Retinoids: Mechanisms of Action in Neuronal Cell Fate Acquisition
Abstract
1. Introduction
2. Retinoic Acid Uptake and Metabolism
3. Retinoic Acid Receptors
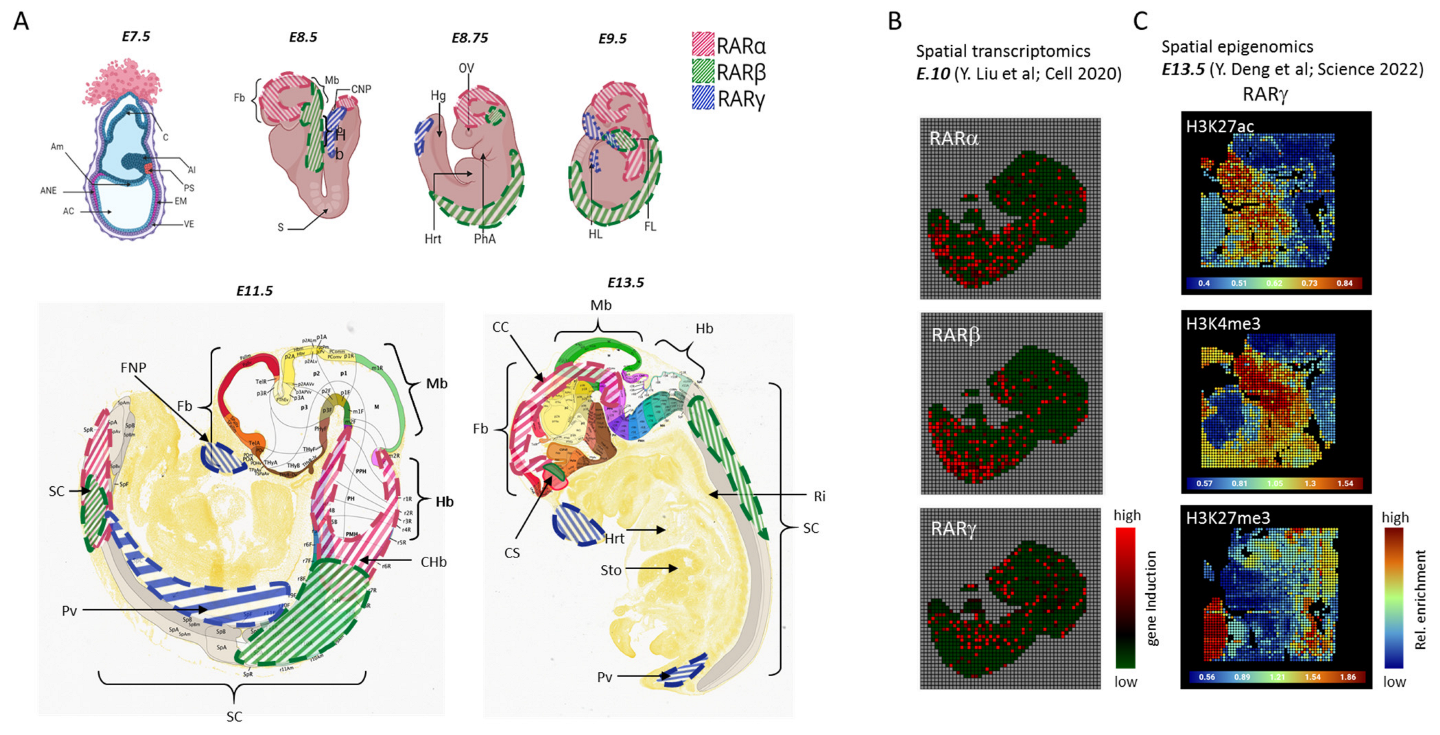
4. The Role and Distribution of Retinoic Acid in Embryogenesis
5. Neuronal Cell Specialization Studies in Animal Models
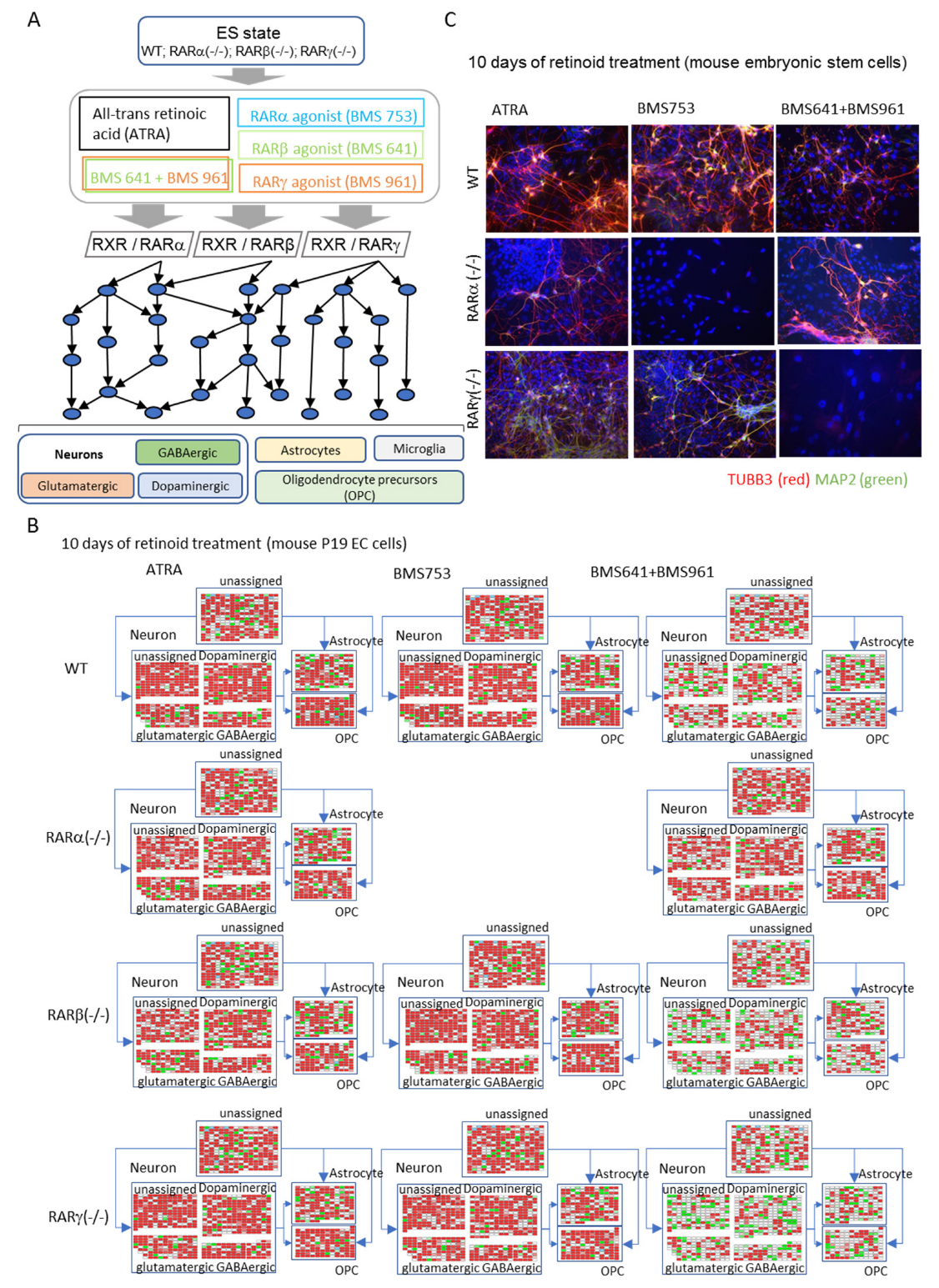
6. In Vitro Studies Modeling Neuronal Cell Specialization
7. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Semba, R.D. On the “discovery” of Vitamin A. Ann. Nutr. Metab. 2012, 61, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, F.G. Feeding Experiments Illustrating the Importance of Accessory Factors in Normal Dietaries. J. Physiol. 1912, 44, 425–460. [Google Scholar] [CrossRef] [PubMed]
- Wolbach, S.B.; Howe, P.R. Tissue Changes Following Deprivation of Fat-Soluble A Vitamin. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef] [PubMed]
- Giguère, V.; Evans, R.M. Chronicle of a Discovery: The Retinoic Acid Receptor. J. Mol. Endocrinol. 2022, 69, T1–T11. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, R.; Blomhoff, H.K. Overview of Retinoid Metabolism and Function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Duester, G. Retinoic Acid Synthesis and Signaling during Early Organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Ang, H.L.; Deltour, L.; Hayamizu, T.F.; Zgombić-Knight, M.; Duester, G. Retinoic Acid Synthesis in Mouse Embryos during Gastrulation and Craniofacial Development Linked to Class IV Alcohol Dehydrogenase Gene Expression. J. Biol. Chem. 1996, 271, 9526–9534. [Google Scholar] [CrossRef]
- Deltour, L.; Foglio, M.H.; Duester, G. Metabolic Deficiencies in Alcohol Dehydrogenase Adh1, Adh3, and Adh4 Null Mutant Mice. Overlapping Roles of Adh1 and Adh4 in Ethanol Clearance and Metabolism of Retinol to Retinoic Acid. J. Biol. Chem. 1999, 274, 16796–16801. [Google Scholar] [CrossRef]
- Molotkov, A.; Fan, X.; Duester, G. Excessive Vitamin A Toxicity in Mice Genetically Deficient in Either Alcohol Dehydrogenase Adh1 or Adh3. Eur. J. Biochem. 2002, 269, 2607–2612. [Google Scholar] [CrossRef]
