Neural Mobilization for Reducing Pain and Disability in Patients with Lumbar Radiculopathy: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Primary Outcome Measurements
2.4. Secondary Outcome Measurements
2.5. Data Extraction
2.6. Assessment and Quality Classification
2.7. Statistical Analysis
3. Results
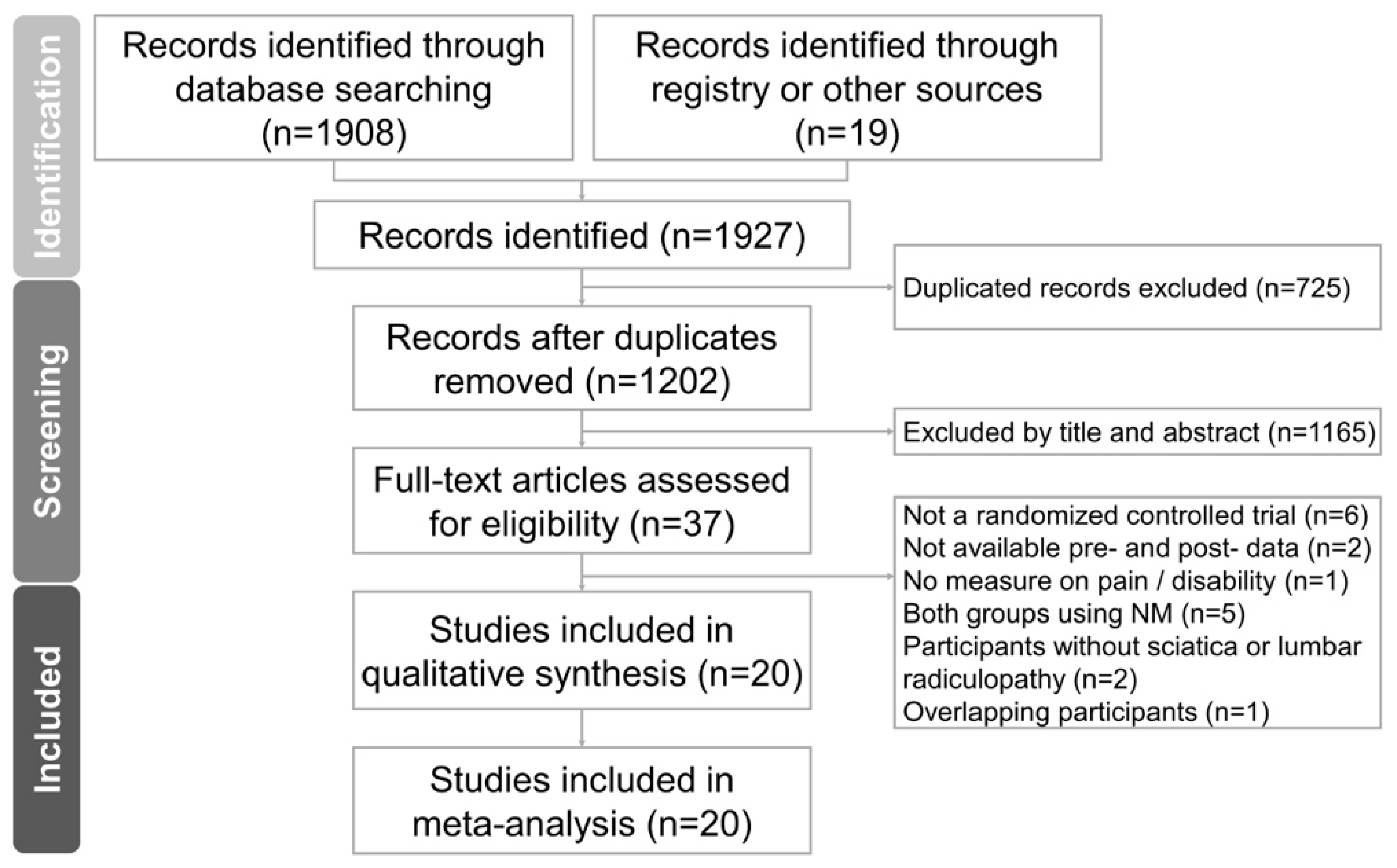
3.1. Study Identification and Selection
3.2. Methodological Quality of the Included Studies
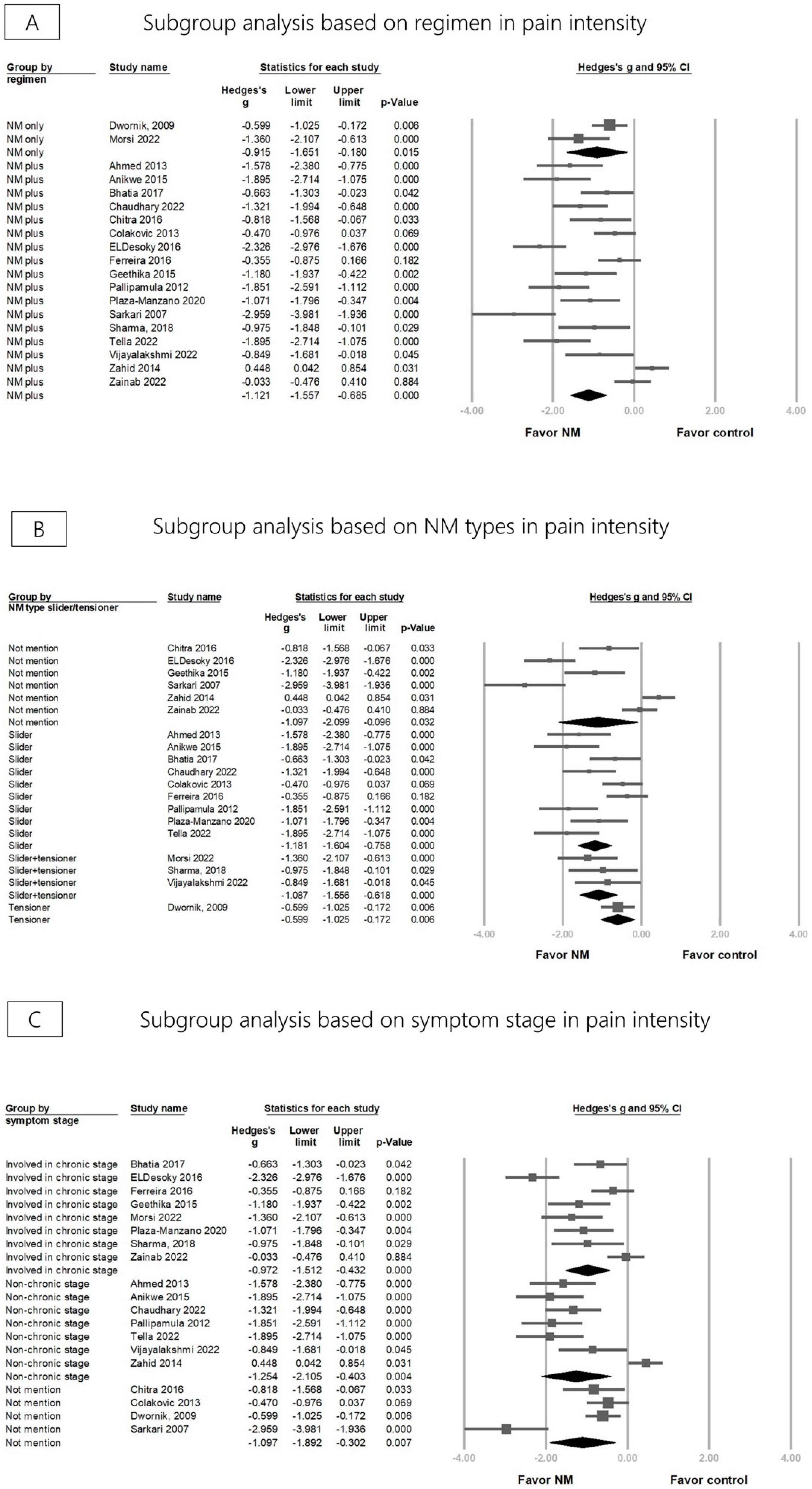
3.3. Effectiveness of NM on Pain Reduction
3.4. Effectiveness of NM on Disability
3.5. Publication Bias
4. Discussion
4.1. Mechanisms of NM
4.2. Effectiveness of NM on Pain Reduction and Disability Improvement
4.3. Adverse Events
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, J.A.; Elia, C.; Saini, H.S.; Miulli, D.E. A Review of Lumbar Radiculopathy, Diagnosis, and Treatment. Cureus 2019, 11, e5934. [Google Scholar] [CrossRef] [PubMed]
- Tarulli, A.W.; Raynor, E.M. Lumbosacral radiculopathy. Neurol. Clin. 2007, 25, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Taylor, J.; Wright, A.; Milosavljevic, S.; Goode, A.; Whitford, M. Risk factors for first time incidence sciatica: A systematic review. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2014, 19, 65–78. [Google Scholar] [CrossRef]
- Lewis, R.A.; Williams, N.H.; Sutton, A.J.; Burton, K.; Din, N.U.; Matar, H.E.; Hendry, M.; Phillips, C.J.; Nafees, S.; Fitzsimmons, D. Comparative clinical effectiveness of management strategies for sciatica: Systematic review and network meta-analyses. Spine J. 2015, 15, 1461–1477. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, M.A.; Stefanakis, M.; Savva, C.; Giakas, G. Effectiveness of neural mobilization in patients with spinal radiculopathy: A critical review. J. Bodyw. Mov. Ther. 2015, 19, 205–212. