Baseline Corticosterone, Stress Responses, and Leukocyte Profiles in Chicks of Precocial Birds in Rural and Urban Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biology of the Southern Lapwing and the Study Sites
2.2. Capture Procedures and Blood Sampling
2.3. Heterophil/Lymphocyte Ratio
2.4. Hormone Assay
2.5. Data Analysis
3. Results
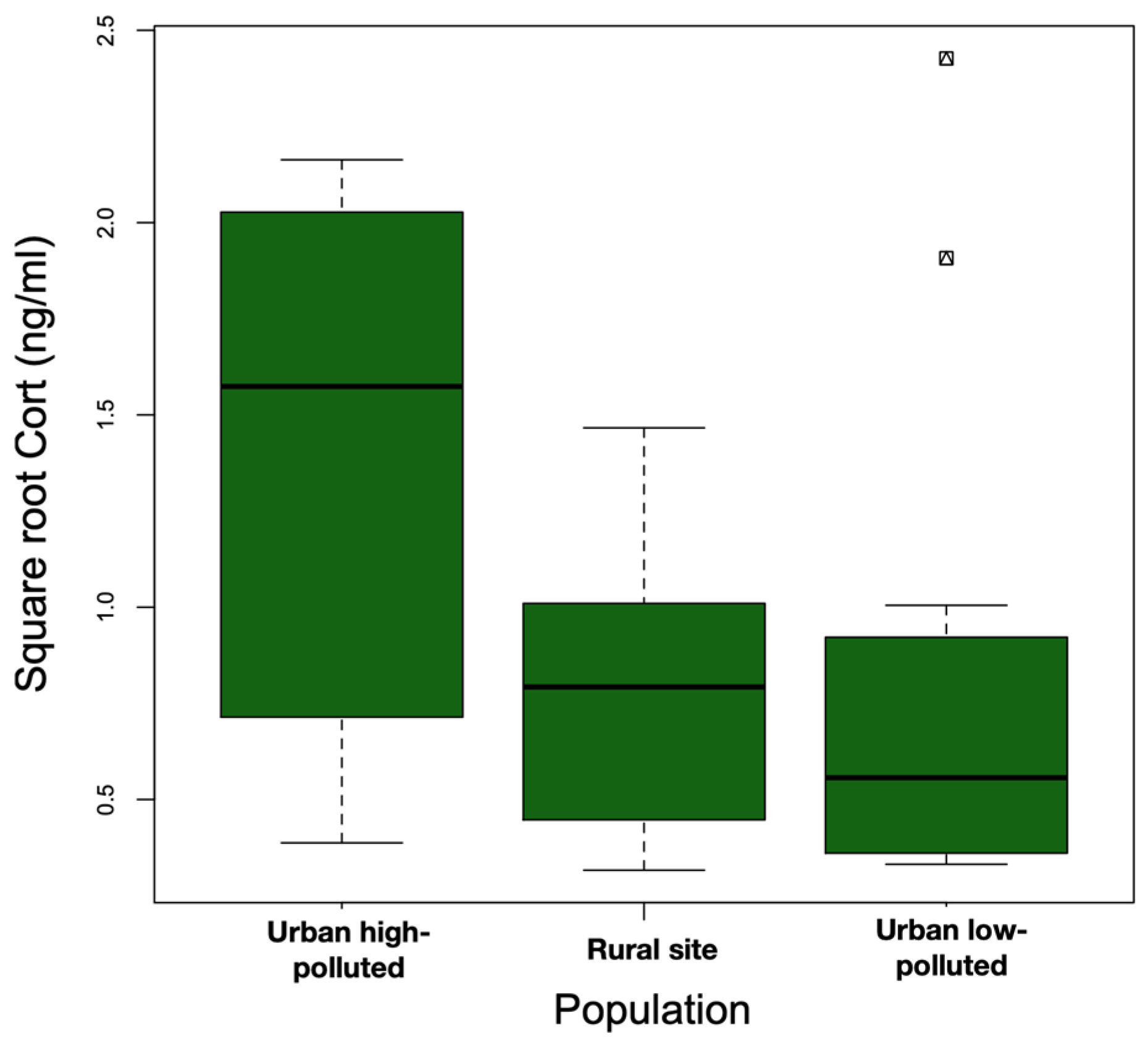
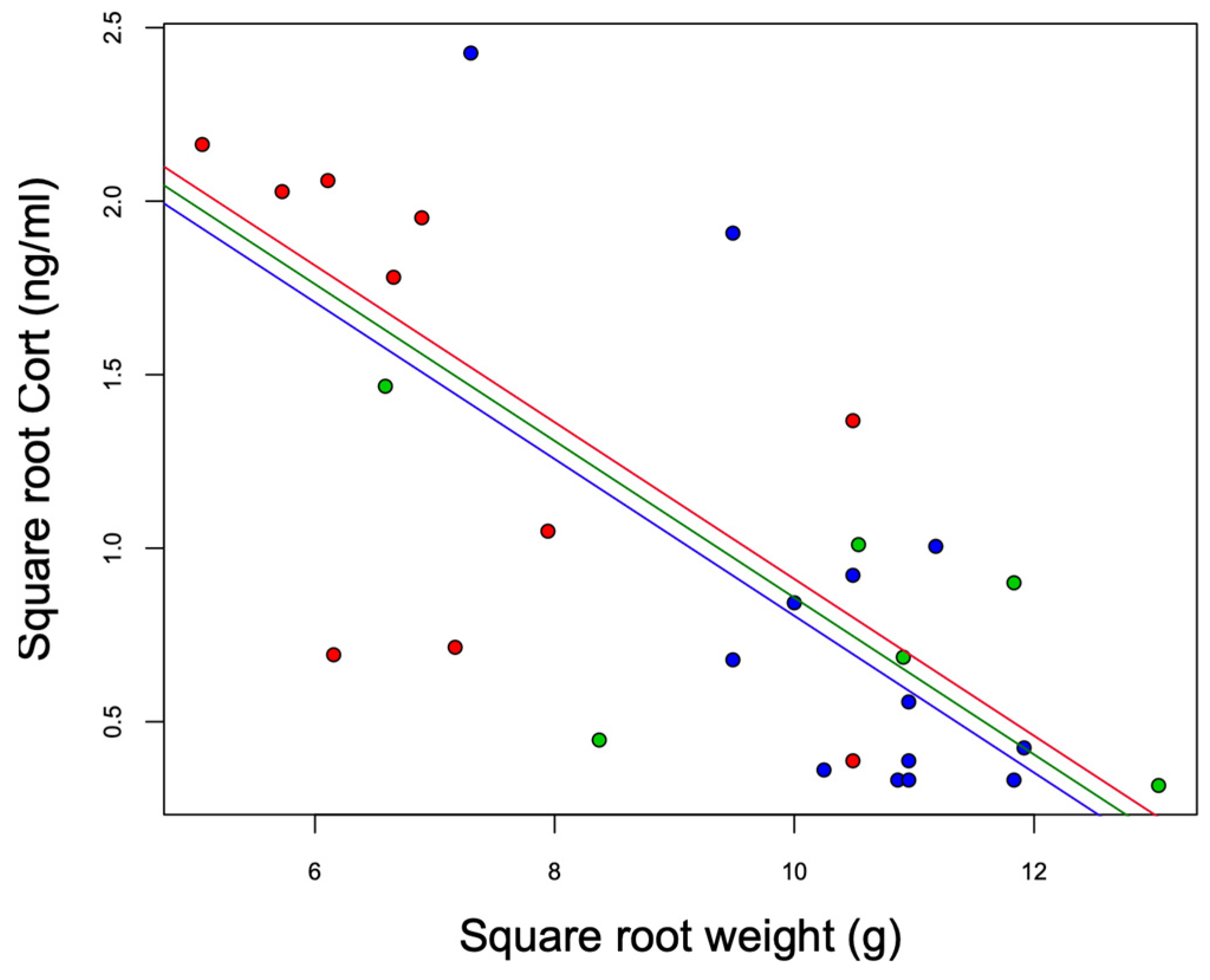
3.1. Body Mass, H/L Ratio, and Baseline Cort
3.2. Stress Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress-responses? Integrating permissive, suppressive, stimulatory, and adaptive actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- Bonier, F. Hormones in the city: Endocrine ecology of urban birds. Horm. Behav. 2012, 61, 763–772. [Google Scholar] [CrossRef]
- Madliger, C.L.; Love, O.P. Do baseline glucocorticoids simultaneously represent fitness and environmental quality in a declining aerial insectivore? Oikos 2016, 125, 1824–1837. [Google Scholar] [CrossRef]
- Injaian, A.S.; Francis, C.D.; Ouyang, J.Q.; Dominoni, D.M.; Donald, J.W.; Fuxjager, M.J.; Vitousek, M.N. Baseline and stress-induced corticosterone levels across birds and reptiles do not reflect urbanization levels. Conserv. Physiol. 2020, 8, coz110. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Carrasco, M.; Aich, U.; Jennions, M.D.; Head, M.L. Stress in the city: Meta-analysis indicates no overall evidence for stress in urban vertebrates: Stressful cities: A meta-analysis. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201754. [Google Scholar]
