Can Isotopologues Be Used as Biosignature Gases in Exoplanet Atmospheres?
Abstract
1. Introduction
2. Isotopes as Biosignatures in the Solar System: Possible Evidence of Metabolic Processes on Nearby Worlds from Surface and Atmospheric Measurements
3. Detectability of Isotopes in Exoplanet Atmospheres
3.1. Recent Measurements and Simulated Detections
3.2. Why CO Is the Most Detectable Isotopologue from Space
3.3. Formation of Carbon Isotopes
4. Major Challenges with Using Isotopologues as Biosignature Gases
4.1. Establishing an Abiotic Baseline for C/C in Exoplanet Atmospheres
4.2. Disentangling Abiotic and Biotic Isotope Fractionation
4.3. Limited Number of Known and Anticipated Targets
5. Carbon Isotope Ratios as Supportive Evidence for Life
5.1. Seasonality of Carbon Isotope Fractionation: A Possible Solution
5.2. The Case for Observing Carbon Isotope Fractionation in Non-Terrestrial Planet Atmospheres: Isotopes as a Tool to Discern Formation Mechanisms
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, J.P.; Mather, J.C.; Clampin, M.; Doyon, R.; Greenhouse, M.A.; Hammel, H.B.; Hutchings, J.B.; Jakobsen, P.; Lilly, S.J.; Long, K.S.; et al. The James Webb space telescope. Space Sci. Rev. 2006, 123, 485–606. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Arrhenius, G.; McKeegan, K.D.; Harrison, T.M.; Nutman, A.P.; Friend, C.R.L. Evidence for Life on Earth Before 3800 Million Years Ago. Nature 1996, 384, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Eiler, J.M.; Mojzsis, S.J.; Arrhenius, G. Carbon Isotope Evidence for Early Life. Nature 1997, 386, 665. [Google Scholar] [CrossRef]
- Rothschild, L.J.; Desmarais, D. Stable Carbon Isotope Fractionation in The Search for Life on Early Mars. Adv. Space Ret 1989, 9, 159. [Google Scholar] [CrossRef]
- Garcia, A.K.; Cavanaugh, C.M.; Kacar, B. The Curious Consistency of Carbon Biosignatures Over Billions of Years of Earth-Life Coevolution. ISME J. 2021, 15, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Boehnke, P.; Harrison, T.M.; Mao, W.L. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl. Acad. Sci. USA 2015, 112, 14518–14521. [Google Scholar] [CrossRef]
- Hare, V.J.; Loftus, E.; Jeffrey, A.; Ramsey, C.B. Atmospheric CO2 Effect on Stable Carbon Isotope Composition of Terrestrial Fossil Archives. Nat. Commun. 2018, 9, 252. [Google Scholar] [CrossRef]
- Keeling, C.D. The Concentration and Isotopic Abundances of Atmospheric Carbon Dioxide in Rural Areas. Geochim. Cosmochim. Acta 1958, 13, 322–334. [Google Scholar] [CrossRef]
- Neveu, M.; Hays, L.E.; Voytek, M.A.; New, M.H.; Schulte, M.D. The Ladder of Life Detection. Astrobiology 2018, 18, 1375–1402. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.V.; Skemer, A.J.; Miles, B.E.; Line, M.R.; Lopez, E.D.; Brogi, M.; Freedman, R.S.; Marley, M.S. Measuring the D/H Ratios of Exoplanets and Brown Dwarfs. Astrophys. J. 2019, 882, L29. [Google Scholar] [CrossRef]