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Hough, A.D.; Dilley, A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: An in vivo study using dynamic ultrasound imaging. J. Orthop. Sports Phys. Ther. 2009, 39, 164–171. [Google Scholar] [CrossRef]
- Shacklock, M. Clinical Neurodynamics: A New System of Neuromusculoskeletal Treatment; Elsevier Health Sciences: Edinburgh, UK, 2005. [Google Scholar]
- Gilbert, K.K.; Roger James, C.; Apte, G.; Brown, C.; Sizer, P.S.; Brismée, J.M.; Smith, M.P. Effects of simulated neural mobilization on fluid movement in cadaveric peripheral nerve sections: Implications for the treatment of neuropathic pain and dysfunction. J. Man. Manip. Ther. 2015, 23, 219–225. [Google Scholar] [CrossRef]
- Beneciuk, J.M.; Bishop, M.D.; George, S.Z. Effects of upper extremity neural mobilization on thermal pain sensitivity: A sham-controlled study in asymptomatic participants. J. Orthop. Sports Phys. Ther. 2009, 39, 428–438. [Google Scholar] [CrossRef]
- Zhu, G.C.; Tsai, K.L.; Chen, Y.W.; Hung, C.H. Neural Mobilization Attenuates Mechanical Allodynia and Decreases Proinflammatory Cytokine Concentrations in Rats With Painful Diabetic Neuropathy. Phys. Ther. 2018, 98, 214–222. [Google Scholar] [CrossRef]
- Neto, T.; Freitas, S.R.; Marques, M.; Gomes, L.; Andrade, R.; Oliveira, R. Effects of lower body quadrant neural mobilization in healthy and low back pain populations: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2017, 27, 14–22. [Google Scholar] [CrossRef]