- Monaghan, P.; Haussmann, M.F. The positive and negative consequences of stressors during early life. Early Hum. Dev. 2015, 91, 643–647. [Google Scholar] [CrossRef]
- Crino, O.L.; Johnson, E.E.; Blickley, J.L.; Patricelli, G.L.; Breuner, C.W. Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J. Exp. Biol. 2013, 216, 2055–2062. [Google Scholar] [CrossRef]
- Angelier, F.; Meillère, A.; Grace, J.K.; Trouvé, C.; Brischoux, F. No evidence for an effect of traffic noise on the development of the corticosterone stress response in an urban exploiter. Gen. Comp. Endocrinol. 2016, 232, 43–50. [Google Scholar] [CrossRef]
- Kleist, N.J.; Guralnick, R.P.; Cruz, A.; Lowry, C.A.; Francis, C.D. Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community. Proc. Natl. Acad. Sci. USA 2018, 115, E648–E657. [Google Scholar] [CrossRef]
- Løseth, M.E.; Briels, N.; Eulaers, I.; Nygård, T.; Malarvannan, G.; Poma, G.; Jaspers, V.L. Plasma concentrations of organohalogenated contaminants in white-tailed eagle nestlings–The role of age and diet. Environ. Pollut. 2019, 246, 527–534. [Google Scholar] [CrossRef]
- Ortiz-Santaliestra, M.E.; Tauler-Ametller, H.; Lacorte, S.; Hernández-Matías, A.; Real, J.; Mateo, R. Accumulation of pollutants in nestlings of an endangered avian scavenger related to territory urbanization and physiological biomarkers. Environ. Pollut. 2019, 252, 1801–1809. [Google Scholar] [CrossRef]
- Isaksson, C. Urbanization, oxidative stress and inflammation: A question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 2015, 29, 913–923. [Google Scholar] [CrossRef]
