COVID-19 and Kidney: The Importance of Follow-Up and Long-Term Screening
Abstract
:1. Introduction
2. Materials and Methods
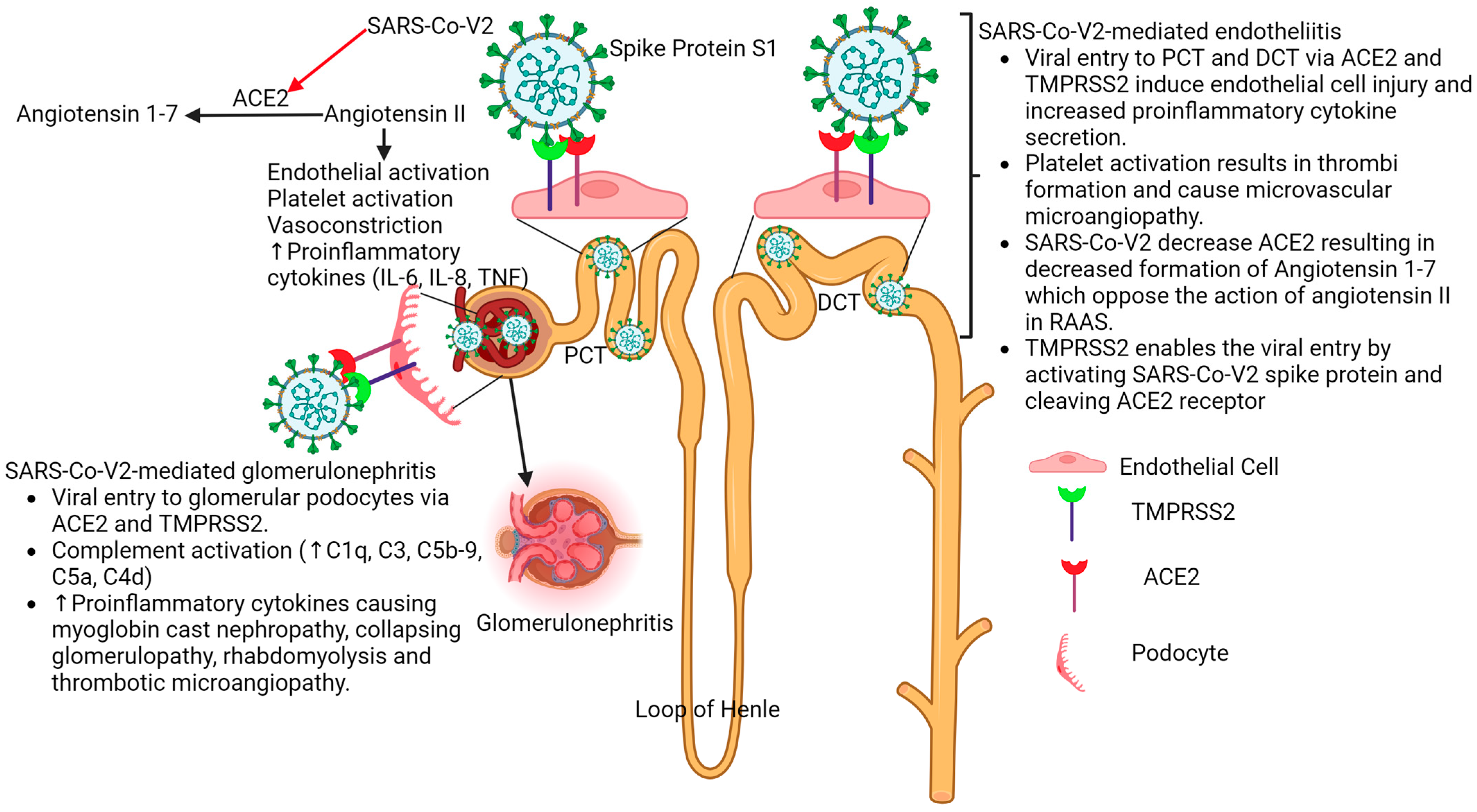
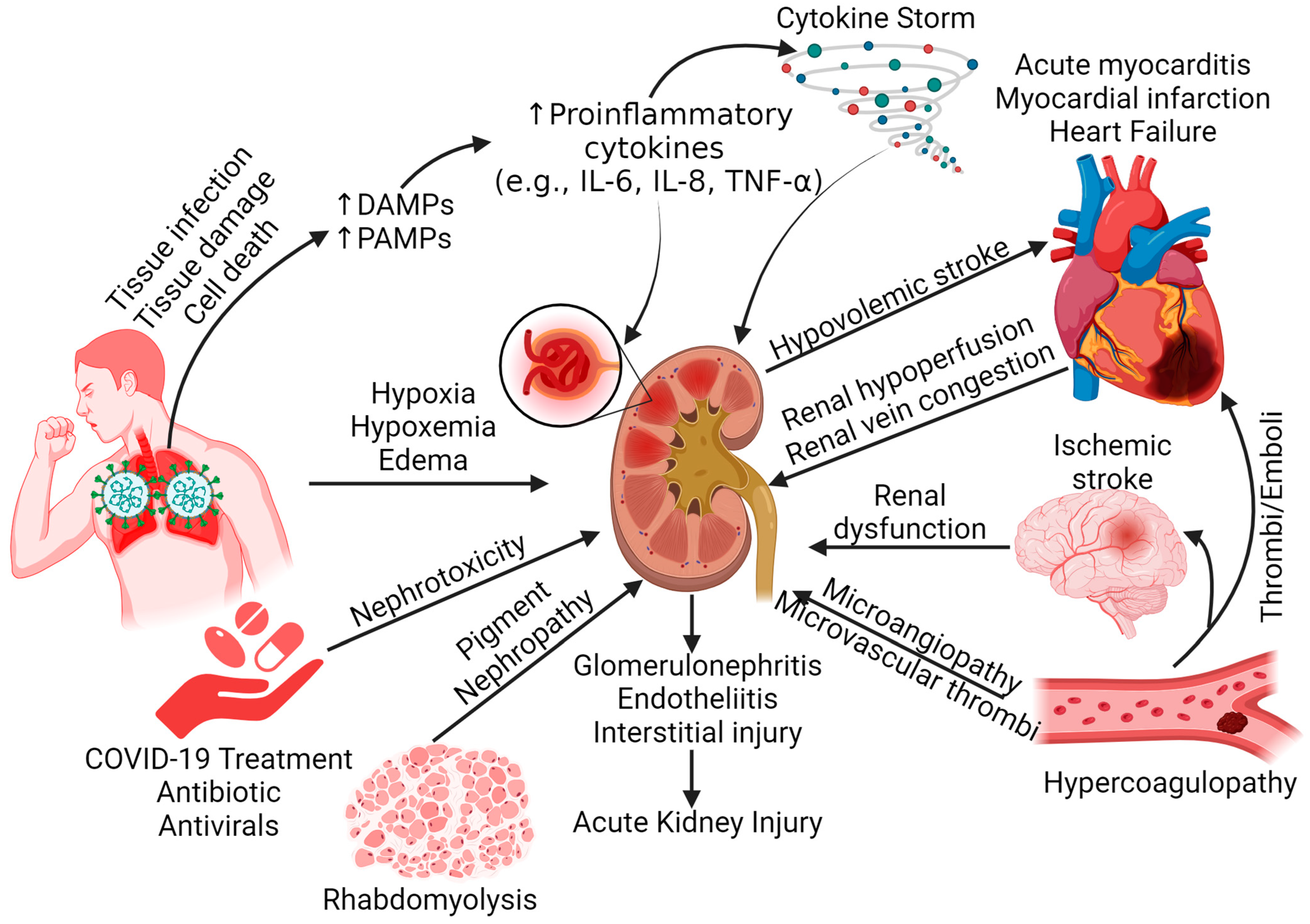
3. Acute Kidney Injury in COVID-19
4. Chronic Kidney Disease in COVID-19
5. Management and Treatment of AKI
6. COVID-19 in Patients with CKD
7. Biomarkers of Kidney Injury in COVID-19
8. Future Perspectives
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiffl, H.; Lang, S.M. Long-term interplay between COVID-19 and chronic kidney disease. Int. Urol. Nephrol. 2023, 55, 1977–1984. [Google Scholar] [CrossRef]
- Zadeh, F.H.; Wilson, D.R.; Agrawal, D.K. Long COVID: Complications, Underlying Mechanisms, and Treatment Strategies. Arch. Microbiol. Immunol. 2023, 7, 36–61. [Google Scholar]
- Yazdani, A.N.; DeMarco, N.; Patel, P.; Abdi, A.; Velpuri, P.; Agrawal, D.K.; Rai, V. Adverse Hematological Effects of COVID-19 Vaccination and Pathomechanisms of Low Acquired Immunity in Patients with Hematological Malignancies. Vaccines 2023, 11, 662. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Wang, H. Treatment and prognosis of COVID-19: Current scenario and prospects (Review). Exp. Ther. Med. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Lee, P.I.; Hsueh, P.R. Treatment options for COVID-19: The reality and challenges. J. Microbiol. Immunol. Infect. 2020, 53, 436–443. [Google Scholar] [CrossRef]
- Torjesen, I. COVID-19: Infection increases the risk of kidney disease even in mild cases, finds study. BMJ 2021, 374, n2189. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Long, J.D.; Strohbehn, I.; Sawtell, R.; Bhattacharyya, R.; Sise, M.E. COVID-19 Survival and its impact on chronic kidney disease. Transl. Res. 2022, 241, 70–82. [Google Scholar] [CrossRef]
- Radotra, B. Pathology of COVID-19 Infection. In Delineating Health and Health System: Mechanistic Insights into Covid 19 Complications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 135–148. [Google Scholar]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef]
- Geetha, D.; Kronbichler, A.; Rutter, M.; Bajpai, D.; Menez, S.; Weissenbacher, A.; Anand, S.; Lin, E.; Carlson, N.; Sozio, S.; et al. Impact of the COVID-19 pandemic on the kidney community: Lessons learned and future directions. Nat. Rev. Nephrol. 2022, 18, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.D.; Cordioli, R.L.; Santos, B.; Guerra, J.C.C.; Rodrigues, R.D.R.; Souza, G.M.; Ashihara, C.; Midega, T.D.; Campos, N.S.; Carneiro, B.V.; et al. COVID-19-associated coagulopathy and acute kidney injury in critically ill patients. Einstein 2023, 21, eAO0119. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, X.; Hu, B.; Li, D.; Chen, L.; Li, Y.; Tu, Y.; Xiong, S.; Wang, G.; Deng, J.; et al. Mechanisms of SARS-CoV-2 Infection-Induced Kidney Injury: A Literature Review. Front. Cell. Infect. Microbiol. 2022, 12, 838213. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.A.G.; Chicano, S.; Resontoc, L.P.; Aragon, E.E. Diffuse proliferative glomerulonephritis in a patient with COVID-19 infection. BMJ Case Rep. 2023, 16, e251962. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.P.; Mangalaparthi, K.K.; Madugundu, A.K.; Moyer, A.M.; Adam, B.A.; Mengel, M.; Singh, S.; Herrmann, S.M.; Rule, A.D.; Cheek, E.H.; et al. Acute Kidney Injury in Severe COVID-19 Has Similarities to Sepsis-Associated Kidney Injury: A Multi-Omics Study. Mayo Clin. Proc. 2021, 96, 2561–2575. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zeng, X.; Chen, H.; Chen, Y.; Yang, D.; Shen, Z.; Wang, X.; Liu, X.; Xiong, M.; et al. Kidney injury molecule-1 is a potential receptor for SARS-CoV-2. J. Mol. Cell Biol. 2021, 13, 185–196. [Google Scholar] [CrossRef]
- Leidinger, G.; Flockerzi, F.; Hohneck, J.; Bohle, R.M.; Fieguth, A.; Tschernig, T. TRPC6 is altered in COVID-19 pneumonia. Chem. Biol. Interact. 2022, 362, 109982. [Google Scholar] [CrossRef]
- Waraich, R.S.; Sohail, F.A.; Khan, G.; Durr, E.S.S.; Khan, B.; Rafi, S.; Nasir, S. Enhanced Expression of RAGE AXIS Is Associated with Severity of COVID-19 in Patients with Comorbidities. Metab. Syndr. Relat. Disord. 2023, 21, 141–147. [Google Scholar] [CrossRef]
- Curran, C.S.; Kopp, J.B. RAGE pathway activation and function in chronic kidney disease and COVID-19. Front. Med. 2022, 9, 970423. [Google Scholar] [CrossRef]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; Del Camino, D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Soleymanlou, N.; McAuley, D.F.; Estrada, V.; Diaz, G.A.; Lacamera, P.; Kaste, R.; Choi, W.; Gupta, A.; Welte, T. TRPC6 inhibitor (BI 764198) to reduce risk and severity of ARDS due to COVID-19: A phase II randomised controlled trial. Thorax 2023, 78, 816–824. [Google Scholar] [CrossRef]
- Brown, B.J.; Boekell, K.L.; Stotter, B.R.; Talbot, B.E.; Schlondorff, J.S. Gain-of-function, focal segmental glomerulosclerosis Trpc6 mutation minimally affects susceptibility to renal injury in several mouse models. PLoS ONE 2022, 17, e0272313. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wan, C.; Wang, Z.D.; Gao, Y.; Li, Y.C.; Tang, F.; Zhu, H.Y.; Yi, L.X.; Zhang, C. Expression of CD147 and Cyclophilin A in Kidneys of Patients with COVID-19. Clin. J. Am. Soc. Nephrol. 2021, 16, 618–619. [Google Scholar] [CrossRef]
