A Behavioral Characteristics Observational Measure of Youth with Somatic Symptom Disorder during Physical Rehabilitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Study Group—Children and Youth with SSD
2.1.2. Comparison Group—Children and Youth with Non-SSD Impairments in Body Functions and Structures
2.2. Outcome Measures
2.2.1. Indirect Measures
2.2.2. Direct Measures
2.3. Procedure
2.3.1. SSD Group
2.3.2. Comparison Group—Children and Youth with Non-SSD Impairments in Body Functions and Structures
2.4. Statistical Analysis
2.4.1. Descriptive Statistics and SBCQ Scores
2.4.2. Reliability Analysis of SBCQ
2.4.3. Convergent and Discriminative Validity of the SBCQ
3. Results
3.1. Participant Demographic and Clinical Characteristics
3.2. SBCQ Reliability and Factor Analysis
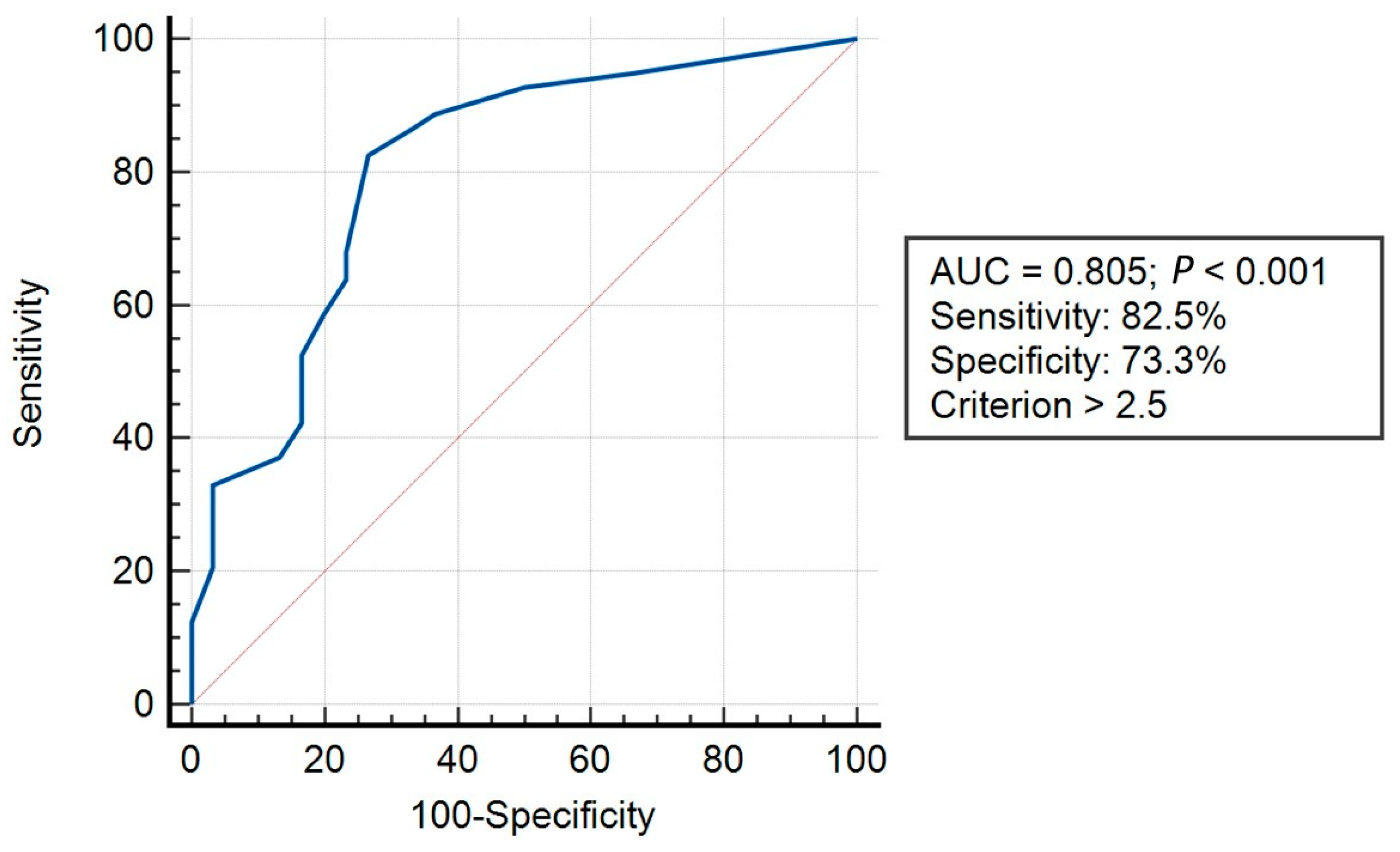
3.3. Convergent Validity
3.4. Discriminative Validity
4. Discussion
4.1. SBCQ Demographic Characteristics—Sex and Age Differences
4.2. SBCQ Reliability
4.3. SBCQ Convergent Validity
4.4. Discriminative Validity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5®]; American Psychiatric Association Publishing: Washington, DC, USA, 2016; Volume 51. [Google Scholar]
- Mohapatra, S.; Deo, S.; Satapathy, A.; Rath, N. Somatoform Disorders in Children and Adolescents. Ger. J. Psychiatry 2014, 17, 7–10. [Google Scholar]
- Gerner, M.; Barak, S.; Landa, J.; Eisenstein, E. Parent-Child Communication-Centered Rehabilitative Approach for Pediatric Functional Somatic Symptoms. Isr. J. Psychiatry 2016, 53, 39–46. [Google Scholar]
- Saunders, N.R.; Gandhi, S.; Chen, S.; Vigod, S.; Fung, K.; De Souza, C.; Saab, H.; Kurdyak, P. Health Care Use and Costs of Children, Adolescents, and Young Adults with Somatic Symptom and Related Disorders. JAMA Netw. Open 2020, 3, e2011295. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.; Stone, J.; Matthews, A.; Brown, M.; Sparkes, C.; Farmer, R.; Masterton, L.; Duncan, L.; Winters, A.; Daniell, L.; et al. Physiotherapy for Functional Motor Disorders: A Consensus Recommendation. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.E.; Pate, J.W.; Richardson, P.A.; Ickmans, K.; Wicksell, R.K.; Simons, L.E. Best-Evidence for the Rehabilitation of Chronic Pain Part 1: Pediatric Pain. J. Clin. Med. 2019, 8, 1267. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.J.; Adams, R.A.; Brown, H.; Parees, I.; Friston, K.J. A Bayesian Account of “Hysteria”. Brain 2012, 135, 3495–3512. [Google Scholar] [CrossRef]
- Karkhanis, D.G.; Winsler, A. Somatization in Children and Adolescents: Practical Implications. J. Indian Assoc. Child Adolesc. Ment. Health 2016, 12, 79–115. [Google Scholar] [CrossRef]
- Kyung, S.E.; Lee, S.M.; Lim, M.H. Child Behavior Check List and Korean Personality Inventory for Children with Functional Visual Loss. Int. J. Psychiatry Clin. Pract. 2014, 18, 197–202. [Google Scholar] [CrossRef]