- Beaugeard, E.; Brischoux, F.; Henry, P.Y.; Parenteau, C.; Trouvé, C.; Angelier, F. Does urbanization cause stress in wild birds during development? Insights from feather corticosterone levels in juvenile house sparrows (Passer domesticus). Ecol. Evol. 2019, 9, 640–652. [Google Scholar] [CrossRef]
- Buxton, V.L.; Santymire, R.M.; Benson, T.J. Mixed effects of urbanization on density, nest survival, and nestling corticosterone of a generalist passerine. Ecosphere 2018, 9, e02517. [Google Scholar] [CrossRef]
- Redondo, I.; Muriel, J.; de Castro Díaz, C.; Aguirre, J.I.; Gil, D.; Pérez-Rodríguez, L. Influence of growing up in the city or near an airport on the physiological stress of tree sparrow nestlings (Passer montanus). Eur. J. Wildl. Res. 2021, 67, 68. [Google Scholar] [CrossRef]
- Cavalli, M.; Baladrón, A.V.; Isacch, J.P.; D’Amico, V.; Bó, M.S. Leukocyte profiles and body condition of free-living Burrowing Owls (Athene cunicularia) from rural and urban areas in the Argentinean Pampas. Rev. Bras. Ornitol. 2018, 26, 45–51. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Love, O.P.; Bird, D.M.; Shutt, L.J. Plasma corticosterone in American kestrel siblings: Effects of age, hatching order, and hatching asynchrony. Horm. Behav. 2003, 43, 480–488. [Google Scholar] [CrossRef]
- Lobato, E.; Merino, S.; Moreno, J.; Morales, J.; Tomás, G.; Martínez-de la Puente, J.; Möstl, E. Corticosterone metabolites in blue tit and pied flycatcher droppings: Effects of brood size, ectoparasites and temperature. Horm. Behav. 2008, 53, 295–305. [Google Scholar] [CrossRef]
- Rensel, M.A.; Wilcoxen, T.E.; Schoech, S.J. The influence of nest attendance and provisioning on nestling stress physiology in the Florida scrub-jay. Horm. Behav. 2010, 57, 162–168. [Google Scholar] [CrossRef]