- Pourahmadi, M.; Hesarikia, H.; Keshtkar, A.; Zamani, H.; Bagheri, R.; Ghanjal, A.; Shamsoddini, A. Effectiveness of Slump Stretching on Low Back Pain: A Systematic Review and Meta-analysis. Pain Med. 2019, 20, 378–396. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bielewicz, J.; Daniluk, B.; Kamieniak, P. VAS and NRS, Same or Different? Are Visual Analog Scale Values and Numerical Rating Scale Equally Viable Tools for Assessing Patients after Microdiscectomy? Pain Res. Manag. 2022, 2022, 5337483. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C. Oswestry disability index. J. Neurosurg. Spine 2014, 20, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Aithala, J.P.; Kumar, S.; Aithal, S.; Kotian, S.M. Development of a Modified Disability Questionnaire for Evaluating Disability Caused by Backache in India and Other Developing Countries. Asian Spine J. 2018, 12, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Irrgang, J.J. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys. Ther. 2001, 81, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.; Fairbank, J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Higgins, J.P.; Eldridge, S.; Li, T. Including variants on randomized trials. Cochrane Handb. Syst. Rev. Interv. 2019, 569–593. [Google Scholar] [CrossRef]
- Higgins, J.P.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. Cochrane Handb. Syst. Rev. Interv. 2019, 143–176. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Assessing risk of bias due to missing results in a synthesis. Cochrane Handb. Syst. Rev. Interv. 2019, 349–374. [Google Scholar] [CrossRef]
- Morsi, H.I.; El Nahass, B.G.E.; Ibrahim, M.M. Effects of Slider, Tensioner Neurodynamic Mobilization Techniques and Stretching Exercises in Treatment of Chronic Discogenic Sciatica: A Comparative Study. 2022. Available online: https://www.researchsquare.com/article/rs-1417011/v1 (accessed on 20 November 2023).