- Lincowski, A.P.; Lustig-Yaeger, J.; Meadows, V.S. Observing Isotopologue Bands in Terrestrial Exoplanet Atmospheres with the James Webb Space Telescope: Implications for Identifying Past Atmospheric and Ocean Loss. Astron. J. 2019, 158, 26. [Google Scholar] [CrossRef]
- Molliere, P.; Snellen, I.A. Detecting Isotopologues in Exoplanet Atmospheres Using Ground-Based High-Dispersion Spectroscopy. Astron. Astrophys. 2019, 622, 139. [Google Scholar] [CrossRef]
- Zhang, Y.; Snellen, I.A.; Bohn, A.J.; Mollière, P.; Ginski, C.; Hoeijmakers, H.J.; Kenworthy, M.A.; Mamajek, E.E.; Meshkat, T.; Reggiani, M.; et al. The 13CO-Rich Atmosphere of a Young Accreting Super-Jupiter. Nature 2021, 595, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Line, M.R.; Brogi, M.; Bean, J.L.; Gandhi, S.; Zalesky, J.; Parmentier, V.; Smith, P.; Mace, G.N.; Mansfield, M.; Kempton, E.M.; et al. A solar C/O and sub-solar metallicity in a hot Jupiter atmosphere. Nature 2021, 598, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Ahrer, E.M.; Alderson, L.; Batalha, N.M.; Batalha, N.E.; Bean, J.L.; Beatty, T.G.; Bell, T.J.; Benneke, B.; Berta-Thompson, Z.K.; Carter, A.L.; et al. Identification of Carbon Dioxide in an Exoplanet Atmosphere. Nature 2023, 614, 649–652. [Google Scholar] [CrossRef]
- Glidden, A.; Seager, S.; Huang, J.; Petkowski, J.J.; Ranjan, S. Can Carbon Fractionation Provide Evidence for Aerial Biospheres in the Atmospheres of Temperate Sub-Neptunes? Astrophys. J. 2022, 930, 62. [Google Scholar] [CrossRef]
- Owen, T. The Composition of the Martian Atmosphere. Adv. Space Res 1982, 2, 75–80. [Google Scholar] [CrossRef]
- Schrey, U.; Rothermel, H.; Kaufl, H.; Drapatz, S. Determination of the 12C/13C and 16O/18O Ratio in the Martian Atmosphere by 10 micron Heterodyne Spectroscopy. Astron. Astrophys. 1986, 155, 200–204. [Google Scholar]
- Niles, P.B.; Boynton, W.V.; Hoffman, J.H.; Ming, D.W.; Hamara, D. Stable Isotope Measurements of Martian Atmospheric CO2 at the Phoenix Landing Site. Science 2010, 329, 1334–1337. [Google Scholar] [CrossRef]
- Webster, C.; Mahaffy, P.R.; Webster, C.; Flesch, G.J.; Niles, P.B.; Jones, J.H.; Leshin, L.A.; Atreya, S.K.; Stern, J.C.; Christensen, L.E.; et al. Isotope Ratios of H, C, and O in CO2 and H2O of the Martian Atmosphere. Science 2013, 341, 260–263. [Google Scholar] [CrossRef]
- Mahaffy, P.R.; Webster, C.R.; Atreya, S.K.; Franz, H.; Wong, M.; Conrad, P.G.; Harpold, D.; Jones, J.J.; Leshin, L.A.; Manning, H.; et al. Abundance and Isotopic Composition of Gases in the Martian Atmosphere from the Curiosity Rover. Sci. Team Source Sci. 2013, 341, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Leshin, L.A.; Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Coll, P.; Conrad, P.G.; Archer, P.D.; Atreya, S.K.; Brunner, A.E.; Buch, A.; et al. Volatile, Isotope, and Organic Analysis of Martian Fines with the Mars Curiosity Rover. Science 2013, 341, 12389. [Google Scholar] [CrossRef] [PubMed]
- Alday, J.; Wilson, C.F.; Irwin, P.G.; Trokhimovskiy, A.; Montmessin, F.; Fedorova, A.A.; Belyaev, D.A.; Olsen, K.S.; Korablev, O.; Lefèvre, F.; et al. Isotopic Composition of CO2 in the Atmosphere of Mars: Fractionation by Diffusive Separation Observed by the ExoMars Trace Gas Orbiter. J. Geophys. Res. Planets 2021, 126, e2021JE006992. [Google Scholar] [CrossRef]
