Frontiers of Collaboration between Primary Care and Specialists in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review
Abstract
:1. Introduction
2. Concepts of NAFLD/NASH, MAFLD, and MASLD
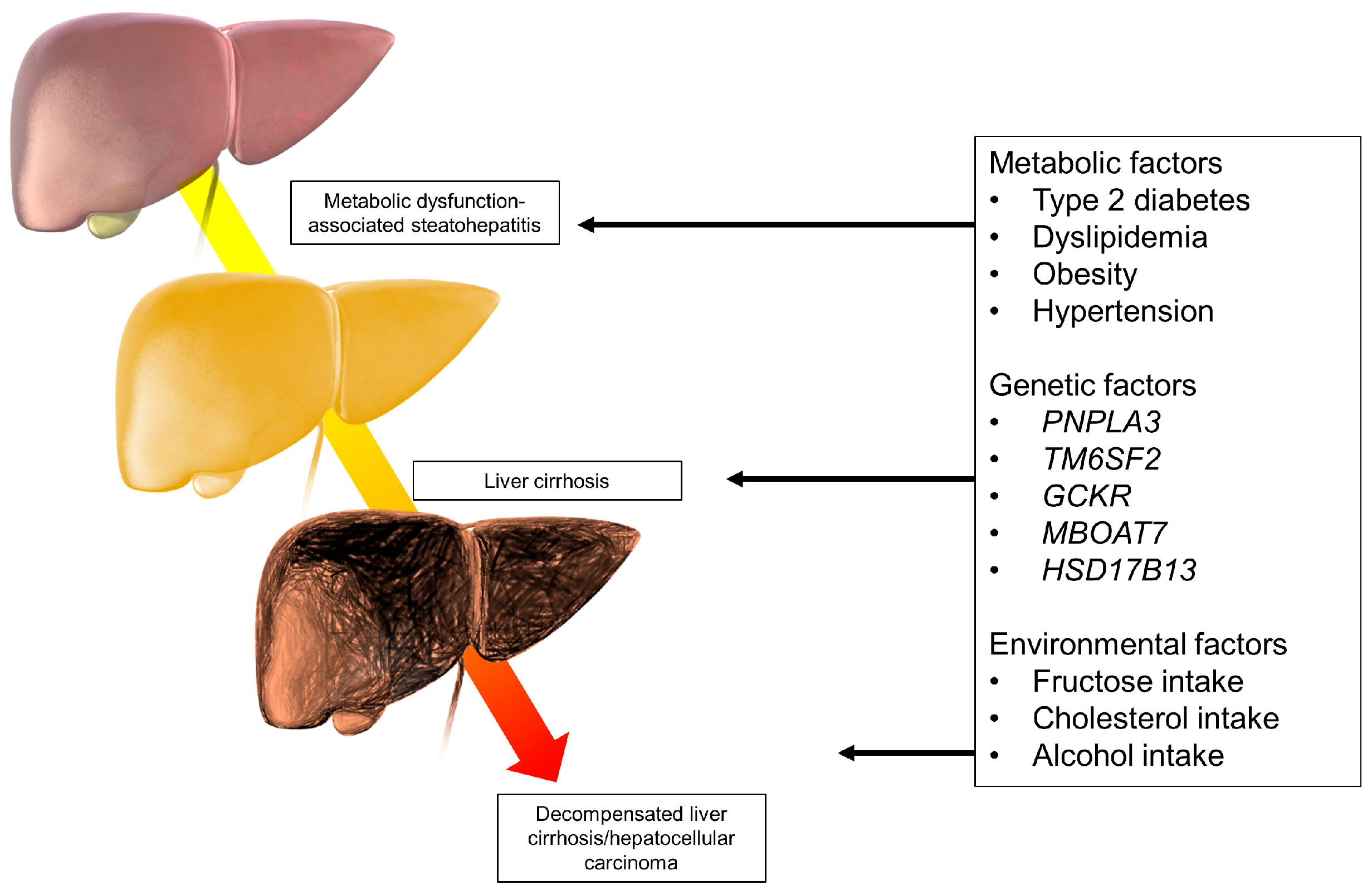
3. Pathogenesis of MASLD/MASH
4. Epidemiology
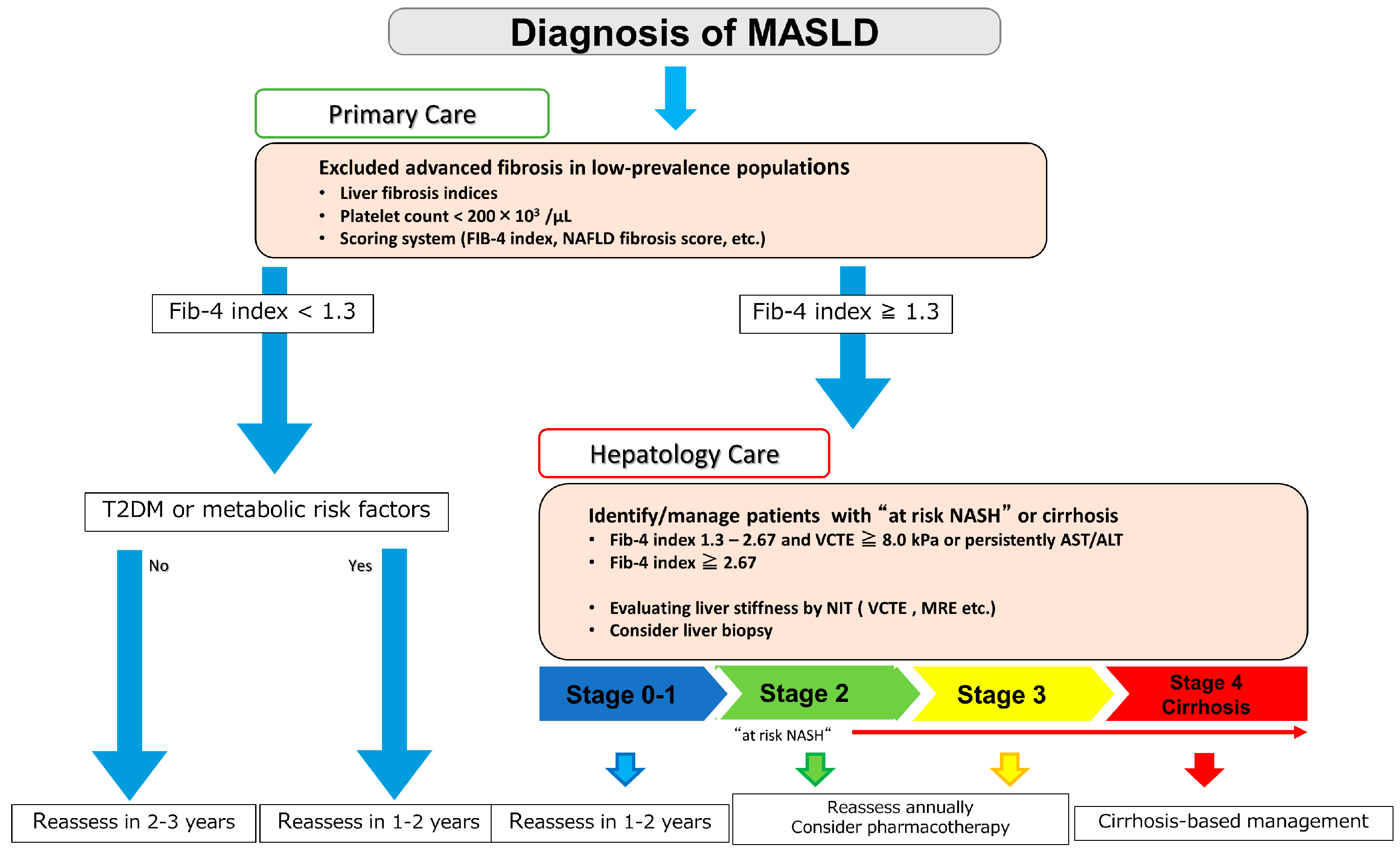
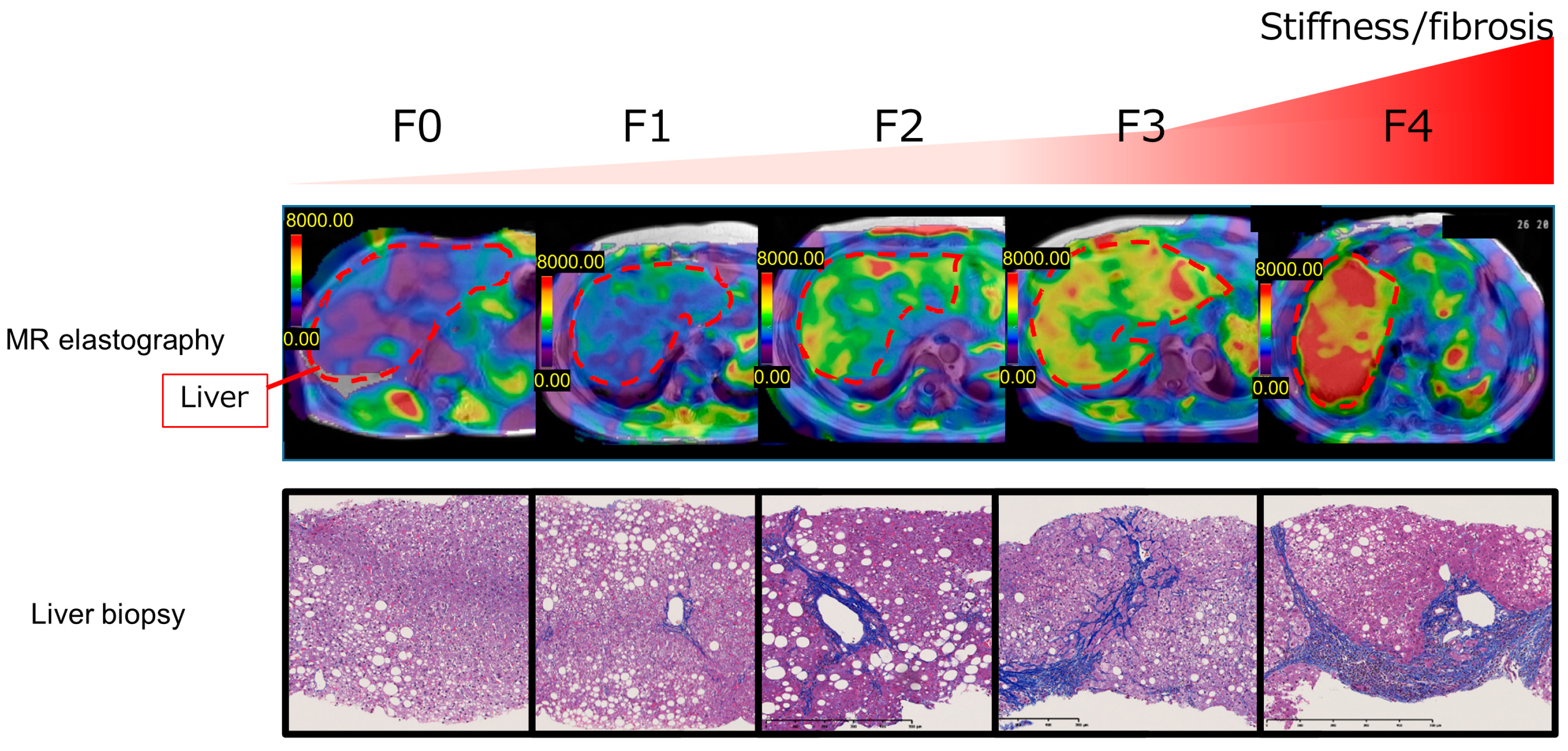
5. Noninvasive Tests (NITs) for Hepatic Fibrosis in Patients with MASLD
6. Secondary Check and Detailed Examination by a Gastroenterologist/Hepatologist
7. Collaboration between Family Doctors and Specialists for Liver Disease in Japan
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multi-society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multi-society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Pennisi, G.; Enea, M.; Falco, V.; Aithal, G.P.; Palaniyappan, N.; Yilmaz, Y.; Boursier, J.; Cassinotto, C.; de Lédinghen, V.; Chan, W.K.; et al. Noninvasive Assessment of Liver Disease Severity in Patients with Nonalcoholic Fatty Liver Disease (NAFLD) and Type 2 Diabetes. Hepatology 2023, 78, 195–211. [Google Scholar] [CrossRef]
- Pisano, M.B.; Giadans, C.G.; Flichman, D.M.; Ré, V.E.; Preciado, M.V.; Valva, P. Viral Hepatitis Update: Progress and Perspectives. World J. Gastroenterol. 2021, 27, 4018–4044. [Google Scholar] [CrossRef]
- Tateishi, R.; Matsumura, T.; Okanoue, T.; Shima, T.; Uchino, K.; Fujiwara, N.; Senokuchi, T.; Kon, K.; Sasako, T.; Taniai, M.; et al. Hepatocellular Carcinoma Development in Diabetic Patients: A Nationwide Survey in Japan. J. Gastroenterol. 2021, 56, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: Results from a Nationwide Cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased Risk of Mortality by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Imajo, K.; Nakajima, A. Non-invasive Diagnosis of Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2018, 113, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Hilliard, M.E.; Isaacs, D.; et al. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S49–S67. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-Based Clinical Practice Guidelines for Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis 2020. J. Gastroenterol. 2021, 56, 951–963. [Google Scholar] [CrossRef]