- Quirici, V.; Guerrero, C.J.; Krause, J.S.; Wingfield, J.C.; Vásquez, R.A. The relationship of telomere length to baseline corticosterone levels in nestlings of an altricial passerine bird in natural populations. Front. Zool. 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H. Vegetación Urbana en Santiago de Chile. 2008. Available online: https://repositorio.uchile.cl/handle/2250/120265 (accessed on 26 July 2023).
- Yáñez Sánchez, J. Cambios en la Estructura Funcional del Espacio Intraurbano del Gran Santiago. Período 1990–2010. 2014. Available online: https://repositorio.uchile.cl/handle/2250/130048 (accessed on 26 July 2023).
- Ubilla-Bravo, G.; Robles-Vargas, R.; González, D.; Garay, N.; Norambuena-Vega, P.; Sandoval-Verdugo, G.; Muñoz-Muñoz, F. Carta de Cobertura y Uso del Suelo en la Región Metropolitana de Santiago. Ph.D. Dissertation, Gobierno Regional Metropolitano de Santiago, Santiago, Chile, 2012. [Google Scholar]
- Hernández, H.J.; Galleguillos, M.; Estades, C. Mapa de Cobertura de Suelos de Chile 2014: Descripción del Producto. Laboratorio GEP. Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Universidad de Chile. 2016. Available online: https://www.gep.uchile.cl/Landcover/Landcover%20de%20Chile%20-%20Descripción%20del%20Producto%20-%20GEP%20UCHILE%202016.pdf (accessed on 28 July 2023).
- Romero, H.; Irarrázaval, F.; Opazo, D.; Salgado, M.; Smith, P. Climas urbanos y contaminación atmosférica en Santiago de Chile. EURE 2010, 36, 35–62. [Google Scholar]
- Ministerio de Medio Ambiente. Available online: https://mma.gob.cl/conoce-cuales-son-los-lugares-mas-ruidosos-de-santiago/ (accessed on 28 July 2023).