- Jakosky, B.M. The CO2 Inventory on Mars. Planet. Space Sci. 2019, 175, 52–59. [Google Scholar] [CrossRef]
- Hu, R.; Kass, D.M.; Ehlmann, B.L.; Yung, Y.L. Tracing the Fate of Carbon and the Atmospheric Evolution of Mars. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Johnson, M.S.; Schinke, R. Carbon Dioxide Photolysis from 150 to 210 nm: Singlet and Triplet Channel Dynamics, UV-spectrum, and Isotope Effects. Proc. Natl. Acad. Sci. USA 2013, 110, 17691–17696. [Google Scholar] [CrossRef]
- House, C.H.; Wong, G.M.; Webster, C.R.; Flesch, G.J.; Franz, H.B.; Stern, J.C.; Pavlov, A.; Atreya, S.K.; Eigenbrode, J.L.; Gilbert, A.; et al. Depleted Carbon Isotope Compositions Observed at Gale Crater, Mars. Proc. Natl. Acad. Sci. USA 2022. [Google Scholar] [CrossRef]
- Yoshida, T.; Aoki, S.; Ueno, Y.; Terada, N.; Nakamura, Y.; Shiobara, K.; Yoshida, N.; Nakagawa, H.; Sakai, S.; Koyama, S. Strong Depletion of 13C in CO Induced by Photolysis of CO2 in the Martian Atmosphere, Calculated by a Photochemical Model. Planet. Sci. J. 2023, 4, 53. [Google Scholar] [CrossRef]
- Liuzzi, G.; Villanueva, G.L.; Stone, S.W.; Faggi, S.; Kofman, V.; Trompet, A.C. Measuring the 13C/12C in CO2 in the Lower Atmosphere of Mars with NOMAD/TGO: Challenges and Interpretation. In Proceedings of the Seventh International Workshop on the Mars Atmosphere: Modelling and Observations, Paris, France, 14–17 June 2022; p. 3532. [Google Scholar] [CrossRef]
- Moreras-Marti, A.; Fox-Powell, M.; Cousins, C.R.; Macey, M.C.; Zerkle, A.L. Sulfur Isotopes as Biosignatures for Mars and Europa Exploration. J. Geol. Soc. 2022, 179, jgs2021-134. [Google Scholar] [CrossRef]
- Sim, M.S.; Bosak, T.; Ono, S. Large Sulfur Isotope Fractionation Does Not Require Disproportionation. New Ser. 2011, 333, 74–77. [Google Scholar] [CrossRef]
- Franz, H.B.; Wu, N.; Farquhar, J.; Irving, A.J. A new type of isotopic anomaly in shergottite sulfides. Meteorit. Planet. Sci. 2019, 54, 3036–3051. [Google Scholar] [CrossRef]
- Becker, T.M.; Trumbo, S.K.; Molyneux, P.M.; Retherford, K.D.; Hendrix, A.R.; Roth, L.; Raut, U.; Alday, J.; McGrath, M.A. Mid-ultraviolet Hubble Observations of Europa and the Global Surface Distribution of SO2. Planet. Sci. J. 2022, 3, 129. [Google Scholar] [CrossRef]
- Chela-Flores, J. Miniaturised Instrumentation for the Detection of Biosignatures in Ocean Worlds of the Solar System. Front. Space Technol. 2021, 2, 703809. [Google Scholar] [CrossRef]
- Arevalo, R.; Ni, Z.; Danell, R.M. Mass spectrometry and planetary exploration: A brief review and future projection. J. Mass Spectrom. 2020, 55, e4454. [Google Scholar] [CrossRef] [PubMed]
- Petkowski, J.J.; Bains, W.; Seager, S. On the Potential of Silicon as a Building Block for Life. Life 2020, 10, 84. [Google Scholar] [CrossRef]
- Crossfield, I.J.; Lothringer, J.D.; Flores, B.; Mills, E.A.; Freedman, R.; Valverde, J.; Miles, B.; Guo, X.; Skemer, A. Unusual Isotopic Abundances in a Fully Convective Stellar Binary. Astrophys. J. 2019, 871, 7. [Google Scholar] [CrossRef]