- Parker, R.O.; Crouch, R.K. Retinol Dehydrogenases (RDHs) in the Visual Cycle. Exp. Eye Res. 2010, 91, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; McCaffery, P.; Dräger, U.C.; Chambon, P.; Dollé, P. Restricted Expression and Retinoic Acid-Induced Downregulation of the Retinaldehyde Dehydrogenase Type 2 (RALDH-2) Gene during Mouse Development. Mech. Dev. 1997, 62, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Rochette-Egly, C. Retinoic Acid Signaling and Mouse Embryonic Stem Cell Differentiation: Cross Talk between Genomic and Non-Genomic Effects of RA. Biochim. Biophys. Acta 2015, 1851, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kam, R.K.T.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic Acid Synthesis and Functions in Early Embryonic Development. Cell Biosci. 2012, 2, 11. [Google Scholar] [CrossRef]
- Balmer, J.E.; Blomhoff, R. Gene Expression Regulation by Retinoic Acid. J. Lipid. Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef]
- Brtko, J.; Dvorak, Z. Natural and Synthetic Retinoid X Receptor Ligands and Their Role in Selected Nuclear Receptor Action. Biochimie 2020, 179, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Thacher, S.; Vasudevan, J.; Chandraratna, R. Therapeutic Applications for Ligands of Retinoid Receptors. CPD 2000, 6, 25–58. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, N.R.; Conda-Sheridan, M. A Review of the Molecular Design and Biological Activities of RXR Agonists. Med. Res. Rev. 2019, 39, 1372–1397. [Google Scholar] [CrossRef]
- Asson-Batres, M.A.; Rochette-Egly, C. (Eds.) The Biochemistry of Retinoic Acid Receptors I: Structure, Activation, and Function at the Molecular Level; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; Volume 70, ISBN 978-94-017-9049-9. [Google Scholar]
- Roy, B.; Taneja, R.; Chambon, P. Synergistic Activation of Retinoic Acid (RA)-Responsive Genes and Induction of Embryonal Carcinoma Cell Differentiation by an RA Receptor Alpha (RAR Alpha)-, RAR Beta-, or RAR Gamma-Selective Ligand in Combination with a Retinoid X Receptor-Specific Ligand. Mol. Cell Biol. 1995, 15, 6481–6487. [Google Scholar] [CrossRef]
- Taneja, R.; Roy, B.; Plassat, J.L.; Zusi, C.F.; Ostrowski, J.; Reczek, P.R.; Chambon, P. Cell-Type and Promoter-Context Dependent Retinoic Acid Receptor (RAR) Redundancies for RAR Beta 2 and Hoxa-1 Activation in F9 and P19 Cells Can Be Artefactually Generated by Gene Knockouts. Proc. Natl. Acad. Sci. USA 1996, 93, 6197–6202. [Google Scholar] [CrossRef]