- Barsky, A.J. Amplification, Somatization, and the Somatoform Disorders. Psychosomatics 1992, 33, 28–34. [Google Scholar] [CrossRef]
- Barsky, A.J.; Goodson, J.D.; Lane, R.S.; Cleary, P.D. The Amplification of Somatic Symptoms. Psychosom. Med. 1988, 50, 510–519. [Google Scholar] [CrossRef]
- Spinhoven, P.; Willem van der Does, A.J. Somatization and Somatosensory Amplification in Psychiatric Outpatients: An Explorative Study. Compr. Psychiatry 1997, 38, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Samuels, A.; Tuvia, T.; Patterson, D.; Briklin, O.; Shaffer, S.; Walker, A. Characteristics of Conversion Disorder in an Urban Academic Children’s Medical Center. Clin. Pediatr. 2019, 58, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.V. Treating Conversion Disorders: Is a Pediatric Rehabilitation Hospital the Place? Rehabil. Psychol. 1998, 43, 56–62. [Google Scholar] [CrossRef]
- Henningsen, P.; Zipfel, S.; Sattel, H.; Creed, F. Management of Functional Somatic Syndromes and Bodily Distress. Psychother. Psychosom. 2018, 87, 12–31. [Google Scholar] [CrossRef]
- Gray, N.; Savage, B.; Scher, S.; Kozlowska, K. Psychologically Informed Physical Therapy for Children and Adolescents with Functional Neurological Symptoms: The Wellness Approach. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Carson, A.J.; Brown, R.; David, A.S.; Duncan, R.; Edwards, M.J.; Goldstein, L.H.; Grunewald, R.; Howlett, S.; Kanaan, R.; Mellers, J.; et al. Functional (Conversion) Neurological Symptoms: Research since the Millennium. J. Neurol. Neurosurg. Psychiatry 2012, 83, 842–850. [Google Scholar] [CrossRef]
- Walker, L.S.; Beck, J.E.; Garber, J.; Lambert, W. Children’s Somatization Inventory: Psychometric Properties of the Revised Form (CSI-24). J. Pediatr. Psychol. 2009, 34, 430–440. [Google Scholar] [CrossRef]
- American Thoracic Society Statement. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- Pfeiffer, K.A.; Pivarnik, J.M.; Womack, C.J.; Reeves, M.J.; Malina, R.M. Reliability and Validity of the Borg and OMNI Rating of Perceived Exertion Scales in Adolescent Girls. Med. Sci. Sports Exerc. 2002, 34, 2057–2061. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Boer, N.F.; Peoples, J.A.; Foreman, A.J.; Dabayebeh, I.M.; Millich, N.B.; Balasekaran, G.; Riechman, S.E.; Gallagher, J.D.; et al. Children’s OMNI Scale of Perceived Exertion: Mixed Gender and Race Validation. Med. Sci. Sports Exerc. 2000, 32, 452–458. [Google Scholar] [CrossRef]
