and the intensity of signs and symptoms in individuals with temporomandibular disorder and sleep bruxism: A randomized clinical trial. Chiropr. Man. Ther. 2014, 22, 43. [Google Scholar] [CrossRef]
- Hernanz, G.S.; Angulo-Carrere, T.; Ardizone-García, I.; Svensson, P.; Álvarez-Méndez, A.M. Pressure Release Technique Versus Placebo Applied to Cervical and Masticatory Muscles in Patients with Chronic Painful Myofascial Temporomandibular Disorder. A Randomized Clinical Trial 2020, PREPRINT (Version 1) available at Research Square. Available online: https://www.researchsquare.com/article/rs-51085/v1 (accessed on 7 December 2020).
- Kalamir, A.; Bonello, R.; Graham, P.; Vitiello, A.L.; Pollard, H. Intraoral Myofascial Therapy for Chronic Myogenous Temporomandibular Disorder: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2012, 35, 26–37. [Google Scholar] [CrossRef]
- Kanhachon, W.; Boonprakob, Y. Modified-Active Release Therapy in Patients with Scapulocostal Syndrome and Masticatory Myofascial Pain: A Stratified-Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 8533. [Google Scholar] [CrossRef]
- La Touche, R.; Paris-Alemany, A.; Mannheimer, J.S.; Angulo-Díaz-Parreño, S.; Bishop, M.; Centeno, A.L.-V.; von Piekartz, H.; Fernandez-Carnero, J. Does Mobilization of the Upper Cervical Spine Affect Pain Sensitivity and Autonomic Nervous System Function in Patients With Cervico-craniofacial Pain? Clin. J. Pain 2013, 29, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Leite, W.B.; Oliveira, M.L.; Ferreira, I.C.; Anjos, C.F.; Barbosa, M.A.; Barbosa, A.C. Effects of 4-Week Diacutaneous Fibrolysis on Myalgia, Mouth Opening, and Level of Functional Severity in Women With Temporomandibular Disorders: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 806–815. [Google Scholar] [CrossRef]
- Lucas, C.; Branco, I.; Silva, M.; Alves, P.; Pereira, Â.M. Benefits of manual therapy in temporomandibular joint dysfunction treatment. In 2nd International Congress of CiiEM-Translational Research and Innovation in Human and Health Science; Campus Egas Moniz: Monte de Caparica, Portugal, 2017. [Google Scholar]
- Nagata, K.; Hori, S.; Mizuhashi, R.; Yokoe, T.; Atsumi, Y.; Nagai, W.; Goto, M. Efficacy of mandibular manipulation technique for temporomandibular disorders patients with mouth opening limitation: A randomized controlled trial for comparison with improved multimodal therapy. J. Prosthodont. Res. 2019, 63, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Packer, A.C.; Pires, P.F.; Dibai-Filho, A.V.; Rodrigues-Bigaton, D. Effect of Upper Thoracic Manipulation on Mouth Opening and Electromyographic Activity of Masticatory Muscles in Women With Temporomandibular Disorder: A Randomized Clinical Trial. J. Manip. Physiol. Ther. 2015, 38, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Puentedura, E.J.; Kolber, M.J.; Cleland, J.A. Effectiveness of Cervical Spine High-Velocity, Low-Amplitude Thrust Added to Behavioral Education, Soft Tissue Mobilization, and Exercise for People With Temporomandibular Disorder With Myalgia: A Randomized Clinical Trial. J. Orthop. Sport. Phys. Ther. 2020, 50, 455–465. [Google Scholar] [CrossRef]
- Rezaie, K.; Amiri, A.; Takamjani, E.E.; Shirani, G.; Salehi, S.; Alizadeh, L. The Efficacy of Neck and Temporomandibular Joint (TMJ) Manual Therapy in Comparison With a Multimodal Approach in the Patients with TMJ Dysfunction: A Blinded Randomized Controlled Trial. Med. J. Islam. Repub. Iran 2022, 36, 328–337. [Google Scholar] [CrossRef]
- Şahin, D.; Mutlu, E.K.; Şakar, O.; Ateş, G.; Inan, Ş.; Taşkıran, H. The effect of the ischaemic compression technique on pain and functionality in temporomandibular disorders: A randomised clinical trial. J. Oral Rehabil. 2021, 48, 531–541. [Google Scholar] [CrossRef]
- de la Serna, P.D.; Plaza-Manzano, G.; Cleland, J.; Fernández-De-Las-Peñas, C.; Martín-Casas, P.; Díaz-Arribas, M.J. Effects of Cervico-Mandibular Manual Therapy in Patients with Temporomandibular Pain Disorders and Associated Somatic Tinnitus: A Randomized Clinical Trial. Pain Med. 2020, 21, 613–624. [Google Scholar] [CrossRef]
- Tuncer, A.; Ergun, N.; Tuncer, A.H.; Karahan, S. Effectiveness of manual therapy and home physical therapy in patients with temporomandibular disorders: A randomized controlled trial. J. Bodyw. Mov. Ther. 2013, 17, 302–308. [Google Scholar] [CrossRef]
- Yoshida, H.; Fukumura, Y.; Suzuki, S.; Fujita, S.; Kenzo, O.; Yoshikado, R.; Nakagawa, M.; Inoue, A.; Sako, J.; Yamada, K.; et al. Simple manipulation therapy for temporomandibular joint internal derangement with closed lock. J. Oral. Maxillofac. Surg. 2005, 17, 256–260. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Conti, P.C.R.; Alyahya, A.; Alkebsi, K.; Elsharkawy, A.; Christidis, N. The hierarchy of different treatments for myogenous temporomandibular disorders: A systematic review and network meta-analysis of randomized clinical trials. Oral Maxillofac. Surg. 2022, 26, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, A.; Costin, B.; Dharamdasani, S.; Page, R.; Purs, N.; Treleaven, J. What conservative interventions improve bite function in those with temporomandibular disorders? A systematic review using self-reported and physical measures. J. Oral Rehabil. 2022, 49, 456–475. [Google Scholar] [CrossRef] [PubMed]
- Asquini, G.; Pitance, L.; Michelotti, A.; Falla, D. Effectiveness of manual therapy applied to craniomandibular structures in temporomandibular disorders: A systematic review. J. Oral Rehabil. 2022, 49, 442–455. [Google Scholar] [CrossRef]
- Calixtre, L.B.; Moreira, R.F.C.; Franchini, G.H.; Alburquerque-Sendín, F.; Oliveira, A.B. Manual therapy for the management of pain and limited range of motion in subjects with signs and symptoms of temporomandibular disorder: A systematic review of randomised controlled trials. J. Oral Rehabil. 2015, 42, 847–861. [Google Scholar] [CrossRef]
- Ferrillo, M.; Ammendolia, A.; Paduano, S.; Calafiore, D.; Marotta, N.; Migliario, M.; Fortunato, L.; Giudice, A.; Michelotti, A.; de Sire, A. Efficacy of rehabilitation on reducing pain in muscle-related temporomandibular disorders: A systematic review and meta-analysis of randomized controlled trials. J. Back Musculoskelet. Rehabil. 2022, 35, 921–936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).