- Woods, P.M. Carbon Isotope Measurements in the Solar System. Technical report. arXiv 2009, arXiv:0901.4513. [Google Scholar] [CrossRef]
- Ayres, T.R.; Plymate, C.; Keller, C.U. Solar Carbon Monoxide, Thermal Profiling, and the Abundances of C, O, and Their Isotopes. Astrophys. J. Suppl. Ser. 2006, 165, 618–651. [Google Scholar] [CrossRef]
- Milam, S.N.; Savage, C.; Brewster, M.A.; Ziurys, L.M.; Wyckoff, S. The 12 C/13 C Isotope Gradient Derived from Millimeter Transitions of CN: The Case for Galactic Chemical Evolution. Astrophys. J. 2005, 634, 1126–1132. [Google Scholar] [CrossRef]
- Zhan, Z.; Seager, S.; Petkowski, J.J.; Sousa-Silva, C.; Ranjan, S.; Huang, J.; Bains, W. Assessment of Isoprene as a Possible Biosignature Gas in Exoplanets with Anoxic Atmospheres. Astrobiology 2021, 21, 765–792. [Google Scholar] [CrossRef]
- Langer, W.D.; Graedel, T.E.; Laboratories, B.; Jet, M.A.F.; Armentrout, P.B. Carbon and Oxygen Isotope Fractionation in Dense Interstellar Clouds. Astrophys. J. 1984, 277, 581–604. [Google Scholar] [CrossRef]
- Charnley, S.B.; Ehrenfreund, P.; Millar, T.J.; Boogert, A.C.A.; Markwick, A.J.; Butner, H.M.; Ruiterkamp, R.; Rodgers, S.D. Observational Tests for Grain Chemistry: Posterior Isotopic Labelling. Mon. Not. R. Astron. Soc 2004, 347, 157–162. [Google Scholar] [CrossRef]
- Jørgensen, J.K.; Van Der Wiel, M.H.; Coutens, A.; Lykke, J.M.; Müller, H.S.; Van Dishoeck, E.F.; Calcutt, H.; Bjerkeli, P.; Bourke, T.L.; Drozdovskaya, M.N.; et al. The ALMA Protostellar Interferometric Line Survey (PILS): First Results from an Unbiased Submillimeter Wavelength Line Survey of the Class 0 Protostellar Binary IRAS 16293–2422 with ALMA. Astron. Astrophys. 2016, 595, A117. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Günther, M.N.; Bains, W.; Mikal-evans, T.; Deming, D. Possibilities for an Aerial Biosphere in Temperate Sub Neptune-sized Exoplanet Atmospheres. Universe 2021, 7, 172. [Google Scholar] [CrossRef]
- Snellen, I.A.; De Kok, R.J.; Le Poole, R.; Brogi, M.; Birkby, J. Finding Extraterrestrial Life using Ground-based High-dispersion Spectroscopy. Astrophys. J. 2013, 764, 182. [Google Scholar] [CrossRef]
- Rodler, F.; López-Morales, M. Feasibility Studies for the Detection of O2 in an Earth-like Exoplanet. Astrophys. J. 2014, 781, 54. [Google Scholar] [CrossRef]
- Serindag, D.B.; Snellen, I.A.G. Testing the Detectability of Extraterrestrial O2 with the Extremely Large Telescopes Using Real Data with Real Noise. Astrophys. J. 2019, 871, L7. [Google Scholar] [CrossRef]
- Rustamkulov, Z.; Sing, D.K.; Mukherjee, S.; May, E.M.; Kirk, J.; Schlawin, E.; Line, M.R.; Piaulet, C.; Carter, A.L.; Batalha, N.E.; et al. Early Release Science of the exoplanet WASP-39b with JWST NIRSpec PRISM. Nature 2023, 614, 659–663. [Google Scholar] [CrossRef]
- Mollière, P.; van Boekel, R.; Bouwman, J.; Henning, T.; Lagage, P.O.; Min, M. Observing transiting planets with JWST-Prime targets and their synthetic spectral observations. Astron. Astrophys. 2017, 600, A10. [Google Scholar] [CrossRef]