- Roemmich, J.N.; Barkley, J.E.; Epstein, L.H.; Lobarinas, C.L.; White, T.M.; Foster, J.H. Validity of PCERT and OMNI Walk/Run Ratings of Perceived Exertion. Med. Sci. Sports Exerc. 2006, 38, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Geenen, R.; Van Ooijen-van der Linden, L.; Lumley, M.A.; Bijlsma, J.W.J.; Van Middendorp, H. The Match–Mismatch Model of Emotion Processing Styles and Emotion Regulation Strategies in Fibromyalgia. J. Psychosom. Res. 2012, 72, 45–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanbara, K.; Fukunaga, M.; Mutsuura, H.; Takeuchi, H.; Kitamura, K.; Nakai, Y. An Exploratory Study of Subgrouping of Patients With Functional Somatic Syndrome Based on the Psychophysiological Stress Response: Its Relationship with Moods and Subjective Variables. Psychosom. Med. 2007, 69, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, K.; English, M.; Savage, B. Connecting Body and Mind: The First Interview with Somatising Patients and Their Families. Clin. Child Psychol. Psychiatry 2013, 18, 224–245. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, K. The Body Comes to Family Therapy: Utilising Research to Formulate Treatment Interventions with Somatising Children and Their Families. Aust. New Zealand J. Fam. Ther. 2016, 37, 6–29. [Google Scholar] [CrossRef]
- Kozlowska, K.; English, M.; Savage, B.; Chudleigh, C. Multimodal Rehabilitation: A Mind-Body, Family-Based Intervention for Children and Adolescents Impaired by Medically Unexplained Symptoms. Part 1: The Program. Am. J. Fam. Ther. 2012, 40, 399–419. [Google Scholar] [CrossRef]
- Häuser, W.; Walitt, B.; Fitzcharles, M.-A.; Sommer, C. Review of Pharmacological Therapies in Fibromyalgia Syndrome. Arthritis Res. Ther. 2014, 16, 201. [Google Scholar] [CrossRef]
- Hollenstein, T.; Granic, I.; Stoolmiller, M.; Snyder, J. Rigidity in Parent–Child Interactions and the Development of Externalizing and Internalizing Behavior in Early Childhood. J. Abnorm. Child. Psychol. 2004, 32, 595–607. [Google Scholar] [CrossRef]
- Feinstein, A. Conversion Disorder: Advances in Our Understanding. Can. Med. Assoc. J. 2011, 183, 915–920. [Google Scholar] [CrossRef]
- Royal College of Nursing. Clinical Practice Guidelines: The Recognition and Assessment of Acute Pain in Children; Royal College of Nursing Publishing: London, UK, 2009. [Google Scholar]
- Carson, A.; Hallett, M.; Stone, J. Assessment of Patients with Functional Neurologic Disorders. Handb. Clin. Neurol. 2016, 139, 169–188. [Google Scholar]
- Vervoort, T.; Craig, K.D.; Goubert, L.; Dehoorne, J.; Joos, R.; Matthys, D.; Buysse, A.; Crombez, G. Expressive Dimensions of Pain Catastrophizing: A Comparative Analysis of School Children and Children with Clinical Pain. Pain 2008, 134, 59–68. [Google Scholar] [CrossRef]
- Yam, A.; Rickards, T.; Pawlowski, C.A.; Harris, O.; Karandikar, N.; Yutsis, M.V. Interdisciplinary Rehabilitation Approach for Functional Neurological Symptom (Conversion) Disorder: A Case Study. Rehabil. Psychol. 2016, 61, 102–111. [Google Scholar] [CrossRef]