- Kalejaiye, T.D.; Bhattacharya, R.; Burt, M.A.; Travieso, T.; Okafor, A.E.; Mou, X.; Blasi, M.; Musah, S. SARS-CoV-2 Employ BSG/CD147 and ACE2 Receptors to Directly Infect Human Induced Pluripotent Stem Cell-Derived Kidney Podocytes. Front. Cell Dev. Biol. 2022, 10, 855340. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.W.L.; Tan, B.W.Q.; Tan, A.L.M.; Schriver, E.R.; Gutierrez-Sacristan, A.; Das, P.; Yuan, W.; Hutch, M.R.; Garcia Barrio, N.; Pedrera Jimenez, M.; et al. Long-term kidney function recovery and mortality after COVID-19-associated acute kidney injury: An international multi-centre observational cohort study. EClinicalMedicine 2023, 55, 101724. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; Parikh, C.R. Long COVID and kidney disease. Nat. Rev. Nephrol. 2021, 17, 792–793. [Google Scholar] [CrossRef]
- Jdiaa, S.S.; Mansour, R.; El Alayli, A.; Gautam, A.; Thomas, P.; Mustafa, R.A. COVID-19 and chronic kidney disease: An updated overview of reviews. J. Nephrol. 2022, 35, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Gibertoni, D.; Reno, C.; Rucci, P.; Fantini, M.P.; Buscaroli, A.; Mosconi, G.; Rigotti, A.; Giudicissi, A.; Mambelli, E.; Righini, M.; et al. COVID-19 incidence and mortality in non-dialysis chronic kidney disease patients. PLoS ONE 2021, 16, e0254525. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Wei, W.; Yi, C.; Pu, Y.; Zhang, L.; Cui, T.; Ma, L.; Zhang, J.; Koyner, J.; et al. Kidney health in the COVID-19 pandemic: An umbrella review of meta-analyses and systematic reviews. Front. Public Health 2022, 10, 963667. [Google Scholar] [CrossRef]
- Hadadi, A.; Farrokhpour, H.; Rashedi, S.; Kafan, S.; Sotoudehnia, M.; Rahimzadeh, H.; Tabatabaei, S.; Razeghi, E.; Aghsaeifard, Z. Long-Term Impact of the COVID-19 Associated AKI: The Relationship between Kidney Recovery and Mortality in a 10-Month Follow-Up Cohort Study. Kidney Blood Press. Res. 2022, 47, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Matthay, M.A.; Aldrich, J.M.; Gotts, J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020, 8, 433–434. [Google Scholar] [CrossRef]
- Shepherd, T.D.; Niaz, T.S.; Yadav, R. Massive pulmonary embolism treated with low-dose thrombolysis on the geriatric ward during the COVID-19 pandemic. BMJ Case Rep. 2022, 15, e248125. [Google Scholar] [CrossRef]
- Harari, A.; Nitenberg, G.; Rapin, M.; Samii, K.; Lemaire, F.; Le Gall, J.R. Hypovolemic shocks simulating severe pulmonary embolism. 20 cases. Nouv Presse Med. 1975, 4, 153–157. [Google Scholar] [PubMed]
- Couturaud, F.; Tromeur, C.; Le Mao, R. Pulmonary embolism in COVID-19 infection: A high case-fatality related to pulmonary embolism characteristics. Eur. Respir. J. 2023, 61, 2202447. [Google Scholar] [CrossRef]