- Epstein, S.; Zeiri, L. Oxygen and Carbon Isotopic Compositions of Gases Respired by Humans. Proc. Natl. Acad. Sci. USA 1988, 85, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E. Biogeochemistry of Sulfur Isotopes. Rev. Mineral. Geochem. 2001, 43, 607–636. [Google Scholar] [CrossRef]
- Nealson, K.H.; Rye, R. Evolution of Metabolism. In Treatise on Geochemistry; Schlesinger, W.H., Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; Volume 8, p. 682. [Google Scholar] [CrossRef]
- Unkovich, M. Isotope Discrimination Provides New Insight into Biological Nitrogen Fixation. New Phytol. 2013, 198, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Brookshire, E.N.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological Interpretations of Nitrogen Isotope Ratios of Terrestrial Plants and Soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Denk, T.R.A.; Mohn, J.; Decock, C.; Lewicka-Szczebak, D.; Harris, E.; Butterbach-Bahl, K.; Kiese, R.; Wolf, B. The Nitrogen Cycle: A Review of Isotope Effects and Isotope Modeling Approaches. Soil Biol. Biochem. 2017, 105, 121–137. [Google Scholar] [CrossRef]
- Bogard, M.J.; Vachon, D.; St.-Gelais, N.F.; del Giorgio, P.A. Using Oxygen Stable Isotopes to Quantify Ecosystem Metabolism in Northern Lakes. Biogeochemistry 2017, 133, 347–364. [Google Scholar] [CrossRef]
- Mooshammer, M.; Alves, R.J.; Bayer, B.; Melcher, M.; Stieglmeier, M.; Jochum, L.; Rittmann, S.K.; Watzka, M.; Schleper, C.; Herndl, G.J.; et al. Nitrogen Isotope Fractionation During Archaeal Ammonia Oxidation: Coupled Estimates From Measurements of Residual Ammonium and Accumulated Nitrite. Front. Microbiol. 2020, 11, 1710. [Google Scholar] [CrossRef] [PubMed]
- Wieloch, T.; Grabner, M.; Augusti, A.; Serk, H.; Ehlers, I.; Yu, J.; Schleucher, J. Metabolism is a major driver of hydrogen isotope fractionation recorded in tree-ring glucose of Pinus nigra. New Phytol. 2022, 234, 449–461. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Kochanov, R.V.; Gordon, I.E.; Rothman, L.S.; Wcislo, P.; Hill, C.; Wilzewski, J. HITRAN Aapplication Programing Interface (HAPI): Extending HITRAN Capabilities. In Proceedings of the 71st International Symposium on Molecular Spectroscopy, Champaign, IL, USA, 20–24 June 2016; Volume 168, pp. 1097–1105. [Google Scholar]
- Rothman, L.S.; Rinsland, C.P.; Goldman, A.; Massie, S.T.; Edwards, D.P.; Flaud, J.M.; Perrin, A.; Camy-Peyret, C.; Dana, V.; Mandin, J.Y.; et al. The HITRAN Molecular Spectroscopic Database and HAWKS (HITRAN ATMOSPHERIC WORKSTATION): 1996 Edition. J. Quant. Spectrosc. Radiat. Transf. 1998, 60, 665–710. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.M.; Conway, E.K.; Hill, C.; Kochanov, R.V.; Tan, Y.; et al. The HITRAN2020 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar] [CrossRef]
- De Biévre, P.; Gallet, M.; Holden, N.E.; Barnes, I.L. Isotopic Abundances and Atomic Weights of the Elements. J. Phys. Chem. Ref. Data 1984, 13, 809–891. [Google Scholar] [CrossRef]