- Lessells, C.M. The mating system of Kentish plovers Charadrius alexandrines. Ibis 1984, 126, 474–483. [Google Scholar] [CrossRef]
- Blomqvist, D.; Kempenaers, B.; Lanctot, R.B.; Sandercock, B.K. Genetic parentage and mate guarding in the arctic-breeding western sandpiper. Auk 2002, 119, 228–233. [Google Scholar] [CrossRef]
- Liker, A.; Székely, T. Parental behaviour in the Lapwing Vanellus vanellus. Ibis 1999, 141, 608–614. [Google Scholar] [CrossRef]
- Liker, A.; Székely, T. Aggression among female lapwings, Vanellus vanellus. Anim. Behav. 1997, 54, 797–802. [Google Scholar] [CrossRef]
- Santos, E.S.A.; Macedo, R.H. Load lightening in Southern Lapwings: Group-living mothers lay smaller eggs than pair-living mothers. Ethology 2011, 117, 1–9. [Google Scholar] [CrossRef]
- Saracura, V.; Macedo, R.H.; Blomqvist, D. Genetic parentage and variable social structure in breeding Southern Lapwings. Condor 2008, 110, 554–558. [Google Scholar] [CrossRef]
- Cerboncini, R.A.; Braga, T.V.; Roper, J.J.; Passos, F.C. Southern Lapwing Vanellus chilensis cooperative helpers at nests are older siblings. Ibis 2020, 162, 227–231. [Google Scholar] [CrossRef]
- Maruyama, P.K.; Cunha, A.F.; Tizo-Pedroso, E.; Del-Claro, K. Relation of group size and daily activity patterns to southern lapwing (Vanellus chilensis) behaviour. J. Ethol. 2010, 28, 339–344. [Google Scholar] [CrossRef]
- Brown, B.A. Hematology: Principles and Procedures. J. Clin. Pathol. 1993, 37, 1419. [Google Scholar]
- Wingfield, J.C.; Vleck, C.M.; Moore, M.C. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran desert. J. Exp. Zool. 1992, 264, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Breuner, C.W.; Hahn, T.P. Integrating stress physiology, environmental change, and behavior in free-living sparrows. Horm. Behav. 2003, 43, 115–123. [Google Scholar] [CrossRef]
- Heidinger, B.J.; Nisbet, I.C.T.; Ketterson, E.D. Older parents are less responsive to a stressor in a long-lived seabird: A mechanism for increased reproductive performance with age? Proc. Biol. Sci. Lond. B 2006, 273, 2227–2231. [Google Scholar] [CrossRef]
- Jakob, E.M.; Marshall, S.D.; Uetz, G. Estimating fitness: A comparation of body condition indices. Oikos 1996, 77, 61–67. [Google Scholar] [CrossRef]
- Green, A.J. Mass/length residuals: Measures of body condition or generators of spurious results? Ecology 2001, 82, 1473–1483. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Fokidis, H.B.; Greiner, E.C.; Deviche, P. Interspecific variation in avian blood para- sites and haematology associated with urbanization in a desert habitat. J. Avian Biol. 2008, 39, 300–310. [Google Scholar] [CrossRef]