- Parmley, L.F., Jr.; North, R.L.; Ott, B.S. Hemodynamic alterations of acute pulmonary thromboembolism. Circ. Res. 1962, 11, 450–465. [Google Scholar] [CrossRef]
- Ottolina, D.; Zazzeron, L.; Trevisi, L.; Agarossi, A.; Colombo, R.; Fossali, T.; Passeri, M.; Borghi, B.; Ballone, E.; Rech, R.; et al. Acute kidney injury (AKI) in patients with COVID-19 infection is associated with ventilatory management with elevated positive end-expiratory pressure (PEEP). J. Nephrol. 2022, 35, 99–111. [Google Scholar] [CrossRef]
- Anumas, S.; Chueachinda, S.; Tantiyavarong, P.; Pattharanitima, P. The Prediction Score of Acute Kidney Injury in Patients with Severe COVID-19 Infection. J. Clin. Med. 2023, 12, 4412. [Google Scholar] [CrossRef]
- Chaibi, K.; Dao, M.; Pham, T.; Gumucio-Sanguino, V.D.; Di Paolo, F.A.; Pavot, A.; Cohen, Y.; Dreyfuss, D.; Perez-Fernandez, X.; Gaudry, S. Severe Acute Kidney Injury in Patients with COVID-19 and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 1299–1301. [Google Scholar] [CrossRef]
- Joannidis, M.; Forni, L.G.; Klein, S.J.; Honore, P.M.; Kashani, K.; Ostermann, M.; Prowle, J.; Bagshaw, S.M.; Cantaluppi, V.; Darmon, M.; et al. Lung-kidney interactions in critically ill patients: Consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020, 46, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Husain-Syed, F.; Ricci, Z.; Brodie, D.; Vincent, J.L.; Ranieri, V.M.; Slutsky, A.S.; Taccone, F.S.; Gattinoni, L.; Ronco, C. Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: From native to artificial organ crosstalk. Intensive Care Med. 2018, 44, 1447–1459. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Gupta, S.; Tighiouart, H.; Goyal, N.; Faugno, A.J.; Tariq, A.; Raichoudhury, R.; Sharma, J.H.; Meyer, L.; Kshirsagar, R.K.; et al. Kidney Recovery and Death in Critically Ill Patients With COVID-19-Associated Acute Kidney Injury Treated with Dialysis: The STOP-COVID Cohort Study. Am. J. Kidney Dis. 2022, 79, 404–416.e401. [Google Scholar] [CrossRef]
- Diamantidis, C.J.; Cook, D.J.; Redelosa, C.K.; Vinculado, R.B.; Cabajar, A.A.; Vassalotti, J.A. CKD and Rapid Kidney Function Decline during the COVID-19 Pandemic. Kidney Med. 2023, 5, 100701. [Google Scholar] [CrossRef]
- Carriazo, S.; Mas-Fontao, S.; Seghers, C.; Cano, J.; Goma, E.; Avello, A.; Ortiz, A.; Gonzalez-Parra, E. Increased 1-year mortality in haemodialysis patients with COVID-19: A prospective, observational study. Clin. Kidney J. 2022, 15, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Sourial, M.Y.; Gone, A.; Uribarri, J.; Srivatana, V.; Sharma, S.; Shimonov, D.; Chang, M.; Mowrey, W.; Dalsan, R.; Sedaliu, K.; et al. Outcomes of PD for AKI treatment during COVID-19 in New York City: A multicenter study. Perit. Dial. Int. 2023, 43, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nordio, M.; Reboldi, G.; Di Napoli, A.; Quintaliani, G.; Alberici, F.; Postorino, M.; Aucella, F.; Messa, P.; Brunori, G.; Italian Society of Nephrology, C.