- Ahmed, N.; Tufel, S.; Khan, M.H.; Bhatnagar, P. Errata: Effectiveness of neural mobilization in the management of sciatica. J. Musculoskelet. Res. 2013, 16, 1392001. [Google Scholar] [CrossRef]
- Chaudhary, K.; Manjunath, H.; Singh, A.K.; Rajbanshi, S.K. Effect of Neurodynamic Slider Technique Combined with Conventional Therapy and Conventional Therapy Alone in Sciatica: A Comparative Study. Indian J. Physiother. Occup. Ther. 2022, 16, 53–62. [Google Scholar]
- Dwornik, M.; Kujawa, J.; Białoszewski, D.; Słupik, A.; Kiebzak, W. Electromyographic and clinical evaluation of the efficacy of neuromobilization in patients with low back pain. Ortop. Traumatol. Rehabil. 2009, 11, 164–176. [Google Scholar] [CrossRef]
- ELDesoky, M.T.M.; Abutaleb, E.E.M. Efficacy of neural mobilization on low back pain with S1 radiculopathy. Int. J. Physiother. 2016, 3, 362–370. [Google Scholar]
- Sharma, S.S.; Sheth, M.S. Effect of neurodynamic mobilization on pain and function in subjects with lumbo-sacral radiculopathy. Med. Sci. 2018, 7, 5–8. [Google Scholar] [CrossRef]
- Zainab, S.A.; Avaid, A.; Fatimah, W.; Perveen, W.; Naseem, N. Effects of Sciatic Nerve Mobilization on Pain, Disability and Range in Patients with Lumbar Radicular Pain. Pak. J. Med. Health Sci. 2022, 16, 97. [Google Scholar]
- Anikwe, E.; Tella, B.; Aiyegbusi, A.; Chukwu, S. Influence of Nerve Flossing Technique on acute sciatica and hip range of motion. Int. J. Med. Biomed. Res. 2015, 4, 91–99. [Google Scholar]
- Čolaković, H.; Avdić, D. Effects of neural mobilization on pain, straight leg raise test and disability in patients with radicular low back pain. J. Health Sci. 2013, 3, 109–112. [Google Scholar] [CrossRef]
- Ferreira, G.; Stieven, F.; Araujo, F.; Wiebusch, M.; Rosa, C.; Plentz, R.; Silva, M. Neurodynamic treatment did not improve pain and disability at two weeks in patients with chronic nerve-related leg pain: A randomised trial. J. Physiother. 2016, 62, 197–202. [Google Scholar] [CrossRef]
- Geethika, M.V.; Kameswari, G.; Srikumari, V.; Madhavi, K. Effectiveness of neural tissue mobilisation on pain, pain free passive slr range of motion and functional disability in low back ache subjects with sciatica. Int. J. Physiother. 2015, 2, 712–717. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Lokesh, R.; Kanthanathan, S.; Aseer, A.; Ramachandran, S. Effects of neural mobilization on sciatic nerve excursion, symptoms, and regional function in individuals with nerve-related low back pain. Physiother. Q. 2022, 30, 27–33. [Google Scholar] [CrossRef]
- Chitra, J.; Joshi, D. Comparison of the Effect of Neural Mobilization and Kinesio Taping on Pain and Quality of Life in Subjects with Sciatica -A Randomized Clinical Trial. Int. J. Sports Phys. Ther. 2016, 2, 19–23. [Google Scholar]