- Eeva, T.; Hasselquist, D.; Langefors, A.; Tummeleht, L.; Nikinmaa, M.; Ilmonen, P. Pollution related effects on immune function and stress in a free-living population of pied flycatcher Ficedula hypoleuca. J. Avian Biol. 2005, 36, 405–412. [Google Scholar] [CrossRef]
- Hinam, H.L.; Clair, C.C.S. High levels of habitat loss and fragmentation limit reproductive success by reducing home range size and provisioning rates of northern saw-whet owls. Biol. Conserv. 2008, 141, 524–535. [Google Scholar] [CrossRef]
- Minias, P. Evolution of heterophil/lymphocyte ratios in response to ecological and life-history traits: A comparative analysis across the avian tree of life. J. Anim. Ecol. 2019, 88, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Gross, W.B.; Siegel, H.S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983, 27, 972–979. [Google Scholar] [CrossRef]
- Davis, A.K.; Cook, K.C.; Altizer, S. Leukocyte profiles in wild house finches with and without mycoplasmal conjunctivitis, a recently emerged bacterial disease. EcoHealth 2004, 1, 362–373. [Google Scholar] [CrossRef]
- Krams, I.; Vrublevska, J.; Cirule, D.; Kivleniece, I.; Krama, T.; Rantala, M.J.; Hõrak, P. Heterophil/lymphocyte ratios predict the magnitude of humoral immune response to a novel antigen in great tits (Parus major). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 161, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Penna, M.; Wingfield, J.C.; Cuevas, E.; Vásquez, R.A.; Quirici, V. Effects of traffic noise exposure on corticosterone, glutathione and tonic immobility in chicks of a precocial bird. Conserv. Physiol. 2019, 7, coz061. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Kitaysky, A.S. Endocrine responses to unpredictable environmental events: Stress or anti-stress hormones. Integr. Comp. Biol. 2002, 42, 600–609. [Google Scholar] [CrossRef]
- MacDougall-Shackleton, S.A.; Bonier, F.; Romero, L.M.; Moore, I.T. Glucocorticoids and “stress” are not synonymous. Int. Org. Biol. 2019, 1, obz017. [Google Scholar] [CrossRef]
- Janin, A.; Léna, J.-P.; Joly, P. Beyond occurrence: Body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad. Biol. Conserv. 2011, 144, 1008–1016. [Google Scholar] [CrossRef]
- Breuner, C.; Greenberg, A.L.; Wingfield, J.C. Noninvasive corticosterone treatment rapidly increases activity in Gambel’s white-crowned sparrow (Zonotrichia leucophris gambelii). Gen. Comp. Endocrinol. 1998, 111, 386–394. [Google Scholar] [CrossRef]
- Astheimer, L.; Buttemer, W.A.; Wingfield, J.C. Interactions of corticosterone with feeding, activity and metabolism in passerine birds. Ornis Scand. 1992, 23, 255–265. [Google Scholar] [CrossRef]