- Mollière, P.; Wardenier, J.P.; Van Boekel, R.; Henning, T.; Molaverdikhani, K.; Snellen, I.A. PetitRADTRANS: A Python radiative transfer package for exoplanet characterization and retrieval. Astron. Astrophys. 2019, 627. [Google Scholar] [CrossRef]
- Madhusudhan, N. C/O ratio as a dimension for characterizing exoplanetary atmospheres. Astrophys. J. 2012, 758, 36. [Google Scholar] [CrossRef]
- Kobayashi, C.; Karakas, A.I.; Umeda, H. The Evolution of Isotope Ratios in the Milky Way Galaxy. Mon. Not. R. Astron. Soc. 2011, 414, 3231–3250. [Google Scholar] [CrossRef]
- Watson, W.D.; Anicich, V.G.; Huntress, W.T. Measurement and Significance of the Reaction 13C+ + 12CO->12C+ + 13CO for Alteration on the 13C/12C Ratio in Interstellar Molecules. Astrophys. J. 1976, 205, 165–168. [Google Scholar] [CrossRef]
- Smith, D.; Adams, N.G. Laboratory Studies of Isotope Fractionation in the Reactions of C+ and HCO+ with CO: Interstellar Implications. Astrophys. J. 1980, 242, 424–431. [Google Scholar] [CrossRef]
- Sheffer, Y.; Federman, S.; Lambert, D.L.; Cardelli, J.A. Sheffer_1992_ApJ_397_482S. Astrophys. J. 1992, 397, 482–491. [Google Scholar] [CrossRef]
- Woods, P.M.; Willacy, K. Carbon Isotope Fractionation in Protoplanetary Disks. Astrophys. J. 2009, 693, 1360–1378. [Google Scholar] [CrossRef]
- Yoshida, T.C.; Nomura, H.; Furuya, K.; Tsukagoshi, T.; Lee, S. A New Method for Direct Measurement of Isotopologue Ratios in Protoplanetary Disks: A Case Study of the 12CO/13CO Ratio in the TW Hya Disk. Astrophys. J. 2022, 932, 126. [Google Scholar] [CrossRef]
- Lyons, J.R.; Gharib-Nezhad, E.; Ayres, T.R. A Light Carbon Isotope Composition for the Sun. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Smith, R.L.; Pontoppidan, K.M.; Young, E.D.; Morris, M.R. HETEROGENEITY in 12CO/13CO ABUNDANCE RATIOS TOWARD SOLAR-TYPE YOUNG STELLAR OBJECTS. Astrophys. J. 2015, 813, 120. [Google Scholar] [CrossRef]
- Hayes, J.M. Fractionation of Carbon and Hydrogen Isotopes in Biosynthetic Processes*. Rev. Mineral. Geochem. 2001, 43, 225–277. [Google Scholar] [CrossRef]
- Zyakun, A.M.; Lunina, O.N.; Prusakova, T.S.; Pimenov, N.V.; Ivanov, M.V. Fractionation of stable carbon isotopes by photoautotrophically growing anoxygenic purple and green sulfur bacteria. Microbiology 2009, 78, 757–768. [Google Scholar] [CrossRef]
- Havig, J.; Raymond, J.; Meyer-Dombard, D.; Zolotova, N.; Shock, E. Merging isotopes and community genomics in a siliceous sinter-depositing hot spring. J. Geophys. Res. Biogeosci. 2011, 116, G01005. [Google Scholar] [CrossRef]
- Seager, S.; Turner, E.L.; Schafer, J.; Ford, E.B. Vegetation’s Red Edge: A Possible Spectroscopic Biosignature of Extraterrestrial Plants. Astrobiology 2005, 5, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Kiang, N.Y.; Segura, A.; Tinetti, G.; Govindjee; Blankenship, R.E.; Cohen, M.; Siefert, J.; Crisp, D.; Meadows, V.S. Spectral Signatures of Photosynthesis. II. Coevolution with Other Stars and the Atmosphere on Extrasolar Worlds. Astrobiology 2007, 7, 252–274. [Google Scholar] [CrossRef]