- Hilden, M.; Juhl, E.; Thomsen, A.C.; Christoffersen, P. Fatty Liver Persisting for up to 33 Years. A Follow-Up of the inversen-roholm Liver Biopsy Material. Acta Med. Scand. 1973, 194, 485–489. [Google Scholar] [CrossRef]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic Steatohepatitis: Mayo Clinic Experiences with a Hitherto Unnamed Disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar]
- Drew, L. Fatty Liver Disease: Turning the Tide. Nature 2017, 550, S101. [Google Scholar] [CrossRef]
- James, O.F.; Day, C.P. Non-alcoholic Steatohepatitis (NASH): A Disease of Emerging Identity and Importance. J. Hepatol. 1998, 29, 495–501. [Google Scholar] [CrossRef]
- Schaffner, F. Nonalcoholic Fatty Liver. In Bockus Gastroenterology, 4th ed.; Berk, J.E., Haubrich, W.S., Kalser, M.H., Eds.; Saunders: London, UK, 1985; pp. 3049–3061. [Google Scholar]
- Bedossa, P.; FLIP Pathology. Consortium Utility and Appropriateness of the Fatty Liver Inhibition of Progression (FLIP) Algorithm and Steatosis, Activity, and Fibrosis (SAF) Score in the Evaluation of Biopsies of Nonalcoholic Fatty Liver Disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef]
- Eslam, M.; George, J. Reply to: Correspondence Regarding “A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement”: Bringing Evidence to the NAFLD-MAFLD Debate. J. Hepatol. 2020, 73, 1575. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited after a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic Steatohepatitis: Association of Insulin Resistance and Mitochondrial Abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Pinto, H.; Camilo, M.E.; Baptista, A.; De Oliveira, A.G.; De Moura, M.C. Non-Alcoholic Fatty Liver: Another Feature of the Metabolic Syndrome? Clin. Nutr. 1999, 18, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin Resistance in Nonalcoholic Fatty Liver Disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Postic, C.; Girard, J. Contribution of de Novo Fatty Acid Synthesis to Hepatic Steatosis and Insulin Resistance: Lessons from Genetically Engineered Mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Barb, D.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Suman, A.; Weber, M.H.; Budd, J.T.; Lupi, M.E.; Cusi, K. Metabolic and Histological Implications of Intrahepatic Triglyceride Content in Nonalcoholic Fatty Liver Disease. Hepatology 2017, 65, 1132–1144. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A Global View of the Interplay Between Non-alcoholic Fatty Liver Disease and Diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Valenzuela-Vallejo, L.; Chrysafi, P.; Kouvari, M.; Guatibonza-Garcia, V.; Mylonakis, S.C.; Katsarou, A.; Verrastro, O.; Markakis, G.; Eslam, M.; Papatheodoridis, G.; et al. Circulating Hormones in Biopsy-Proven Steatotic Liver Disease and Steatohepatitis: A Multicenter Observational Study. Metabolism 2023, 148, 155694. [Google Scholar] [CrossRef]