- Chiba, H.; Clifford, J.; Metzger, D.; Chambon, P. Specific and Redundant Functions of Retinoid X Receptor/Retinoic Acid Receptor Heterodimers in Differentiation, Proliferation, and Apoptosis of F9 Embryonal Carcinoma Cells. J. Cell Biol. 1997, 139, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Parra, M.A.; Walia, M.; Sankar, M.; Gronemeyer, H. Dissecting the Retinoid-Induced Differentiation of F9 Embryonal Stem Cells by Integrative Genomics. Mol. Syst. Biol. 2011, 7, 538. [Google Scholar] [CrossRef]
- Mendoza-Parra, M.-A.; Malysheva, V.; Mohamed Saleem, M.A.; Lieb, M.; Godel, A.; Gronemeyer, H. Reconstructed Cell Fate-Regulatory Programs in Stem Cells Reveal Hierarchies and Key Factors of Neurogenesis. Genome Res. 2016, 26, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Koshy, A.; Mathieux, E.; Stüder, F.; Bramoulle, A.; Lieb, M.; Colombo, B.M.; Gronemeyer, H.; Mendoza-Parra, M.A. Synergistic Activation of RARβ and RARγ Nuclear Receptors Restores Cell Specialization during Stem Cell Differentiation by Hijacking RARα-Controlled Programs. Life Sci. Alliance 2023, 6, e202201627. [Google Scholar] [CrossRef] [PubMed]
- Dollé, P.; Izpisúa-Belmonte, J.C.; Falkenstein, H.; Renucci, A.; Duboule, D. Coordinate Expression of the Murine Hox-5 Complex Homoeobox-Containing Genes during Limb Pattern Formation. Nature 1989, 342, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, E.; Dolle, P.; Krust, A.; Zelent, A.; Morriss-Kay, G.; Chambon, P. Specific Spatial and Temporal Distribution of Retinoic Acid Receptor Gamma Transcripts during Mouse Embryogenesis. Development 1990, 108, 213–222. [Google Scholar] [CrossRef]
- Ruberte, E.; Dolle, P.; Chambon, P.; Morriss-Kay, G. Retinoic Acid Receptors and Cellular Retinoid Binding Proteins. II. Their Differential Pattern of Transcription during Early Morphogenesis in Mouse Embryos. Development 1991, 111, 45–60. [Google Scholar] [CrossRef]
- Hale, L.A.; Tallafuss, A.; Yan, Y.-L.; Dudley, L.; Eisen, J.S.; Postlethwait, J.H. Characterization of the Retinoic Acid Receptor Genes Raraa, Rarab and Rarg during Zebrafish Development. Gene Expr. Patterns 2006, 6, 546–555. [Google Scholar] [CrossRef]
- Tallafuss, A.; Hale, L.A.; Yan, Y.-L.; Dudley, L.; Eisen, J.S.; Postlethwait, J.H. Characterization of Retinoid-X Receptor Genes Rxra, Rxrba, Rxrbb and Rxrg during Zebrafish Development. Gene Expr. Patterns 2006, 6, 556–565. [Google Scholar] [CrossRef]