-R.G. Risk factors and action thresholds for the novel coronavirus pandemic. Insights from the Italian Society of Nephrology COVID-19 Survey. J. Nephrol. 2021, 34, 325–335. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2023, 401, e21–e33. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Hussain, H.; Ravelo, N.; Sriramajayam, K.; Di Gregorio, D.M.; Paulrasu, K.; Chen, P.; Young, K.; Masciarella, A.D.; Jayakumar, A.R.; et al. Kidney Damage in Long COVID: Studies in Experimental Mice. Biology 2023, 12, 1070. [Google Scholar] [CrossRef]
- Nopsopon, T.; Kittrakulrat, J.; Takkavatakarn, K.; Eiamsitrakoon, T.; Kanjanabuch, T.; Pongpirul, K. COVID-19 in end-stage renal disease patients with renal replacement therapies: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009156. [Google Scholar] [CrossRef]
- Pakhchanian, H.; Raiker, R.; Mukherjee, A.; Khan, A.; Singh, S.; Chatterjee, A. Outcomes of COVID-19 in CKD Patients: A Multicenter Electronic Medical Record Cohort Study. Clin. J. Am. Soc. Nephrol. 2021, 16, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Council, E.-E.; Group, E.W. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Brogan, M.; Ross, M.J. The Impact of Chronic Kidney Disease on Outcomes of Patients with COVID-19 Admitted to the Intensive Care Unit. Nephron 2022, 146, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Malani, P.N. COVID-19 in the Fall of 2023-Forgotten but Not Gone. JAMA 2023, 330, 1517–1518. [Google Scholar] [CrossRef]
- Memon, A.A.; Ahmed, H.; Li, Y.; Wongboonsin, J.; Hundert, J.; Benoit, S.; Chaudhari, A.; Sher, J.; Ghimire, P.; Hopkins, R.; et al. A Randomized Control Trial of Ravulizumab for Treatment of Patients With COVID-19 Infection and Kidney Injury. Kidney Int. Rep. 2022, 7, 2714–2717. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Gaggl, M.; Kinlaw, A.C.; Gou, Z.; Xu, Y.; Wei, J.; Wang, T. Remdesivir for COVID-19 and acute kidney injury: Disproportionality analysis of data from the U.S. Food and Drug Administration Adverse Event Reporting System. Int. J. Clin. Pharm. 2023, 45, 509–514. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Peng, Z. The role of kidney injury biomarkers in COVID-19. Ren. Fail. 2022, 44, 1280–1288. [Google Scholar] [CrossRef]
- Filev, R.; Lyubomirova, M.; Hristova, J.; Bogov, B.; Kalinov, K.; Svinarov, D.; Rostaing, L. Serum and Urinary Biomarkers in COVID-19 Patients with or without Baseline Chronic Kidney Disease. J. Pers. Med. 2023, 13, 382. [Google Scholar] [CrossRef]