- Imajo, K.; Fujita, K.; Yoneda, M.; Nozaki, Y.; Ogawa, Y.; Shinohara, Y.; Kato, S.; Mawatari, H.; Shibata, W.; Kitani, H.; et al. Hyperresponsivity to Low-Dose Endotoxin During Progression to Nonalcoholic Steatohepatitis Is Regulated by Leptin-Mediated Signaling. Cell Metab. 2012, 16, 44–54. [Google Scholar] [CrossRef]
- Wang, X.; Wu, N.; Sun, C.; Jin, D.; Lu, H. Effects of SGLT-2 Inhibitors on Adipose Tissue Distribution in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetol. Metab. Syndr. 2023, 15, 113. [Google Scholar] [CrossRef]
- Sandforth, A.; von Schwartzenberg, R.J.; Arreola, E.V.; Hanson, R.L.; Sancar, G.; Katzenstein, S.; Lange, K.; Preißl, H.; Dreher, S.I.; Weigert, C.; et al. Mechanisms of Weight Loss-Induced Remission in People with Prediabetes: A Post-hoc Analysis of the Randomised, Controlled, Multicentre Prediabetes Lifestyle Intervention Study (PLIS). Lancet Diabetes Endocrinol. 2023, 11, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F.; Suzuki, A.; Guy, C.; Unalp-Arida, A.; Colvin, R.; Johnson, R.J.; Diehl, A.M.; Nonalcoholic Steatohepatitis Clinical Research Network. Increased Fructose Consumption Is Associated with Fibrosis Severity in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2010, 51, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fox, C.S.; Jacques, P.F.; Speliotes, E.K.; Hoffmann, U.; Smith, C.E.; Saltzman, E.; McKeown, N.M. Sugar-Sweetened Beverage, Diet Soda, and Fatty Liver Disease in the Framingham Heart Study Cohorts. J. Hepatol. 2015, 63, 462–469. [Google Scholar] [CrossRef]
- Molloy, J.W.; Calcagno, C.J.; Williams, C.D.; Jones, F.J.; Torres, D.M.; Harrison, S.A. Association of Coffee and Caffeine Consumption with Fatty Liver Disease, Nonalcoholic Steatohepatitis, and Degree of Hepatic Fibrosis. Hepatology 2012, 55, 429–436. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwaki, M.; Nakajima, A.; Nogami, A.; Yoneda, M. Current Research on the Pathogenesis of NAFLD/NASH and the Gut-Liver Axis: Gut Microbiota, Dysbiosis, and Leaky-Gut Syndrome. Int. J. Mol. Sci. 2022, 23, 11689. [Google Scholar] [CrossRef] [PubMed]