- Waxman, J.S.; Yelon, D. Comparison of the Expression Patterns of Newly Identified Zebrafish Retinoic Acid and Retinoid X Receptors. Dev. Dyn. 2007, 236, 587–595. [Google Scholar] [CrossRef]
- Blumberg, B.; Mangelsdorf, D.J.; Dyck, J.A.; Bittner, D.A.; Evans, R.M.; De Robertis, E.M. Multiple Retinoid-Responsive Receptors in a Single Cell: Families of Retinoid “X” Receptors and Retinoic Acid Receptors in the Xenopus Egg. Proc. Natl. Acad. Sci. USA 1992, 89, 2321–2325. [Google Scholar] [CrossRef]
- Ellinger-Ziegelbauer, H.; Dreyer, C. A Retinoic Acid Receptor Expressed in the Early Development of Xenopus Laevis. Genes Dev. 1991, 5, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Downes, M.; Chandraratna, R.A.; Blumberg, B.; Umesono, K. Active Repression of RAR Signaling Is Required for Head Formation. Genes Dev. 2001, 15, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Shiotsugu, J.; Katsuyama, Y.; Arima, K.; Baxter, A.; Koide, T.; Song, J.; Chandraratna, R.A.S.; Blumberg, B. Multiple Points of Interaction between Retinoic Acid and FGF Signaling during Embryonic Axis Formation. Development 2004, 131, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
- Biga, L.M.; Bronson, S.; Dawson, S.; Harwell, A.; Hopkins, R.; Kaufmann, J.; LeMaster, M.; Matern, P.; Morrison-Graham, K.; Oja, K.; et al. Anatomy & Physiology; OpenStax/Oregon State University: Corvallis, OR, USA, 2019. [Google Scholar]
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic Retinoic Acid Synthesis Is Essential for Early Mouse Post-Implantation Development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.; Viville, S.; Ward, S.J.; Décimo, D.; Chambon, P.; Dollé, P. Tissue-Specific Expression of Retinoic Acid Receptor Isoform Transcripts in the Mouse Embryo. Mech. Dev. 2000, 94, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, E.; Friederich, V.; Chambon, P.; Morriss-Kay, G. Retinoic Acid Receptors and Cellular Retinoid Binding Proteins III. Their Differential Transcript Distribution during Mouse Nervous System Development. Development 1993, 118, 267–282. [Google Scholar] [CrossRef]
- Dollé, P. Developmental Expression of Retinoic Acid Receptors (RARs). Nucl. Recept. Signal. 2009, 7, e006. [Google Scholar] [CrossRef]
- Allen Institute for Brain Science. Allen Mouse Brain Atlas [Dataset]. 2013. Available online: http://mouse.brain-map.org/ (accessed on 4 January 2022).