- Still, C.; Rastogi, B. What Drives Carbon Isotope Fractionation by the Terrestrial Biosphere? J. Geophys. Res. Biogeosci. 2017, 122, 3108–3110. [Google Scholar] [CrossRef]
- McNevin, D.B.; Badger, M.R.; Whitney, S.M.; Von Caemmerer, S.; Tcherkez, G.G.; Farquhar, G.D. Differences in Carbon Isotope Discrimination of Three Variants of D-ribulose-1,5-bisphosphate Carboxylase/Oxygenase Reflect Differences in Their Catalytic Mechanisms. J. Biol. Chem. 2007, 282, 36068–36076. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon Isotopes in Photosynthesis. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Kirkels, F.M.; De Boer, H.J.; Concha Hernández, P.; Martes, C.R.; Van Der Meer, M.T.; Basu, S.; Usman, M.O.; Peterse, F. Carbon Isotopic Ratios of Modern C3 and C4 Vegetation on the Indian Peninsula and Changes along the Plant-Soil-River Continuum - Implications for Vegetation Reconstructions. Biogeosciences 2022, 19, 4107–4127. [Google Scholar] [CrossRef]
- Mattey, D.P. Carbon Dioxide Solubility and Carbon Isotope Fractionation in Basaltic Melt. Geochim. Cosmochim. Acta 1991, 55, 3467–3473. [Google Scholar] [CrossRef]
- Bada, J.L. Volcanic Island Lightning Prebiotic Chemistry and the Origin of Life in the Early Hadean Eon. Nat. Commun. 2023, 14, 2011. [Google Scholar] [CrossRef]
- Ricci, A.; Fiebig, J.; Severi, P.; Tassi, F.; Hofmann, S.; Capecchiacci, F.; Vaselli, O. Extremely deuterium depleted methane revealed in high-temperature volcanic gases. Geochim. Cosmochim. Acta 2024, 364, 148–165. [Google Scholar] [CrossRef]
- Leman, L.; Orgel, L.; Ghadiri, M.R. Carbonyl Sulfide: Mediated Prebiotic Formation of Peptides. Science 2004, 306, 283–286. [Google Scholar] [CrossRef]
- Kempton, E.M.; Bean, J.L.; Louie, D.R.; Deming, D.; Koll, D.D.; Mansfield, M.; Christiansen, J.L.; López-Morales, M.; Swain, M.R.; Zellem, R.T.; et al. A Framework for Prioritizing the TESS Planetary Candidates Most Amenable to Atmospheric Characterization. Publ. Astron. Soc. Pac. 2018, 130, 1–14. [Google Scholar] [CrossRef]
- Ricker, G.R.; Winn, J.N.; Vanderspek, R.; Latham, D.W.; Bakos, G.; Bean, J.L.; Berta-Thompson, Z.K.; Brown, T.M.; Buchhave, L.; Butler, N.R.; et al. Transiting Exoplanet Survey Satellite. J. Astron. Telesc. Instruments Syst. 2014, 1, 014003. [Google Scholar] [CrossRef]
- Kunimoto, M.; Winn, J.; Ricker, G.R.; Vanderspek, R.K. Predicting the Exoplanet Yield of the TESS Prime and Extended Missions through Years 1–7. Astron. J. 2022, 163, 290. [Google Scholar] [CrossRef]
- Keeling, C.D.; Piper, S.C. Interannual Variations of Exchanges of Atmospheric CO2 and 13CO2 with the Terrestrial Biosphere and Oceans from 1978 to 2000; Scripps Institution of Oceanography, University of California: San Diego, CA, USA, 2000. [Google Scholar]
- NOAA. NOAA Global Monitoring Lab: Earth Systems Research Laboratories; Scripps Institution of Oceanography, University of California: San Diego, CA, USA, 2020. [Google Scholar]
- Kurganova, I.N.; Lopes de Gerenyu, V.O. Contribution of Abiotic Factors to CO2 Emission from Soils in the Freeze–Thaw Cycles. Eurasian Soil Sci. 2015, 48, 1009–1015. [Google Scholar] [CrossRef]