- Shchepalina, A.; Chebotareva, N.; Akulkina, L.; Brovko, M.; Sholomova, V.; Androsova, T.; Korotchaeva, Y.; Kalmykova, D.; Tanaschuk, E.; Taranova, M.; et al. Acute Kidney Injury in Hospitalized Patients with COVID-19: Risk Factors and Serum Biomarkers. Biomedicines 2023, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, A.; Singh, K. Biomarkers of renal injury as early predictors of COVID-19 associated AKI: A prospective observational trial (BRICOAKI-trial). In Proceedings of the Critical Care Conference: 42nd International Symposium on Intensive Care and Emergency Medicine Brussels Belgium, Brussels, Belgium, 21–24 March 2023. [Google Scholar]
- Menez, S.; Moledina, D.G.; Thiessen-Philbrook, H.; Wilson, F.P.; Obeid, W.; Simonov, M.; Yamamoto, Y.; Corona-Villalobos, C.P.; Chang, C.; Garibaldi, B.T.; et al. Prognostic Significance of Urinary Biomarkers in Patients Hospitalized With COVID-19. Am. J. Kidney Dis. 2022, 79, 257–267.e251. [Google Scholar] [CrossRef]
- Sathe, N.A.; Mostaghim, A.; Barnes, E.; O’Connor, N.G.; Sahi, S.K.; Sakr, S.S.; Zahlan, J.M.; Smith, C.H.; Fitzpatrick, M.; Morrell, E.D.; et al. Biomarker Signatures of Severe Acute Kidney Injury in a Critically Ill Cohort of COVID-19 and Non-COVID-19 Acute Respiratory Illness. Crit. Care Explor. 2023, 5, e0945. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, T.; Guimaraes, G.M.C.; Carvalho, F.R.; Alves, L.S.; Faustino, R.; Campi-Azevedo, A.C.; Peruhype-Magalhaes, V.; Teixeira-Carvalho, A.; de Souza Gomes, M.; Rodrigues do Amaral, L.; et al. Acute kidney injury associated to COVID-19 leads to a strong unbalance of circulant immune mediators. Cytokine 2022, 157, 155974. [Google Scholar] [CrossRef]
- Hilton, J.; Boyer, N.; Nadim, M.K.; Forni, L.G.; Kellum, J.A. COVID-19 and Acute Kidney Injury. Crit. Care Clin. 2022, 38, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Ceresa, F.; Campo, S.; Barbera, G.; Caruso, D.; Palazzo, E.; Patane, F.; Monardo, P. Acute Kidney Injury and Sepsis after Cardiac Surgery: The Roles of Tissue Inhibitor Metalloproteinase-2, Insulin-like Growth Factor Binding Protein-7, and Mid-Regional Pro-Adrenomedullin. J. Clin. Med. 2023, 12, 5193. [Google Scholar] [CrossRef] [PubMed]
- Copur, S.; Berkkan, M.; Basile, C.; Tuttle, K.; Kanbay, M. Post-acute COVID-19 syndrome and kidney diseases: What do we know? J. Nephrol. 2022, 35, 795–805. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Hu, D.; Pan, P.; Liang, L.; Wu, W.; Tang, Y.; Huang, X.R.; Yu, X.; Wu, J.; et al. SARS-CoV-2 N Protein Induces Acute Kidney Injury via Smad3-Dependent G1 Cell Cycle Arrest Mechanism. Adv. Sci. 2022, 9, e2103248. [Google Scholar] [CrossRef]