- Liu, Y.; Yang, M.; Deng, Y.; Su, G.; Enninful, A.; Guo, C.C.; Tebaldi, T.; Zhang, D.; Kim, D.; Bai, Z.; et al. High-Spatial-Resolution Multi-Omics Sequencing via Deterministic Barcoding in Tissue. Cell 2020, 183, 1665–1681.e18. [Google Scholar] [CrossRef]
- Moehlin, J.; Mollet, B.; Colombo, B.M.; Mendoza-Parra, M.A. Inferring Biologically Relevant Molecular Tissue Substructures by Agglomerative Clustering of Digitized Spatial Transcriptomes with Multilayer. Cell Syst. 2021, 12, 694–705.e3. [Google Scholar] [CrossRef]
- Protocol for Using MULTILAYER to Reveal Molecular Tissue Substructures from Digitized Spatial Transcriptomes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34585159/ (accessed on 4 January 2022).
- Deng, Y.; Bartosovic, M.; Kukanja, P.; Zhang, D.; Liu, Y.; Su, G.; Enninful, A.; Bai, Z.; Castelo-Branco, G.; Fan, R. Spatial-CUT&Tag: Spatially Resolved Chromatin Modification Profiling at the Cellular Level. Science 2022, 375, 681–686. [Google Scholar] [CrossRef]
- Barnett, J.; Sotudeh, N.; Rao, P.; Silverman, J.; Jafar, T.; Wang, L. AtlasXplore: A Web Platform for Visualizing and Sharing Spatial Epigenome Data. Bioinformatics 2023, 39, btad447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cooper, S.; Brockdorff, N. The Interplay of Histone Modifications—Writers That Read. EMBO Rep. 2015, 16, 1467–1481. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Yashiro, K.; Mamiya, S.; Nishino, J.; Chambon, P.; Dolle, P.; Sakai, Y. CYP26A1 and CYP26C1 Cooperatively Regulate Anterior-Posterior Patterning of the Developing Brain and the Production of Migratory Cranial Neural Crest Cells in the Mouse. Dev. Biol. 2007, 302, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.E.; Putzke, A.P.; Myers, J.P.; Margaretha, L.; Moens, C.B. Cyp26 Enzymes Generate the Retinoic Acid Response Pattern Necessary for Hindbrain Development. Development 2007, 134, 177–187. [Google Scholar] [CrossRef]
- Ribes, V.; Otto, D.M.E.; Dickmann, L.; Schmidt, K.; Schuhbaur, B.; Henderson, C.; Blomhoff, R.; Wolf, C.R.; Tickle, C.; Dollé, P. Rescue of Cytochrome P450 Oxidoreductase (Por) Mouse Mutants Reveals Functions in Vasculogenesis, Brain and Limb Patterning Linked to Retinoic Acid Homeostasis. Dev. Biol. 2007, 303, 66–81. [Google Scholar] [CrossRef]
- Novitch, B.G.; Wichterle, H.; Jessell, T.M.; Sockanathan, S. A Requirement for Retinoic Acid-Mediated Transcriptional Activation in Ventral Neural Patterning and Motor Neuron Specification. Neuron 2003, 40, 81–95. [Google Scholar] [CrossRef]
- Wilson, L.; Maden, M. The Mechanisms of Dorsoventral Patterning in the Vertebrate Neural Tube. Dev. Biol. 2005, 282, 1–13. [Google Scholar] [CrossRef]
- Diez del Corral, R.; Storey, K.G. Opposing FGF and Retinoid Pathways: A Signalling Switch That Controls Differentiation and Patterning Onset in the Extending Vertebrate Body Axis. Bioessays 2004, 26, 857–869. [Google Scholar] [CrossRef]
- Sockanathan, S.; Jessell, T.M. Motor Neuron-Derived Retinoid Signaling Specifies the Subtype Identity of Spinal Motor Neurons. Cell 1998, 94, 503–514. [Google Scholar] [CrossRef]
- Sockanathan, S.; Perlmann, T.; Jessell, T.M. Retinoid Receptor Signaling in Postmitotic Motor Neurons Regulates Rostrocaudal Positional Identity and Axonal Projection Pattern. Neuron 2003, 40, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Holzschuh, J.; Barrallo-Gimeno, A.; Ettl, A.-K.; Durr, K.; Knapik, E.W.; Driever, W. Noradrenergic Neurons in the Zebrafish Hindbrain Are Induced by Retinoic Acid and Require Tfap2a for Expression of the Neurotransmitter Phenotype. Development 2003, 130, 5741–5754. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, C.; Brade, T.; Duester, G. Retinoic Acid Functions as a Key GABAergic Differentiation Signal in the Basal Ganglia. PLoS Biol. 2011, 9, e1000609. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Kong, J.; Moore, S.; Milton, C.; Sasai, N.; Gonzalez-Quevedo, R.; Terriente, J.; Imayoshi, I.; Kageyama, R.; Wilkinson, D.G.; et al. Retinoid Acid Specifies Neuronal Identity through Graded Expression of Ascl1. Curr. Biol. 2013, 23, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Rataj-Baniowska, M.; Niewiadomska-Cimicka, A.; Paschaki, M.; Szyszka-Niagolov, M.; Carramolino, L.; Torres, M.; Dollé, P.; Krężel, W. Retinoic Acid Receptor β Controls Development of Striatonigral Projection Neurons through FGF-Dependent and Meis1-Dependent Mechanisms. J. Neurosci. 2015, 35, 14467–14475. [Google Scholar] [CrossRef]
- Lobjois, V.; Bel-Vialar, S.; Trousse, F.; Pituello, F. Forcing Neural Progenitor Cells to Cycle Is Insufficient to Alter Cell-Fate Decision and Timing of Neuronal Differentiation in the Spinal Cord. Neural Dev. 2008, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Dasen, J.S.; De Camilli, A.; Wang, B.; Tucker, P.W.; Jessell, T.M. Hox Repertoires for Motor Neuron Diversity and Connectivity Gated by a Single Accessory Factor, FoxP1. Cell 2008, 134, 304–316. [Google Scholar] [CrossRef]
- Sagner, A.; Gaber, Z.B.; Delile, J.; Kong, J.H.; Rousso, D.L.; Pearson, C.A.; Weicksel, S.E.; Melchionda, M.; Mousavy Gharavy, S.N.; Briscoe, J.; et al. Olig2 and Hes Regulatory Dynamics during Motor Neuron Differentiation Revealed by Single Cell Transcriptomics. PLoS Biol. 2018, 16, e2003127. [Google Scholar] [CrossRef]
- Carcagno, A.L.; Di Bella, D.J.; Goulding, M.; Guillemot, F.; Lanuza, G.M. Neurogenin3 Restricts Serotonergic Neuron Differentiation to the Hindbrain. J. Neurosci. 2014, 34, 15223–15233. [Google Scholar] [CrossRef]
- Casarosa, S.; Fode, C.; Guillemot, F. Mash1 Regulates Neurogenesis in the Ventral Telencephalon. Development 1999, 126, 525–534. [Google Scholar] [CrossRef]
- Murakami, A.; Grinberg, D.; Thurlow, J.; Dickson, C. Identification of Positive and Negative Regulatory Elements Involved in the Retinoic Acid/cAMP Induction of Fgf-3 Transcription in F9 Cells. Nucleic Acids Res. 1993, 21, 5351–5359. [Google Scholar] [CrossRef][Green Version]
- McBurney, M.W. P19 Embryonal Carcinoma Cells. Int. J. Dev. Biol. 1993, 37, 135–140. [Google Scholar]
- Bain, G.; Ray, W.J.; Yao, M.; Gottlieb, D.I. From Embryonal Carcinoma Cells to Neurons: The P19 Pathway. Bioessays 1994, 16, 343–348. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W.; Jones-Villeneuve, E.M.; Edwards, M.K.; Anderson, P.J. Control of Muscle and Neuronal Differentiation in a Cultured Embryonal Carcinoma Cell Line. Nature 1982, 299, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.K.; McBurney, M.W. The Concentration of Retinoic Acid Determines the Differentiated Cell Types Formed by a Teratocarcinoma Cell Line. Dev. Biol. 1983, 98, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Reuhl, K.R.; Craig, J.; McBurney, M.W. The Role of Aggregation in Embryonal Carcinoma Cell Differentiation. J. Cell Physiol. 1987, 131, 74–84. [Google Scholar] [CrossRef]
- Monzo, H.J.; Park, T.I.H.; Montgomery, J.M.; Faull, R.L.M.; Dragunow, M.; Curtis, M.A. A Method for Generating High-Yield Enriched Neuronal Cultures from P19 Embryonal Carcinoma Cells. J. Neurosci. Methods 2012, 204, 87–103. [Google Scholar] [CrossRef]
- Mendoza-Parra, M.-A.; Gronemeyer, H. Integrative Genomics to Dissect Retinoid Functions. In The Biochemistry of Retinoic Acid Receptors I: Structure, Activation, and Function at the Molecular Level; Asson-Batres, M.A., Rochette-Egly, C., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; pp. 181–202. ISBN 978-94-017-9050-5. [Google Scholar]