- Kaufman, J.N.; Lahey, S.; Slomine, B.S. Pediatric Rehabilitation Psychology: Rehabilitating a Moving Target. Rehabil. Psychol. 2017, 62, 223–226. [Google Scholar] [CrossRef]
- Lavrakas, P.J. Encyclopedia of Survey Research Methods; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics: And Sex and Drugs and Rock “n” Roll, 4th ed.; SAGE Publications, Limited: London, UK, 2015. [Google Scholar]
- Harlow, L.L. Using Multivariate Statistics, 4th ed.; Structural Equation Modeling; Allyn & Bacon: Boston, MA, USA, 2002; Volume 9. [Google Scholar]
- Allison, P.D. Logistic Regression Using SAS: Theory and Application; SAS Institute: Carry, NC, USA, 2012. [Google Scholar]
- Velicer, W.F.; Fava, J.L. Affects of Variable and Subject Sampling on Factor Pattern Recovery. Psychol Methods 1998, 3, 231–251. [Google Scholar] [CrossRef]
- Costello, A.B.; Osborne, J.W. Best Practices in Exploratory Factor Analysis: Four Recommendations for Getting the Most from Your Analysis. Pract. Assess. Res. Eval. 2005, 10, 1–9. [Google Scholar]
- Metz, C.E. Basic Principles of ROC Analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Zweig, M.H.; Campbell, G. Receiver-Operating Characteristic (ROC) Plots: A Fundamental Evaluation Tool in Clinical Medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and Practical Application of the Receiver-Operating Characteristic Analysis for Diagnostic Tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef]
- Kawai, T.; Suzuki, Y.; Hatanaka, C.; Konakawa, H.; Tanaka, Y.; Uchida, A. Gender Differences in Psychological Symptoms and Psychotherapeutic Processes in Japanese Children. Int. J. Environ. Res. Public Health 2020, 17, 9113. [Google Scholar] [CrossRef]
- Abdelmoula, M.; Chakroun, W.; Akrout, F. The effect of sample size and the number of items on reliability coefficients: Alpha and rhô: A meta-analysis. Int. J. Numer. Methods Appl. 2015, 13, 1–20. [Google Scholar] [CrossRef]
- Erpelding, N.; Simons, L.; Lebel, A.; Serrano, P.; Pielech, M.; Prabhu, S.; Becerra, L.; Borsook, D. Rapid Treatment-Induced Brain Changes in Pediatric CRPS. Brain Struct. Funct. 2016, 221, 1095–1111. [Google Scholar] [CrossRef]
- Borg, E.; Borg, G.; Larsson, K.; Letzter, M.; Sundblad, B.-M. An Index for Breathlessness and Leg Fatigue. Scand. J. Med. Sci. Sports 2010, 20, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Faull, O.K.; Cox, P.J.; Pattinson, K.T.S. Psychophysical Differences in Ventilatory Awareness and Breathlessness between Athletes and Sedentary Individuals. Front. Physiol. 2016, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Faull, O.K.; Dearlove, D.J.; Clarke, K.; Cox, P.J. Beyond RPE: The Perception of Exercise Under Normal and Ketotic Conditions. Front. Physiol. 2019, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Carrieri-Kohlman, V.; Donesky-Cuenco, D.; Park, S.K.; Mackin, L.; Nguyen, H.Q.; Paul, S.M. Additional Evidence for the Affective Dimension of Dyspnea in Patients with COPD. Res. Nurs. Health 2010, 33, 4–19. [Google Scholar] [CrossRef]