- Olson, S.L.; Schwieterman, E.W.; Reinhard, C.T.; Ridgwell, A.; Kane, S.R.; Meadows, V.S.; Lyons, T.W. Atmospheric Seasonality as an Exoplanet Biosignature. Astrophys. J. 2018, 858, L14. [Google Scholar] [CrossRef]
- Meadows, V.S.; Reinhard, C.T.; Arney, G.N.; Parenteau, M.N.; Schwieterman, E.W.; Domagal-Goldman, S.D.; Lincowski, A.P.; Stapelfeldt, K.R.; Rauer, H.; Dassarma, S.; et al. Exoplanet Biosignatures: Understanding Oxygen as a Biosignature in the Context of Its Environment. Astrobiology 2018, 18, 630–662. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Furuya, K.; Cordiner, M.A.; Charnley, S.B.; Alexander, C.M.O.; Nixon, C.A.; Guzman, V.V.; Yurimoto, H.; Tsukagoshi, T.; Iino, T. The Isotopic Links from Planet Forming Regions to the Solar System. arXiv 2022, arXiv:2203.10863. [Google Scholar] [CrossRef]
- Brown, P.D.; Millar, T.J. Models of the Gas-Grain Interaction—Deuterium Chemistry. MNRAS 1989, 237, 661–671. [Google Scholar] [CrossRef][Green Version]
- Yurimoto, H.; Kuramoto, K. Molecular Cloud Origin for the Oxygen Isotope Heterogeneity in the Solar System. Science 2004, 305, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.L.; van Dishoeck, E.F.; Tabone, B.; Gasman, D.; Henning, T.; Kamp, I.; Güdel, M.; Lagage, P.O.; Bettoni, G.; Perotti, G.; et al. MINDS. The Detection of 13CO2 with JWST-MIRI Indicates Abundant CO2 in a Protoplanetary Disk. Astrophys. J. Lett. 2023, 947, L6. [Google Scholar] [CrossRef]
- McClure, M.K.; Rocha, W.R.; Pontoppidan, K.M.; Crouzet, N.; Chu, L.E.; Dartois, E.; Lamberts, T.; Noble, J.A.; Pendleton, Y.J.; Perotti, G.; et al. An Ice Age JWST Inventory of Dense Molecular Cloud Ices. Nat. Astron. 2023, 7, 431–443. [Google Scholar] [CrossRef]
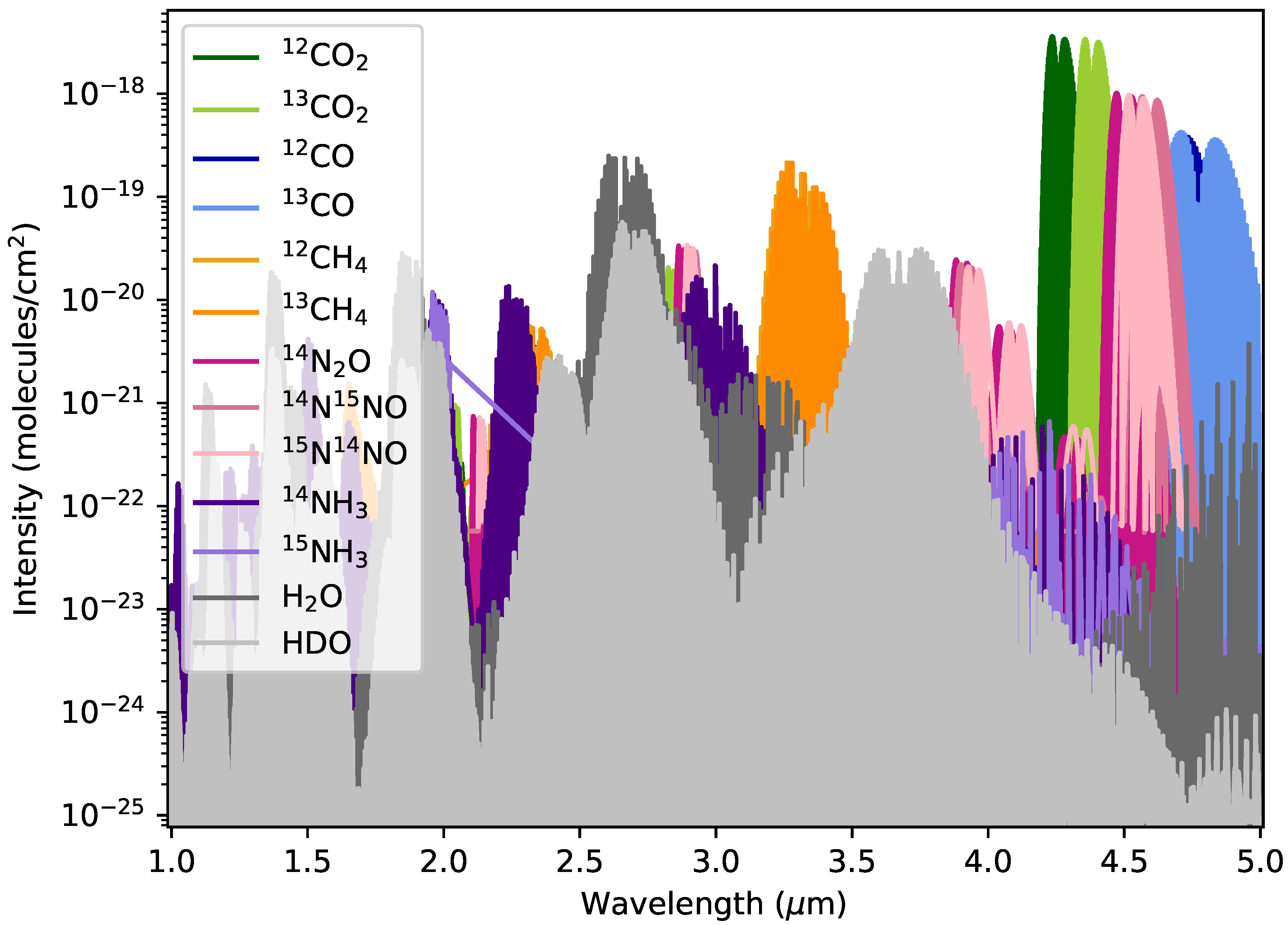
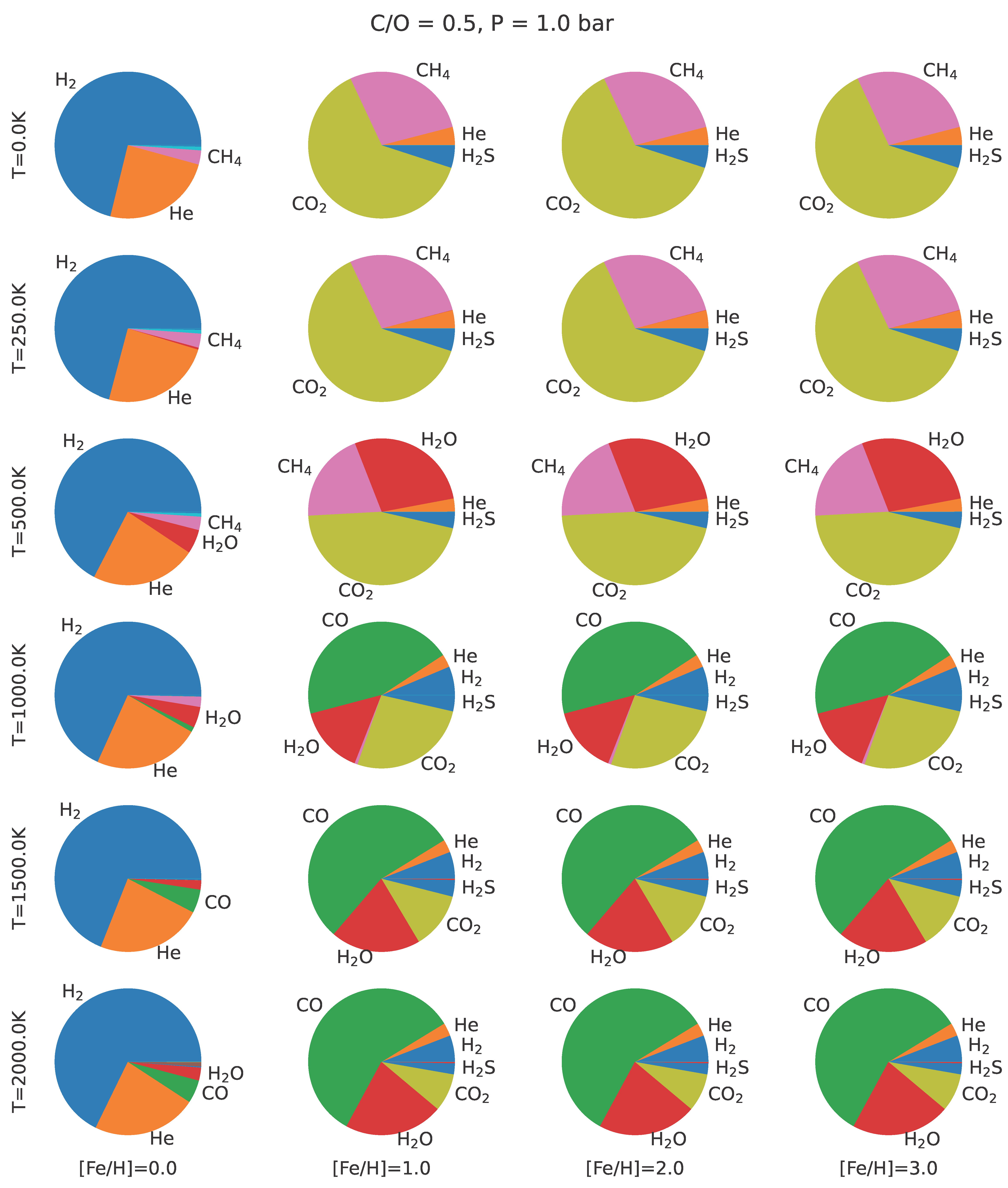
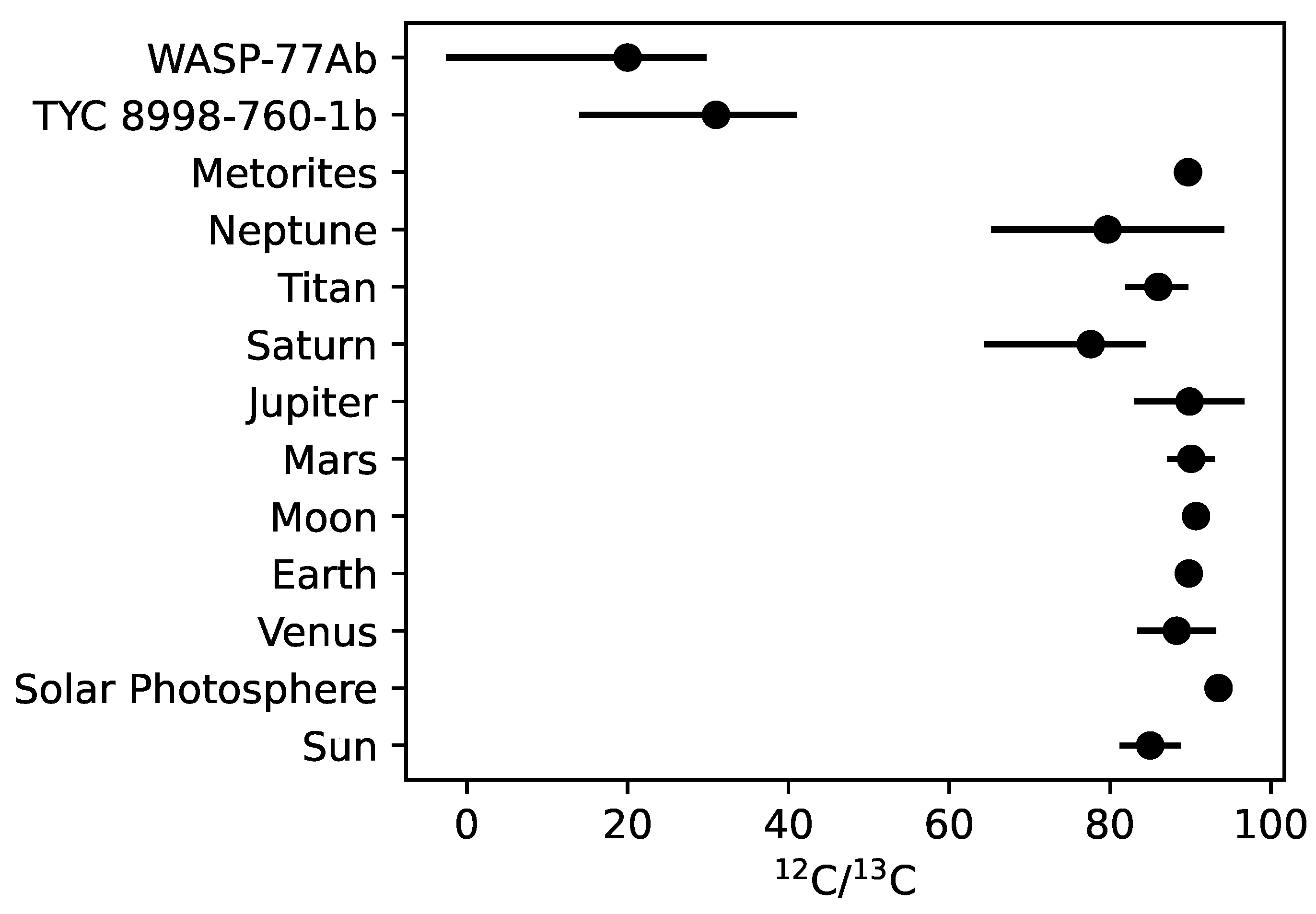
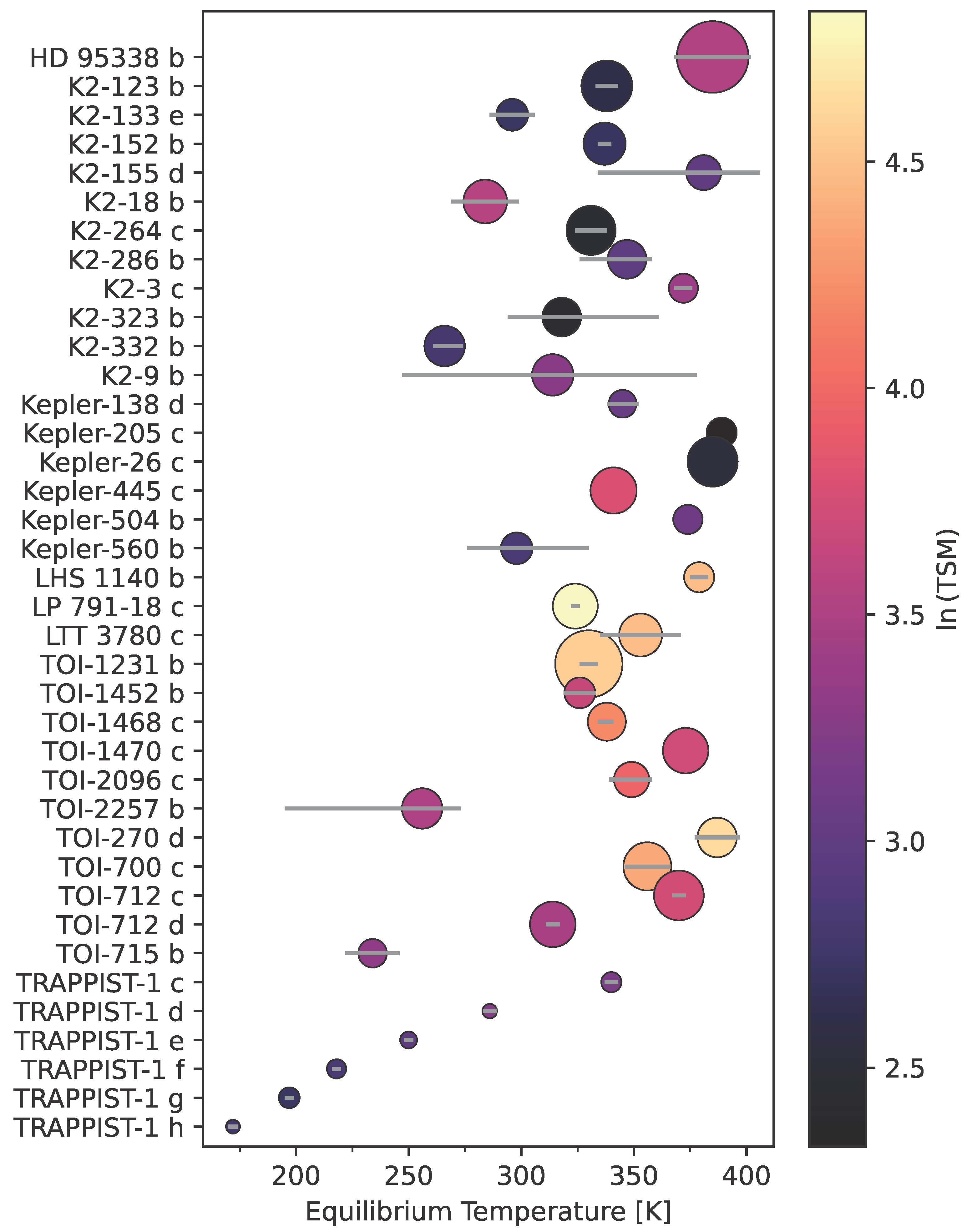
| Object | C/C |
|---|---|
| Sun 1 | |
| Venus 2 | |
| Earth 2 | |
| Moon 2 | |
| Mars 2 | |
| Terrestrial 2 | |
| Jupiter 2 | |
| Saturn 2 | |
| Neptune 2 | |
| TYC 8998-760-1 b 3 | |
| WASP-77A b 4 | 10.2–42.6 at 68% confidence |
| GJ 745A 5 | |
| GJ 745B 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glidden, A.; Seager, S.; Petkowski, J.J.; Ono, S. Can Isotopologues Be Used as Biosignature Gases in Exoplanet Atmospheres? Life 2023, 13, 2325. https://doi.org/10.3390/life13122325
Glidden A, Seager S, Petkowski JJ, Ono S. Can Isotopologues Be Used as Biosignature Gases in Exoplanet Atmospheres? Life. 2023; 13(12):2325. https://doi.org/10.3390/life13122325
Chicago/Turabian StyleGlidden, Ana, Sara Seager, Janusz J. Petkowski, and Shuhei Ono. 2023. "Can Isotopologues Be Used as Biosignature Gases in Exoplanet Atmospheres?" Life 13, no. 12: 2325. https://doi.org/10.3390/life13122325
APA StyleGlidden, A., Seager, S., Petkowski, J. J., & Ono, S. (2023). Can Isotopologues Be Used as Biosignature Gases in Exoplanet Atmospheres? Life, 13(12), 2325. https://doi.org/10.3390/life13122325