- Jeong, U.C.; Kim, C.Y.; Park, Y.H.; Hwang-Bo, G.; Nam, C.W. The effects of self-mobilization techniques for the sciatic nerves on physical functions and health of low back pain patients with lower limb radiating pain. J. Phys. Ther. Sci. 2016, 28, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Manzano, G.; Cancela-Cilleruelo, I.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L.; Thoomes-de-Graaf, M.; Ortega-Santiago, R. Effects of Adding a Neurodynamic Mobilization to Motor Control Training in Patients With Lumbar Radiculopathy Due to Disc Herniation: A Randomized Clinical Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 124–132. [Google Scholar] [CrossRef]
- Bhatia, S.; Bid, D.; Thangamani Ramalingam, A. Effectiveness of nerve flossing technique in chronic lumbar radiculopathy. Indian J. Physiother. Occup. Ther. 2017, 11, 44–49. [Google Scholar]
- Pallipamula, K.; Singaravelan, R. Efficacy of nerve flossing technique on improving sciatic nerve function in patients with sciatica—A randomized controlled trial. Rom. J. Phys. Ther./Rev. Romana De Kinetoterapie 2012, 18, 13–22. [Google Scholar]
- Sarkari, E.; Multani, N. Efficacy of neural mobilisation in sciatica. J. Exerc. Sci. Physiother. 2007, 3, 136–141. [Google Scholar]
- Zahid, S.; Nizami, G.N. Effectiveness of neural mobilization and stretching exercise for the management of sciatica. Pak. J. Rehabil. 2014, 3, 11–15. [Google Scholar] [CrossRef]
- Tella, B.; Aiyegbusi, A.; Anekwe, E. Efficacy of Nerve Flossing Technique in the Management of Acute Sciatica. Rom. J. Phys. Ther. 2022, 23, 14–23. [Google Scholar]
- Santos, F.M.; Silva, J.T.; Giardini, A.C.; Rocha, P.A.; Achermann, A.P.; Alves, A.S.; Britto, L.R.; Chacur, M. Neural mobilization reverses behavioral and cellular changes that characterize neuropathic pain in rats. Mol. Pain 2012, 8, 57. [Google Scholar] [CrossRef]
- da Silva, J.T.; Santos, F.M.; Giardini, A.C.; Martins Dde, O.; de Oliveira, M.E.; Ciena, A.P.; Gutierrez, V.P.; Watanabe, I.S.; Britto, L.R.; Chacur, M. Neural mobilization promotes nerve regeneration by nerve growth factor and myelin protein zero increased after sciatic nerve injury. Growth Factors 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef]
- Cleland, J.; Hunt, G.C.; Palmer, J. Effectiveness of neural mobilization in the treatment of a patient with lower extremity neurogenic pain: A single-case design. J. Man. Manip. Ther. 2004, 12, 143–152. [Google Scholar] [CrossRef]



| First Author (Year) | Country | Participants (Female/Male) | Age | Diagnosis | Duration of Symptom |
|---|---|---|---|---|---|
| Ahmed (2013) [28] | India | 30 | NM: 53.00 ± 1.91 Control: 52.60 ± 1.60 | Sciatica | 2 weeks–3 months |
| Anikwe (2015) [34] | Nigeria | 32 (19/13) | NM: 53.50 ± 8.65 Control: 51.87 ± 10.29 | Sciatica due to intervertebral disc pathology | Less than 6 weeks |
| Bhatia (2017) [42] | India | 38 | NM: 34.11 ± 8.36 Control: 35.47 ± 8.40 | Lumbar radiculopathy | NM: 7.37 ± 2.85 (months) Control: 7.26 ± 2.56 (months) |
| Chaudhary (2022) [29] | Nepal | 40 (14/26) | NM: 40.45 ± 7.3 Control: 41.5 ± 6.2 | Sciatica | 2 weeks–3 months |
| Chitra (2016) [39] | India | 30 a | NM: 32 ± 12.47 Control: 43.34 ± 13.12 | Sciatica | NA |
| Čolaković (2013) [35] | Balkans | 60 (33/27) | NM: 42.3 ± 6 Control: 43.1 ± 6.4 | Lumbar radiculopathy | NA |
| Dwornik (2009) [30] | Poland | 87 (52/34) | 43 ± 10 | Low back pain and neurogenic pain referred to the lower extremities | Chronic stage |