- Landa, J.; Gerner, M.; Eisenstein, E.; Barak, S. Pediatric Functional Neurological Symptoms Disorder: Walking Ability and Perceived Exertion Post-Pediatric Rehabilitation. Int. J. Environ. Res. Public Health 2023, 20, 1631. [Google Scholar] [CrossRef]
- Koekkoek, B.; Hutschemaekers, G.; Van Meijel, B.; Schene, A. How Do Patients Come to Be Seen as ‘Difficult’?: A Mixed-Methods Study in Community Mental Health Care. Soc. Sci. Med. 2011, 72, 504–512. [Google Scholar] [CrossRef]
- Kallivayalil, R.; Punnoose, V. Understanding and Managing Somatoform Disorders: Making Sense of Non-Sense. Indian J. Psychiatry 2010, 52, 240. [Google Scholar] [CrossRef]
- Janssens, K.A.M.; Oldehinkel, A.J.; Rosmalen, J.G.M. Parental Overprotection Predicts the Development of Functional Somatic Symptoms in Young Adolescents. J. Pediatr. 2009, 154, 918–923.e1. [Google Scholar] [CrossRef]
- Parikh, R.; Mathai, A.; Parikh, S.; Sekhar, G.C.; Thomas, R. Understanding and Using Sensitivity, Specificity and Predictive Values. Indian J. Ophthalmol. 2008, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Naeger, D.M.; Kohi, M.P.; Webb, E.M.; Phelps, A.; Ordovas, K.G.; Newman, T.B. Correctly Using Sensitivity, Specificity, and Predictive Values in Clinical Practice: How to Avoid Three Common Pitfalls. Am. J. Roentgenol. 2013, 200, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.G.; Bossuyt, P.M.M.; Irwig, L. Diagnostic Test Accuracy May Vary with Prevalence: Implications for Evidence-Based Diagnosis. J. Clin. Epidemiol. 2009, 62, 5–12. [Google Scholar] [CrossRef] [PubMed]
| Behavioral Characteristic | Description | Reference(s) |
|---|---|---|
| Behavioral lability | The child exhibits pronounced behavioral lability during the evaluation. For instance, within the session, the child may shift from anger to indifferent expressions or complain of a high pain level and then suddenly smiles at another child in the room. | [16,23,24] |
| Cooperation with therapist | The child is unwilling to partake in the physical activity to improve function or decrease pain. | [25,26,27] |
| Analgesic resistance | The child exhibits analgesic resistance and complains that the medications given to them are not useful. | [28] |
| Resentment towards activity variations | The child is unwilling to make variations in activity. This behavior may represent behavioral rigidity, a feature common to many psychopathologies. | [29] |