- Shreya, S.; Grosset, C.F.; Jain, B.P. Unfolded Protein Response Signaling in Liver Disorders: A 2023 Updated Review. Int. J. Mol. Sci. 2023, 24, 14066. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, Q.; Li, L.; Cheng, Y.; Chen, Y.; Lv, M.; Xiang, X. Role of Endoplasmic Reticulum Stress in Hepatic Glucose and Lipid Metabolism and Therapeutic Strategies for Metabolic Liver Disease. Int. Immunopharmacol. 2022, 113, 109458. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic Variation in PNPLA3 Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Trépo, E.; Valenti, L. Update on NAFLD Genetics: From New Variants to the Clinic. J. Hepatol. 2020, 72, 1196–1209. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-Wide Association Study Identifies a TM6SF2 Variant That Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishigami, M.; Zou, B.; Tanaka, T.; Takahashi, H.; Kurosaki, M.; Maeda, M.; Thin, K.N.; Tanaka, K.; Takahashi, Y.; et al. The Epidemiology of NAFLD and Lean NAFLD in Japan: A Meta-analysis with Individual and Forecasting Analysis, 1995–2040. Hepatol. Int. 2021, 15, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD Disease Burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the Period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Harrison, S.A.; Gawrieh, S.; Roberts, K.; Lisanti, C.J.; Schwope, R.B.; Cebe, K.M.; Paradis, V.; Bedossa, P.; Aldridge Whitehead, J.M.; Labourdette, A.; et al. Prospective Evaluation of the Prevalence of Non-alcoholic Fatty Liver Disease and Steatohepatitis in a Large Middle-Aged US Cohort. J. Hepatol. 2021, 75, 284–291. [Google Scholar] [CrossRef]
- Stepanova, M.; Kabbara, K.; Mohess, D.; Verma, M.; Roche-Green, A.; AlQahtani, S.; Ong, J.; Burra, P.; Younossi, Z.M. Nonalcoholic Steatohepatitis Is the Most Common Indication for Liver Transplantation Among the Elderly: Data from the United States Scientific Registry of Transplant Recipients. Hepatol. Commun. 2022, 6, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Tsochatzis, E.A. Case-Finding Strategies in Non-alcoholic Fatty Liver Disease. JHEP Rep. 2021, 3, 100219. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Ohtakaki, Y.; Kikuchi, H.; Uchino, H.; Isomura, M.; Aoyagi, K.; Oya, H.; Katayama, T.; Mitsumoto, Y.; Mizuno, M.; et al. A Novel Rapid Immunoassay of Serum Type IV Collagen 7S for the Diagnosis of Fibrosis Stage of Nonalcoholic Fatty Liver Diseases. Hepatol. Res. 2021, 51, 263–276. [Google Scholar] [CrossRef]