| ELDesoky (2016) [31] | Egypt | 60 (22/38) | NM: 41.56 ± 4.09 Control: 40.8 ± 5.37 | Herniated or bulged disc, or foraminal stenosis at L5-S1 level were the causes of radiculopathy | More than 3 months |
| Ferreira (2016) [36] | Brazil | 60 (45/15) a | NM: 43.9 ± 14.5 Control: 40.3 ± 12.9 | Unilateral nerve-related leg pain | At least 12 weeks |
| Geethika (2015) [37] | India | 30 | 30–50 | Pain or paresthesia in lumbar spine with radiating pain to lower extremity | Sub-acute or chronic stage |
| Jeong (2016) [40] | Korea | 30 (14/16) | NM: 35.1 ± 6.4 Control: 41.6 ± 11.1 | Low back pain patients with radiating lower limb pain | NA |
| Morsi (2022) [27] | Egypt | 24 (22/14) | NM: 34.38 ± 7.25 Control: 34.92 ± 6.46 | Discogenic sciatica | More than 12 weeks up to 1 year |
| Pallipamula (2012) [43] | India | 42 a | NM: 42.53 ± 6.99 Control: 40.2 ± 7.55 | Sciatica | NM: 63.63 ± 13.20 (days) Control: 62.4 ± 12.58 (days) |
| Plaza-Manzano (2020) [41] | Spain | 32 (16/16) | NM: 47.0 ± 8.0 Control: 45.5 ± 6.0 | Disc herniation between L4 and S1 levels with lumbar radiating pain to one lower limb including the foot | NM: 17.2 ± 1.5 (months) Control: 17.3 ± 1.4 (months) |
| Sarkari (2007) [44] | India | 30 (16/14) | NM: 56.1 ± 4.95 Control: 58.3 ± 4.37 | Sciatica | NA |
| Sharma (2018) [32] | India | 21 (13/11) | NM: 38.50 ± 5.73 Control: 37.55 ± 7.59 | Lumbosacral radiculopathy | NM: 3.5 + 1.00 (months) Control: 4.0 + 1.00 (months) |
| Tella (2022) [46] | Nigeria | 32 (19/13) | NM: 53.50 ± 8.65 Control: 51.87 ± 10.29 | Sciatica due to intervertebral disc pathology | Acute stage |
| Vijayalakshmi (2022) [38] | India | 23 (15/8) | NM: 41.1 ± 8.3 Control: 40.2 ± 6.2 | Low back pain with radiating pain distal to leg | Less than 3 months |
| Zahid (2014) [45] | Pakistan | 94 | 20–60 | Sciatica | 2 weeks–3 months |
| Zainab (2022) [33] | Pakistan | 80 a | NM: 39.42 ± 7.62 Control: 38.13 ± 8.03 | Lumbosacral radiculopathy | More than 2 months |
| First Author, Year | NM Group (Per-Protocol N) | Control Group (Per-Protocol N) | NM Treatment Protocol | Outcome Measurement |
|---|---|---|---|---|
| Ahmed (2013) [28] | NM + conventional treatment (15) | Conventional treatments (15) (Lumbar extension/flexion exercise plus TENS) | Total: 2 weeks, 3 days/week NM: SLR technique, slider, 2 sets of 20 repetitions | NRS SF-12 |
| Anikwe (2015) [34] | NM + physical agents + massage (16) | Physical agents + massage (16) | Total intervention period: 2 weeks, 3 days/week NM: slump technique, slider, 15 times for 3 sets with an interval of 5 min between each set | NRS |
| Bhatia (2017) [42] | NM + lumbar stabilization exercise (19) | Lumbar stabilization exercise (19) | Total: 4 weeks, 5 days/week NM: slump technique, slider, 5 sets of 15 repetitions | NRS RMDQ |
| Chaudhary (2022) [29] | NM + conventional treatment (20) | Conventional treatments (20) (Physical agents, piriformis stretch, lumbar extension exercise) | Total: 4 weeks, 3 days/week NM: SLR technique, slider, repetitions not mentioned | VAS ODI |