| Lack of trust | The child exhibits a lack of trust in their therapist. For instance, the child may use statements that imply distrust (e.g., “I said that I can’t do it; why do you have to test it?”). | [17,26] |
| “La belle indifference” | The child exhibits “la belle indifference”: an apparent lack of concern or distress shown by some patients toward their symptoms. It is often regarded as a typical characteristic of conversion symptoms/hysteria. | [13] |
| Amplification of symptoms | The child exhibits an intensification of the symptoms of either pain or dysfunction (e.g., mobility level and ability to conduct activities of daily living). The concept of symptom intensification may reflect an exaggeration of negative affect and illness states. | [16,27] |
| Gap between formal and informal evaluation | Inconsistencies between the child’s motor impairment (direct) and level of activity (indirect), as suggested by the Bayesian approach. | [7] |
| Mismatch between etiology and clinical presentation | Youth with somatic symptoms disorder commonly do not present the expected recovery pattern. For example, 6 weeks after an ankle sprain, the patient still complains of an inability to walk, stand, touch the affected foot, and wash it. | [30] |
| Physiological markers of pain | Child’s nonverbal pain expressions. For example, smiling may represent a negative marker of pain (i.e., no pain). However, grimacing and tears may represent a positive marker of pain. | [31] |
| Detailed description of painful event | The child provides detailed descriptions of the event responsible for the presenting problem/pain including the hour of the day, the weather on that day, the object responsible for the injury, etc. | [32,33] |
| Characteristics | Somatic Symptom Disorder Group (N = 80) | Non-Somatic Symptom Disorder Group (N = 31) | Between-Group Differences: t (p-Value) OR Chi-Squared (p-Value) | ||
|---|---|---|---|---|---|
| Mean (SD) OR n (%) | Range | Mean (SD) OR n (%) | Range | ||
| Age, years: mean (SD) | 13.91 (2.72) | 8.00–17.90 | 14.00 (3.21) | 7.50–16.50 | 1.51 (0.13) |
| Sex | |||||
| Females, n (%) | 61.00 (76.2) | - | 14.00 (45.16) | - | 9.65 (0.001) |
| Males, n (%) | 19.00 (23.7) | - | 17.00 (54.83) | - | |
| Hospitalization duration, months: mean (SD) | 5.50 (3.61) | 0.50–10.00 | 5.65 (2.21) | 1.00–11.00 | 0.23 (0.81) |
| Children’s Somatization Inventory—child, score: mean (SD) | 28.29 (13.97) | 2.00–59.00 | - | - | - |
| Rate of perceived exertion, OMNI scale: mean (SD) | 5.33 (2.66) | 0.00–10.00 | 3.23 (1.26) | 0.00–5.00 | −4.31 (<0.001) |
| Six-minute walk test distance, meters: mean (SD) | 280.12 (120.13) | 0.00–700.00 | 282.21 (150.45) | 50.12–600.25 | 0.07 (0.94) |
| Somatic Symptom Disorder Group (N = 80) | Non-Somatic Symptom Disorder Group (N = 31) | ||||
|---|---|---|---|---|---|
| Alpha | Alpha Change | Alpha | Alpha Change | ||