- Liang, L.; Wang, W.; Chen, J.; Wu, W.; Huang, X.R.; Wei, B.; Zhong, Y.; Ma, R.C.W.; Yu, X.; Lan, H.Y. SARS-CoV-2 N protein induces acute kidney injury in diabetic mice via the Smad3-Ripk3/MLKL necroptosis pathway. Signal. Transduct. Target. Ther. 2023, 8, 147. [Google Scholar] [CrossRef]
- Fearn, A.; Sheerin, N.S. Complement activation in progressive renal disease. World J. Nephrol. 2015, 4, 31–40. [Google Scholar] [CrossRef]
- Trambas, I.A.; Coughlan, M.T.; Tan, S.M. Therapeutic Potential of Targeting Complement C5a Receptors in Diabetic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 8758. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Description | Why a Biomarker of AKI in COVID-19 |
|---|---|---|
| Cystatin C | An endogenous cysteine proteinase inhibitor. Filtered through the glomerulus and then reabsorbed and catabolized in the proximal tubule completely. | Serum CysC had a high predictive value for COVID-19-related AKI. Moderately predictive of disease severity. Independently related to the risks of critical illness and mortality among COVID-19 patients. |
| KIM-1 | A transmembrane protein with an extracellular immunoglobulin-like domain over top of a long mucin-like domain | Increased levels are associated with ischemia-, nephrotoxicants-, sepsis-, and immune-related injuries of the kidney proximal tubule. Renal KIM-1 mRNA levels increase 24-fold in patients with COVID-19 with bacterial sepsis. KIM-1 is a receptor for SARS-CoV-2 in the lung and kidney epithelium. KIM-1 may be a biomarker for early-stage AKI and predict a higher risk for clinical deterioration. Urine KIM-1/creatinine ratio is associated with COVID-19-specific death. |
| l-FABP | A lipid-binding protein that can be localized predominantly in the proximal tubule. A promising biomarker for kidney disorders and also attenuates renal injury. | l-FABP concentration substantially decreases in COVID-19 patients. Increasing l-FABP levels are associated with severity of disease and adverse clinical outcomes. Higher l-FABP levels are associated with death, pulmonary embolism, stroke, myocardial disease, prolonged hospitalization, and mechanical ventilation in COVID-19. |
| IL-18 | A cytokine of IL-1 superfamily is activated by caspase-1 and subsequently secreted by renal tubular cells and macrophages. | Increased urinary level of IL-18 is an early diagnostic and prognostic marker of AKI. Serum IL-18 concentrations correlate with the severity of COVID-19. |