- Jenni, L.; Jenni-Eiermann, S.J.; Spina, F.; Schwabl, H. Regulation of protein breakdown and adrenocortical response to stress in birds during migratory night. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1182–R1189. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.M. Effect of ACTH and glucocorticoids on lipid metabolism in the Japanese quail, Coturnix coturnix japonica. Comp Biochem. Physiol. A 1993, 105, 689–696. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.W.; Leppert, L.L.; Dufty, A.M., Jr. Effects of small increases in corticosterone levels on morphology, immune function, and feather development. Physiol. Biochem. Zoc. 2010, 83, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Nesan, D.; Vijayan, M.M. Role of glucocorticoid in developmental programming: Evidence from zebra fish. Gen. Comp. Endocrinol. 2005, 181, 35–44. [Google Scholar] [CrossRef]
- Spencer, K.A.; Evans, N.P.; Monaghan, P. Postnatal stress in birds: A novel model of glucocorticoid programming of the hypothalamic– pituitary–adrenal axis. Endocrinology 2009, 150, 1931–1934. [Google Scholar] [CrossRef]
- Marasco, V.; Robinson, J.; Herzyk, P.; Spencer, K.A. Pre- and post-natal stress in context: Effects on the stress physiology in a precocial bird. J. Exp. Biol. 2012, 215, 3955–3964. [Google Scholar] [CrossRef]
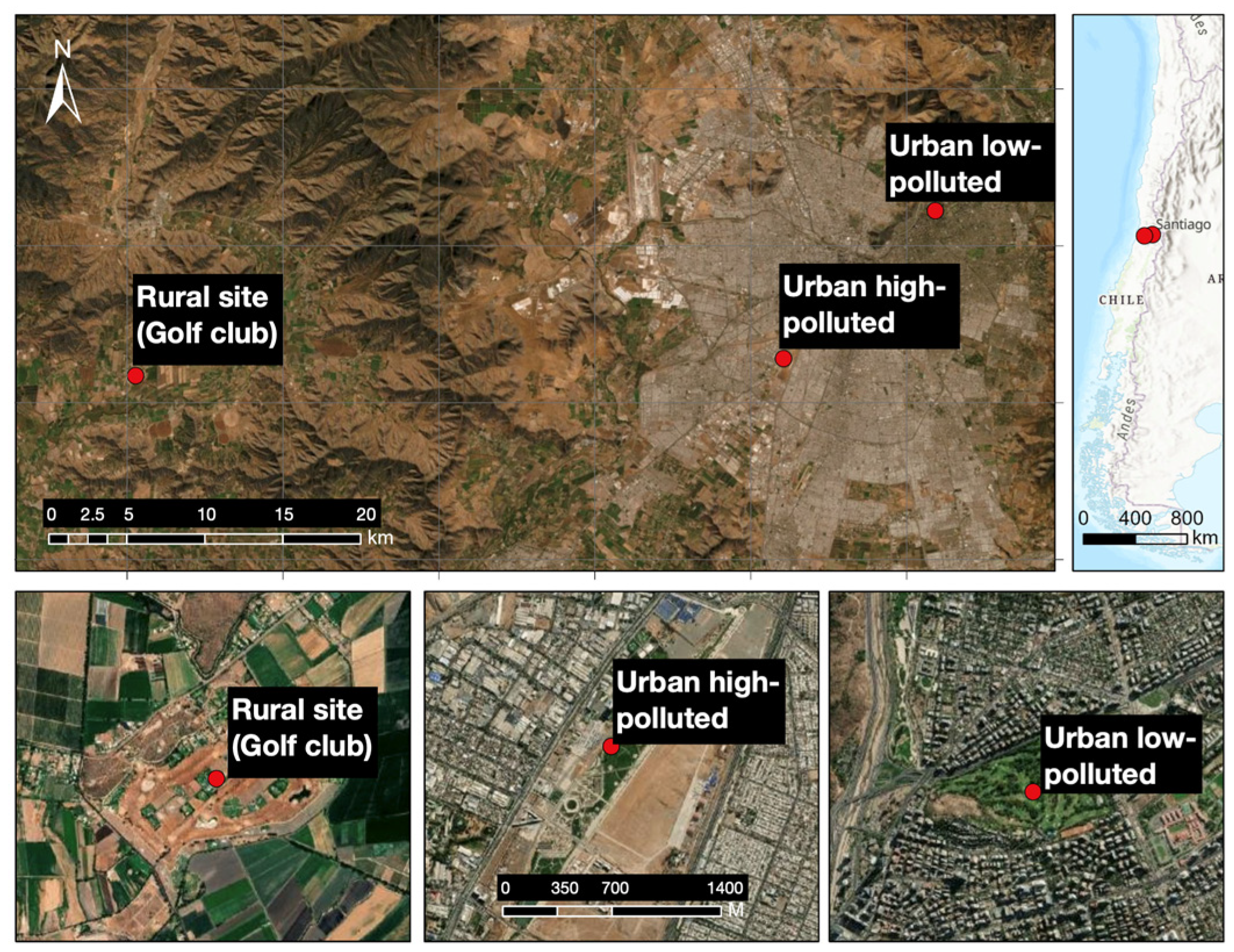



| Sampling Site | Land Use [24] | Private/Public | Green Area (%) [25] | Noise Pollution [27] | Chemical Pollution [26] |
|---|---|---|---|---|---|
| Urban Low-Polluted (Golf Club) | Residential | Private | Close to 50% | Lower | Lower |
| Urban High-Polluted (Public park) | Industrial | Public | Less than 10% | Higher | Higher |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quirici, V.; Valeris-Chacín, C.E.; Parada, P.; Cuevas, E.; Wingfield, J.C. Baseline Corticosterone, Stress Responses, and Leukocyte Profiles in Chicks of Precocial Birds in Rural and Urban Environments. Life 2023, 13, 2138. https://doi.org/10.3390/life13112138
Quirici V, Valeris-Chacín CE, Parada P, Cuevas E, Wingfield JC. Baseline Corticosterone, Stress Responses, and Leukocyte Profiles in Chicks of Precocial Birds in Rural and Urban Environments. Life. 2023; 13(11):2138. https://doi.org/10.3390/life13112138
Chicago/Turabian StyleQuirici, Verónica, Carlos E. Valeris-Chacín, Pablo Parada, Elfego Cuevas, and John C. Wingfield. 2023. "Baseline Corticosterone, Stress Responses, and Leukocyte Profiles in Chicks of Precocial Birds in Rural and Urban Environments" Life 13, no. 11: 2138. https://doi.org/10.3390/life13112138
APA StyleQuirici, V., Valeris-Chacín, C. E., Parada, P., Cuevas, E., & Wingfield, J. C. (2023). Baseline Corticosterone, Stress Responses, and Leukocyte Profiles in Chicks of Precocial Birds in Rural and Urban Environments. Life, 13(11), 2138. https://doi.org/10.3390/life13112138