| Total Cronbach’s alpha | Total | 0.76 | - | 0.82 | - |
| 95% lower confidence limit | 0.67 | - | 0.78 | - | |
| Effect of dropping variables | Q1 | 0.70 | −0.02 | 0.81 | −0.01 |
| Q2 | 0.71 | −0.02 | 0.81 | −0.01 | |
| Q3 | 0.70 | −0.02 | 0.81 | −0.01 | |
| Q4 | 0.76 | 0.02 | 0.80 | −0.01 | |
| Q5 | 0.71 | −0.01 | 0.83 | 0.00 | |
| Q6 | 0.74 | 0.00 | 0.81 | −0.01 | |
| Q7 | 0.66 | −0.07 | 0.81 | −0.01 | |
| Q8 | 0.68 | −0.04 | 0.83 | 0.00 | |
| Q9 | 0.67 | −0.06 | 0.82 | 0.00 | |
| Q10 | 0.77 | 0.03 | 0.81 | −0.01 | |
| Q11 | 0.72 | −0.01 | 0.82 | −0.00 | |
| Item Number and Description | Factor Loadings | ||||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Total Variance Explained | ||
| 11 | Provides detailed description of painful event | 0.78 | |||
| 7 | Exhibits intensification of the symptoms | 0.68 | |||
| 10 | Physiological markers of pain | 0.62 | |||
| 3 | Exhibits analgesic resistance | 0.52 | |||
| 8 | There is a gap between the formal and informal evaluation | 0.68 | |||
| 6 | Exhibits “la belle indifference” | 0.67 | |||
| 9 | Mismatch between etiology and clinical presentation | 0.64 | |||
| 1 | Exhibits pronounced behavioral lability | 0.63 | |||
| 5 | Exhibits lack of trust in the therapist | 0.60 | |||
| 2 | Not willing to partake in the activity | 0.55 | |||
| 4 | Willing to make variations in the activity | 0.44 | |||
| Variance explained | 27.26% | 21.45% | 13.39% | 62.11% | |
| Item Description | Response | SSD (N = 80): n (%) | Non-SSD (N = 31): n (%) | Within-Group Differences—SSD: Chi-Square (p-Value) | Within-Group Differences—Non-SSD: Chi-Square (p-Value) | Between-Group Differences: Chi-Square (p-Value) |
|---|---|---|---|---|---|---|
| Exhibits pronounced behavioral lability | Yes | 31 (38.75) b | 2 (6.45) b,c | 31.52 (<0.01) | 13.64 (<0.01) | 10.99 (<0.01) |
| Sometimes | 8 (10.00) a,c | 14 (45.16) a | 17.09 (<0.01) | |||
| No | 41 (51.25) b | 15 (48.38) a | 0.07 (0.77) | |||
| Not willing to partake in the activity | Yes | 49 (61.25) b,c | 2 (6.45) c | 35.70 (<0.01) | 34.07 (<0.01) | 26.99 (<0.01) |
| Sometimes | 19 (23.75) a | 4 (12.90) c | 1.67 (0.19) | |||
| No | 12 (15.00) a | 25 (80.64) a,b | 42.21 (<0.01) | |||
| Exhibits analgesic resistance | Yes | 34 (42.50) | 6 (19.35) b,c | 36.10 (<0.01) | 40.66 (<0.01) | 5.11 (0.02) |
| No | 44 (55.00) | 25 (80.64) a,c | 5.83 (0.01) | |||
| N/A | 2 (2.50) | 0 (0.00) a,b | 0.78 (0.37) | |||
| Willing to make variations in the activity | Yes | 40 (50.00) b,c | 23 (74.19) b,c | 14.60 (0.01) | 32.46 (<0.01) | 5.19 (0.02) |
| Sometimes | 23 (28.75) a | 1 (3.22) a,c | 8.33 (<0.01) | |||
| No | 17 (21.25) a | 7 (22.58) a,b | 0.01 (0.90) | |||
| Exhibits lack of trust in the therapist | Yes | 52 (65.00) b,c | 0 (0.00) c | 39.60 (<0.01) | 61.00 (<0.01) | 37.56 (<0.01) |