| suPAR | UPAR is a membrane-bound receptor and is cleaved in response to inflammatory stimuli. | SuPAR levels are highly increased in COVID-19 and mediate AKI. SuPAR levels are predictive of in-hospital AKI and the need for dialysis in COVID-19. UPAR is a predictor of disease progression biomarkers in COVID-19. SuPAR has prognostic utility in COVID-19 hospitalized patients to predict severe complications. |
| NGAL | A protein of the lipocalin family. | Urinary NGAL levels are elevated in patients developing AKI in ICU. Maximum urinary NGAL values are correlated with the length of mechanical ventilation. Can be an AKI biomarker in patients with COVID-19 and is strongly linked to AKI diagnosis and prediction of the duration of AKI and outcomes, including death, dialysis, shock, and longer hospital stay. May be the best marker to predict the probability of AKI in COVID-19 patients. |
| TIMP-2 and IGFBP7 | TIMP2 is a protein-coding gene for protein-inhibiting metalloproteinases IGFBP7 is a protein, which regulates morphological changes in glandular cells. | Higher [TIMP-2]*[IGFBP7] levels were associated with adverse clinical outcomes, including the severity of AKI, requirement of RRT, and death. Elevated urinary [TIMP-2]*[IGFBP7] is a risk factor for AKI. |
| IL-6 | An inflammatory cytokine | Higher levels are associated with AKI in COVID-19 patients. |
| Angiopoetin-1 | Normally expressed by periendothelial cells and has a vascular protective effect. | Significantly higher levels of angiopoietin-1 are associated with AKI in COVID-19. |
| Neutrophil elastase 2 | Secreted by neutrophils during inflammation and are involved in regulating chronic inflammation. | Increased neutrophil elastase 2 is associated with more severe AKI stages 2–3. Neutrophil elastase level 2 is significantly associated with markers of inflammation. |
| sTNFR-1 | A transmembrane receptor that plays a key role in the regulation of the inflammatory pathway. | Associated with higher risk for severe AKI. |
| sTREM-1 | A transmembrane receptor of the immunoglobulin superfamily plays a role in inflammatory response. | Associated with higher risk for severe AKI. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, V. COVID-19 and Kidney: The Importance of Follow-Up and Long-Term Screening. Life 2023, 13, 2137. https://doi.org/10.3390/life13112137
Rai V. COVID-19 and Kidney: The Importance of Follow-Up and Long-Term Screening. Life. 2023; 13(11):2137. https://doi.org/10.3390/life13112137
Chicago/Turabian StyleRai, Vikrant. 2023. "COVID-19 and Kidney: The Importance of Follow-Up and Long-Term Screening" Life 13, no. 11: 2137. https://doi.org/10.3390/life13112137
APA StyleRai, V. (2023). COVID-19 and Kidney: The Importance of Follow-Up and Long-Term Screening. Life, 13(11), 2137. https://doi.org/10.3390/life13112137