| Chitra (2016) [39] | NM + TENS (14) | Kinesio taping (14) | Total: 2 weeks, 3 days/week NM: technique not mentioned, grade 4 Maitland mobilization for all branches of sciatic nerve, repetitions not mentioned | VAS |
| Čolaković (2013) [35] | NM + lumbar stabilization exercise (30) | Active ROM exercise + lumbar stabilization exercises (30) | Total intervention period: 4 weeks, 3 days/week NM: side-lying SLR technique, slider, repeated 3 times with 10 oscillatory movements | VAS |
| Dwornik (2009) [30] | NM only (42) | Conventional treatments (45) (Physical agent and lumbar exercise) | Total: 2 weeks NM: tensioner and mobilization techniques, repetitions not mentioned | VAS |
| ELDesoky, 2016 [31] | NM + conventional treatments (30) | Conventional treatments (30) (physical agents, lumbar extension) | Total: 6 weeks, 3 days/week NM: SLR technique, including 30 s oscillations and 1 min rest in each session | VAS ODI |
| Ferreira (2016) [36] | NM + lumbar mobilization (28) | Education about ADL (28) | Total intervention period: 2 weeks, 2 days/week NM: side-lying SLR and slump, slider, two sets of 30 repetitions | VAS ODI |
| Geethika (2015) [37] | NM + conventional treatments + hamstring stretching + trigger release (15) | Conventional treatments + hamstring stretching + trigger release (lumbar traction + cryotherapy + back-strengthening exercises) (15) | Total intervention period: 3 weeks, 3 days/week NM: SLR technique, 10 min per session including 30 s hold and 1 min rest | VAS ODI |
| Jeong (2016) [40] | NM + lumbar segmental stabilization exercise (15) | Lumbar segmental stabilization exercise (15) | Total intervention period: 6 weeks, 3 days/week NM: technique and number of repetitions not mentioned | SF-36 |
| Morsi (2022) [27] | NM only (24) | Stretching lower extremity muscle (12) | Total: 2 weeks, 3 days per week NM: slump technique, slider and tensioner, 3 sets in every session | VAS ODI |
| Pallipamula (2012) [43] | NM + physical agents (19) | Physical agents (20) | Total intervention period: 6 days, once daily NM: slump technique, slider, participant performs knee extension with neck extension with hold for 5 s and then flexes both the knee and neck simultaneously and holds it for 5 s | VAS MODI |
| Plaza-Manzano (2020) [41] | NM+ lumbar stabilization exercise (16) | Lumbar stabilization exercise (16) | Total intervention period: 4 weeks, 2 days/week NM: SLR technique, slider, 3 sets of 10 repetitions in each treatment session | NRS RMDQ |
| Sarkari (2007) [44] | NM + physical agents (15) | Physical agents (15) | Total: 9 sessions NM: SLR, 10 min per session including 30 s hold and 1 min rest | VAS |
| Sharma (2018) [32] | NM + conventional treatments (11) | Conventional treatment (10) (Hot back, lumbar strengthening) | Total: 6 sessions NM: slider and tensioner techniques, number of repetitions not mentioned | NRS MODI |
| Tella (2022) [46] | NM+ conventional treatment + massage (16) | Conventional treatment + massage (16) (TENS + lumbar extension exercise) | Total: 2 weeks, 3 days/week NM: slump technique, slider, 15 times for 3 sets with an interval of 5 min | NRS |