- Mendoza-Parra, M.-A.; Bourguet, W.; de Lera, A.R.; Gronemeyer, H. Retinoid Receptor-Selective Modulators. In The Retinoids; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 165–192. ISBN 978-1-118-62800-3. [Google Scholar]
- Podleśny-Drabiniok, A.; Sobska, J.; de Lera, A.R.; Gołembiowska, K.; Kamińska, K.; Dollé, P.; Cebrat, M.; Krężel, W. Distinct Retinoic Acid Receptor (RAR) Isotypes Control Differentiation of Embryonal Carcinoma Cells to Dopaminergic or Striatopallidal Medium Spiny Neurons. Sci. Rep. 2017, 7, 13671. [Google Scholar] [CrossRef]
- Million, K.; Tournier, F.; Houcine, O.; Ancian, P.; Reichert, U.; Marano, F. Effects of Retinoic Acid Receptor–Selective Agonists on Human Nasal Epithelial Cell Differentiation. Am. J. Respir. Cell Mol. Biol. 2001, 25, 744–750. [Google Scholar] [CrossRef]
- Ericson, J.; Morton, S.; Kawakami, A.; Roelink, H.; Jessell, T.M. Two Critical Periods of Sonic Hedgehog Signaling Required for the Specification of Motor Neuron Identity. Cell 1996, 87, 661–673. [Google Scholar] [CrossRef]
- Ericson, J.; Briscoe, J.; Rashbass, P.; van Heyningen, V.; Jessell, T.M. Graded Sonic Hedgehog Signaling and the Specification of Cell Fate in the Ventral Neural Tube. Cold Spring Harb. Symp. Quant. Biol. 1997, 62, 451–466. [Google Scholar]
- Okada, Y.; Shimazaki, T.; Sobue, G.; Okano, H. Retinoic-Acid-Concentration-Dependent Acquisition of Neural Cell Identity during in Vitro Differentiation of Mouse Embryonic Stem Cells. Dev. Biol. 2004, 275, 124–142. [Google Scholar] [CrossRef]
- Jessell, T.M. Neuronal Specification in the Spinal Cord: Inductive Signals and Transcriptional Codes. Nat. Rev. Genet. 2000, 1, 20–29. [Google Scholar] [CrossRef]
- Pierani, A.; Moran-Rivard, L.; Sunshine, M.J.; Littman, D.R.; Goulding, M.; Jessell, T.M. Control of Interneuron Fate in the Developing Spinal Cord by the Progenitor Homeodomain Protein Dbx1. Neuron 2001, 29, 367–384. [Google Scholar] [CrossRef]
- Brown, C.R.; Butts, J.C.; McCreedy, D.A.; Sakiyama-Elbert, S.E. Generation of V2a Interneurons from Mouse Embryonic Stem Cells. Stem. Cells Dev. 2014, 23, 1765–1776. [Google Scholar] [CrossRef]
- Wichterle, H.; Lieberam, I.; Porter, J.A.; Jessell, T.M. Directed Differentiation of Embryonic Stem Cells into Motor Neurons. Cell 2002, 110, 385–397. [Google Scholar] [CrossRef]
- Salero, E.; Hatten, M.E. Differentiation of ES Cells into Cerebellar Neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 2997–3002. [Google Scholar] [CrossRef]
- Li, X.-J.; Du, Z.-W.; Zarnowska, E.D.; Pankratz, M.; Hansen, L.O.; Pearce, R.A.; Zhang, S.-C. Specification of Motoneurons from Human Embryonic Stem Cells. Nat. Biotechnol. 2005, 23, 215–221. [Google Scholar] [CrossRef]
- Amoroso, M.W.; Croft, G.F.; Williams, D.J.; O’Keeffe, S.; Carrasco, M.A.; Davis, A.R.; Roybon, L.; Oakley, D.H.; Maniatis, T.; Henderson, C.E.; et al. Accelerated High-Yield Generation of Limb-Innervating Motor Neurons from Human Stem Cells. J. Neurosci. 2013, 33, 574–586. [Google Scholar] [CrossRef]
- Kitano, O.; Nakazawa, K. Neuronal Differentiation of NT2 Cells in Monolayer and Spheroid Cultures. MATEC Web Conf. 2021, 333, 07008. [Google Scholar] [CrossRef]
- Coyle, D.E.; Li, J.; Baccei, M. Regional Differentiation of Retinoic Acid-Induced Human Pluripotent Embryonic Carcinoma Stem Cell Neurons. PLoS ONE 2011, 6, e16174. [Google Scholar] [CrossRef]
- Zeller, M.; Strauss, W.L. Retinoic Acid Induces Cholinergic Differentiation of NTera 2 Human Embryonal Carcinoma Cells. Int. J. Dev. Neurosci. 1995, 13, 437–445. [Google Scholar] [CrossRef]