- Guha, I.N.; Parkes, J.; Roderick, P.; Chattopadhyay, D.; Cross, R.; Harris, S.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; et al. Noninvasive Markers of Fibrosis in Nonalcoholic Fatty Liver Disease: Validating the European Liver Fibrosis Panel and Exploring Simple Markers. Hepatology 2008, 47, 455–460. [Google Scholar] [CrossRef]
- Birch-Machin, I.; Gao, S.; Huen, D.; McGirr, R.; White, R.A.; Russell, S. Genomic Analysis of Heat-Shock Factor Targets in Drosophila. Genome Biol. 2005, 6, R63. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Z.; Zhao, Y.; Tao, C. Wisteria floribunda Agglutinin-Positive Mac-2-binding Protein as a Diagnostic Biomarker in Liver Cirrhosis: An Updated Meta-analysis. Sci. Rep. 2020, 10, 10582. [Google Scholar] [CrossRef]
- Tanwar, S.; Trembling, P.M.; Guha, I.N.; Parkes, J.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; Day, C.P.; Rosenberg, W.M. Validation of terminal peptide of procollagen III for the detection and assessment of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease. Hepatology 2013, 57, 103–111. [Google Scholar] [CrossRef]
- Davyduke, T.; Tandon, P.; Al-Karaghouli, M.; Abraldes, J.G.; Ma, M.M. Impact of Implementing a “FIB-4 First” Strategy on a Pathway for Patients with NAFLD Referred from Primary Care. Hepatol. Commun. 2019, 3, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Validation of the FIB4 Index in a Japanese Nonalcoholic Fatty Liver Disease Population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD Fibrosis Score: A Noninvasive System That Identifies Liver Fibrosis in Patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Chow, K.W.; Futela, P.; Saharan, A.; Saab, S. Comparison of Guidelines for the Screening, Diagnosis, and Noninvasive Assessment of Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2023, 13, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Fujii, H.; Sumida, Y.; Hyogo, H.; Itoh, Y.; Ono, M.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Platelet Count for Predicting Fibrosis in Nonalcoholic Fatty Liver Disease. J. Gastroenterol. 2011, 46, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic Accuracy of Non-invasive Tests for Advanced Fibrosis in Patients with NAFLD: An Individual Patient Data Meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef]