| Sometimes | 15 (18.75) a | 0 (0.00) c | 6.35 (0.01) | |||
| No | 13 (16.25) a | 31 (100.00) a,b | 65.39 (<0.01) | |||
| Exhibits “la belle indifference” | Yes | 52 (65.00) b,c | 1 (3.22) b,c | 76.55 (<0.01) | 32.46 (<0.01) | 34.11 (<0.01) |
| Sometimes | 0 (0.00) a,c | 7 (22.58) a,c | 18.58 (<0.01) | |||
| No | 28 (35.00) a,b | 23 (74.19) a,b | 13.56 (<0.01) | |||
| Exhibits intensification of the symptoms | Yes | 50 (62.50) b,c | 5 (16.12) c | 46.65 (<0.01) | 37.24 (<0.01) | 18.74 (<0.01) |
| Sometimes | 8 (10.00) a,c | 1 (3.22) c | 1.46 (0.22) | |||
| No | 22 (27.50) a,b | 25 (80.64) a,b | 25.56 (<0.01) | |||
| There is a gap between the formal and informal evaluation | Yes | 40 (50.00) b | 0 (0.00) c | 53.00 (<0.01) | 56.30 (<0.01) | 24.01 (<0.01) |
| Sometimes | 0 (0.00) a,c | 1 (3.22) c | 2.39 (0.12) | |||
| No | 40 (50.00) b | 30 (96.77) a,b | 20.06 (<0.01) | |||
| Mismatch between etiology and clinical presentation | Yes | 58 (72.50) b,c | 1 (3.22) c | 89.43 (<0.01) | 46.39 (<0.01) | 42.29 (<0.01) |
| Sometimes | 0 (0.00) a,c | 2 (6.45) c | 4.38 (0.02) | |||
| No | 22 (27.50) a,b | 28 (90.32) a,b | 35.56 (<0.01) | |||
| Physiological markers of pain | Yes | 42 (52.50) b,c | 9 (29.03) | 16.48 (<0.01) | 0.55 (0.45) | 4.72 (0.02) |
| Sometimes | 17 (21.25) a | 12 (38.70) | 3.34 (0.06) | |||
| No | 21 (26.25) a | 10 (32.25) | 0.39 (0.52) | |||
| Provides detailed description of painful event | Yes | 51 (63.75) b,c | 11 (35.48) b,c | 73.11 (<0.01) | 23.57 (<0.01) | 7.01 (<0.01) |
| Sometimes | 0 (0.00) a,c | 1 (3.22) a,c | 2.39 (0.12) | |||
| No | 29 (36.25) a,b | 19 (61.29) a,b | 5.64 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barak, S.; Landa, J.; Gerner, M.; Eisenstein, E.; Arzoni Bardach, C.; Silberg, T. A Behavioral Characteristics Observational Measure of Youth with Somatic Symptom Disorder during Physical Rehabilitation. Life 2023, 13, 2078. https://doi.org/10.3390/life13102078
Barak S, Landa J, Gerner M, Eisenstein E, Arzoni Bardach C, Silberg T. A Behavioral Characteristics Observational Measure of Youth with Somatic Symptom Disorder during Physical Rehabilitation. Life. 2023; 13(10):2078. https://doi.org/10.3390/life13102078
Chicago/Turabian StyleBarak, Sharon, Jana Landa, Maya Gerner, Etzyona Eisenstein, Chen Arzoni Bardach, and Tamar Silberg. 2023. "A Behavioral Characteristics Observational Measure of Youth with Somatic Symptom Disorder during Physical Rehabilitation" Life 13, no. 10: 2078. https://doi.org/10.3390/life13102078
APA StyleBarak, S., Landa, J., Gerner, M., Eisenstein, E., Arzoni Bardach, C., & Silberg, T. (2023). A Behavioral Characteristics Observational Measure of Youth with Somatic Symptom Disorder during Physical Rehabilitation. Life, 13(10), 2078. https://doi.org/10.3390/life13102078