| Vijayalakshmi (2022) [38] | NM + conventional treatments + hamstring stretching (13) | Conventional treatments + hamstring stretching (10) (interferential therapy lumbar strengthening) | Total: 3 weeks, total 10 sessions NM: slump technique, both sliders and tensioners, nerve sliding technique was applied for 20–30 repetitions in 2–3 sets per day for 10 sessions, and nerve tensioning technique was also implemented for 15–25 s in 5–7 repetitions in sessions 8–10. | NRS ODI |
| Zahid (2014) [45] | NM + physical agents (47) | Physical agents (47) | Total: 9 sessions NM: SLR technique, neural mobilization was given for 10 min/session, including 30 s hold and 1 min rest | NRS QBPDS |
| Zainab (2022) [33] | NM + conventional treatments (40) | Conventional treatments (37) (Physical agents, lumbar strengthening) | Total: 2 weeks, 3 days/week NM: SLR technique, 3 sets of 10 oscillatory movements | NRSMODI |
| First Author | Year | Randomization Process | Intervention Adherence | Missing Outcome Data | Outcome Measurement | Selective Reporting | Overall RoB |
|---|---|---|---|---|---|---|---|
| Ahmed | 2013 | H 1,4 | L | H 5 | L | L | H |
| Anikwe | 2015 | L | L | L | L | L | L |
| Bhatia | 2017 | S 4 | L | H 5 | L | L | H |
| Chaudhary | 2022 | S 4 | L | H 5 | L | L | H |
| Chitra | 2016 | S 4 | L | S 3 | L | L | H |
| Čolaković | 2013 | S 1 | L | H 5 | L | L | H |
| Dwornik | 2009 | H 1,4 | L | H 5 | L | L | H |
| ELDesoky | 2016 | L | L | H 5 | L | L | H |
| Ferreira | 2016 | S 4 | L | S 2 | L | L | H |
| Geethika | 2015 | H 1,4 | L | H 5 | L | L | H |
| Jeong | 2016 | S 1 | L | H 5 | L | L | H |
| Morsi | 2022 | L | L | H 5 | L | L | H |
| Pallipamula | 2012 | L | L | S 6 | L | L | S |
| Plaza-Manzano | 2020 | L | L | L | L | L | L |
| Sarkari | 2007 | S 1 | L | L | L | L | S |
| Sharma | 2018 | S 1 | L | S 6 | L | L | H |
| Tella | 2022 | L | L | S 3 | L | L | S |
| Vijayalakshmil | 2022 | L | L | L | L | L | L |
| Zahid | 2014 | S 4 | L | H 5 | L | L | H |
| Zainab | 2022 | L | L | S 6 | L | L | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.-H.; Lin, T.-Y.; Chang, K.-V.; Wu, W.-T.; Özçakar, L. Neural Mobilization for Reducing Pain and Disability in Patients with Lumbar Radiculopathy: A Systematic Review and Meta-Analysis. Life 2023, 13, 2255. https://doi.org/10.3390/life13122255
Lin L-H, Lin T-Y, Chang K-V, Wu W-T, Özçakar L. Neural Mobilization for Reducing Pain and Disability in Patients with Lumbar Radiculopathy: A Systematic Review and Meta-Analysis. Life. 2023; 13(12):2255. https://doi.org/10.3390/life13122255
Chicago/Turabian StyleLin, Long-Huei, Ting-Yu Lin, Ke-Vin Chang, Wei-Ting Wu, and Levent Özçakar. 2023. "Neural Mobilization for Reducing Pain and Disability in Patients with Lumbar Radiculopathy: A Systematic Review and Meta-Analysis" Life 13, no. 12: 2255. https://doi.org/10.3390/life13122255
APA StyleLin, L.-H., Lin, T.-Y., Chang, K.-V., Wu, W.-T., & Özçakar, L. (2023). Neural Mobilization for Reducing Pain and Disability in Patients with Lumbar Radiculopathy: A Systematic Review and Meta-Analysis. Life, 13(12), 2255. https://doi.org/10.3390/life13122255