- Chan, W.K.; Treeprasertsuk, S.; Goh, G.B.; Fan, J.G.; Song, M.J.; Charatcharoenwitthaya, P.; Duseja, A.; Dan, Y.Y.; Imajo, K.; Nakajima, A.; et al. Optimizing Use of Nonalcoholic Fatty Liver Disease Fibrosis Score, fibrosis-4 Score, and Liver Stiffness Measurement to Identify Patients with Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2019, 17, 2570–2580. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Vali, Y.; Alzoubi, O.; Staufer, K.; Trauner, M.; Paternostro, R.; Stauber, R.E.; Holleboom, A.G.; van Dijk, A.M.; et al. Performance of Non-invasive Tests and Histology for the Prediction of Clinical Outcomes in Patients with Nonalcoholic Fatty Liver Disease: An Individual Participant Data Meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 704–713. [Google Scholar] [CrossRef]
- Singh, S.; Venkatesh, S.K.; Loomba, R.; Wang, Z.; Sirlin, C.; Chen, J.; Yin, M.; Miller, F.H.; Low, R.N.; Hassanein, T.; et al. Magnetic Resonance Elastography for Staging Liver Fibrosis in Non-alcoholic Fatty Liver Disease: A Diagnostic Accuracy Systematic Review and Individual Participant Data Pooled Analysis. Eur. Radiol. 2016, 26, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Loomba, R.R.; Imajo, K.; Madamba, E.; Gandhi, S.; Bettencourt, R.; Singh, S.; Hernandez, C.; Valasek, M.A.; Behling, C.; et al. MRE Combined with FIB-4 (MEFIB) Index in Detection of Candidates for Pharmacological Treatment of NASH-Related Fibrosis. Gut 2021, 70, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel; Chair: EASL Governing Board Representative. EASL Clinical Practice Guidelines on Non-invasive Tests for Evaluation of Liver Disease Severity and Prognosis—2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Seko, Y.; Imai, N.; Hosaka, T.; Kobayashi, M.; Saitoh, S.; Sezaki, H.; Akuta, N.; et al. Large-Scale Long-Term Follow-Up Study of Japanese Patients with Non-alcoholic Fatty Liver Disease for the Onset of Hepatocellular Carcinoma. Am. J. Gastroenterol. 2012, 107, 253–261. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Adams, L.A.; Lindor, K.D.; Angulo, P. The Prevalence of Autoantibodies and Autoimmune Hepatitis in Patients with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2004, 99, 1316–1320. [Google Scholar] [CrossRef]
- Yatsuji, S.; Hashimoto, E.; Kaneda, H.; Taniai, M.; Tokushige, K.; Shiratori, K. Diagnosing Autoimmune Hepatitis in Nonalcoholic Fatty Liver Disease: Is the International Autoimmune Hepatitis Group Scoring System Useful? J. Gastroenterol. 2005, 40, 1130–1138. [Google Scholar] [CrossRef]