- Cheung, W.M.W.; Chu, P.W.K.; Lung, C.H.; Ip, N.Y. Expression of Retinoid Receptors During the Retinoic Acid-Induced Neuronal Differentiation of Human Embryonal Carcinoma Cells. J. Neurochem. 2000, 75, 34–40. [Google Scholar] [CrossRef]
- Tonge, P.D.; Andrews, P.W. Retinoic Acid Directs Neuronal Differentiation of Human Pluripotent Stem Cell Lines in a Non-Cell-Autonomous Manner. Differentiation 2010, 80, 20–30. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Sloan, S.A.; Andersen, J.; Pașca, A.M.; Birey, F.; Pașca, S.P. Generation and Assembly of Human Brain Region-Specific Three-Dimensional Cultures. Nat. Protoc. 2018, 13, 2062–2085. [Google Scholar] [CrossRef]
- Kanton, S.; Boyle, M.J.; He, Z.; Santel, M.; Weigert, A.; Sanchís-Calleja, F.; Guijarro, P.; Sidow, L.; Fleck, J.S.; Han, D.; et al. Organoid Single-Cell Genomic Atlas Uncovers Human-Specific Features of Brain Development. Nature 2019, 574, 418–422. [Google Scholar] [CrossRef]
- Li, C.; Fleck, J.S.; Martins-Costa, C.; Burkard, T.R.; Themann, J.; Stuempflen, M.; Peer, A.M.; Vertesy, Á.; Littleboy, J.B.; Esk, C.; et al. Single-Cell Brain Organoid Screening Identifies Developmental Defects in Autism. Nature 2023, 621, 373–380. [Google Scholar] [CrossRef]
- Gabriel, E.; Albanna, W.; Pasquini, G.; Ramani, A.; Josipovic, N.; Mariappan, A.; Schinzel, F.; Karch, C.M.; Bao, G.; Gottardo, M.; et al. Human Brain Organoids Assemble Functionally Integrated Bilateral Optic Vesicles. Cell Stem. Cell 2021, 28, 1740–1757.e8. [Google Scholar] [CrossRef]
- Lozachmeur, G.; Bramoulle, A.; Aubert, A.; Stüder, F.; Moehlin, J.; Madrange, L.; Yates, F.; Deslys, J.-P.; Mendoza-Parra, M.A. Three-Dimensional Molecular Cartography of Human Cerebral Organoids Revealed by Double-Barcoded Spatial Transcriptomics. Cell Rep. Methods 2023, 3, 100573. [Google Scholar] [CrossRef]
- Uzquiano, A.; Kedaigle, A.J.; Pigoni, M.; Paulsen, B.; Adiconis, X.; Kim, K.; Faits, T.; Nagaraja, S.; Antón-Bolaños, N.; Gerhardinger, C.; et al. Proper Acquisition of Cell Class Identity in Organoids Allows Definition of Fate Specification Programs of the Human Cerebral Cortex. Cell 2022, 185, 3770–3788.e27. [Google Scholar] [CrossRef]
- He, Z.; Maynard, A.; Jain, A.; Gerber, T.; Petri, R.; Lin, H.-C.; Santel, M.; Ly, K.; Dupré, J.-S.; Sidow, L.; et al. Lineage Recording in Human Cerebral Organoids. Nat. Methods 2022, 19, 90–99. [Google Scholar] [CrossRef]
- Oldak, B.; Wildschutz, E.; Bondarenko, V.; Comar, M.-Y.; Zhao, C.; Aguilera-Castrejon, A.; Tarazi, S.; Viukov, S.; Pham, T.X.A.; Ashouokhi, S.; et al. Complete Human Day 14 Post-Implantation Embryo Models from Naïve ES Cells. Nature 2023, 622, 562–573. [Google Scholar] [CrossRef]
- Weatherbee, B.A.T.; Gantner, C.W.; Iwamoto-Stohl, L.K.; Daza, R.M.; Hamazaki, N.; Shendure, J.; Zernicka-Goetz, M. Pluripotent Stem Cell-Derived Model of the Post-Implantation Human Embryo. Nature 2023, 622, 584–593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshy, A.M.; Mendoza-Parra, M.A. Retinoids: Mechanisms of Action in Neuronal Cell Fate Acquisition. Life 2023, 13, 2279. https://doi.org/10.3390/life13122279
Koshy AM, Mendoza-Parra MA. Retinoids: Mechanisms of Action in Neuronal Cell Fate Acquisition. Life. 2023; 13(12):2279. https://doi.org/10.3390/life13122279
Chicago/Turabian StyleKoshy, Aysis Maria, and Marco Antonio Mendoza-Parra. 2023. "Retinoids: Mechanisms of Action in Neuronal Cell Fate Acquisition" Life 13, no. 12: 2279. https://doi.org/10.3390/life13122279
APA StyleKoshy, A. M., & Mendoza-Parra, M. A. (2023). Retinoids: Mechanisms of Action in Neuronal Cell Fate Acquisition. Life, 13(12), 2279. https://doi.org/10.3390/life13122279