- The 59th Annual Meeting of the Japan Society of Hepatology. Available online: https://site2.convention.co.jp/jsh59/nara_sengen/iryou.html (accessed on 28 August 2023).
- Hardy, T.; Wonders, K.; Younes, R.; Aithal, G.P.; Aller, R.; Allison, M.; Bedossa, P.; Betsou, F.; Boursier, J.; Brosnan, M.J.; et al. The European NAFLD Registry: A Real-World Longitudinal Cohort Study of Nonalcoholic Fatty Liver Disease. Contemp. Clin. Trials 2020, 98, 106175. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ong, J.P.; Takahashi, H.; Yilmaz, Y.; Eguc Hi, Y.; El Kassas, M.; Buti, M.; Diago, M.; Zheng, M.H.; Fan, J.G.; et al. A Global Survey of Physicians Knowledge About Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2022, 20, e1456–e1468. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Hallsworth, K.; Lynch, N.; Hauvespre, A.; Mansour, E.; Kozma, S.; Marino, J.P.; Bottomley, J.; Piercy, J.; Higgins, V. Real-World Management of Non-alcoholic Steatohepatitis Differs from Clinical Practice Guideline Recommendations and Across Regions. JHEP Rep. 2022, 4, 100411. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, K.; Nagai, K.; Iwaki, M.; Kobayashi, T.; Nogami, A.; Oka, M.; Saito, S.; Yoneda, M. Frontiers of Collaboration between Primary Care and Specialists in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Life 2023, 13, 2144. https://doi.org/10.3390/life13112144
Nagai K, Nagai K, Iwaki M, Kobayashi T, Nogami A, Oka M, Saito S, Yoneda M. Frontiers of Collaboration between Primary Care and Specialists in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Life. 2023; 13(11):2144. https://doi.org/10.3390/life13112144
Chicago/Turabian StyleNagai, Koki, Kazuki Nagai, Michihiro Iwaki, Takashi Kobayashi, Asako Nogami, Masanao Oka, Satoru Saito, and Masato Yoneda. 2023. "Frontiers of Collaboration between Primary Care and Specialists in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review" Life 13, no. 11: 2144. https://doi.org/10.3390/life13112144
APA StyleNagai, K., Nagai, K., Iwaki, M., Kobayashi, T., Nogami, A., Oka, M., Saito, S., & Yoneda, M. (2023). Frontiers of Collaboration between Primary Care and Specialists in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Life, 13(11), 2144. https://doi.org/10.3390/life13112144







