Meckel’s Diverticulum Causing Ileal Volvulus and Peritonitis after a Recent Appendectomy: A Case Report and Literature Review—We Should Likely Resect an Incidental MD
Abstract
1. Introduction
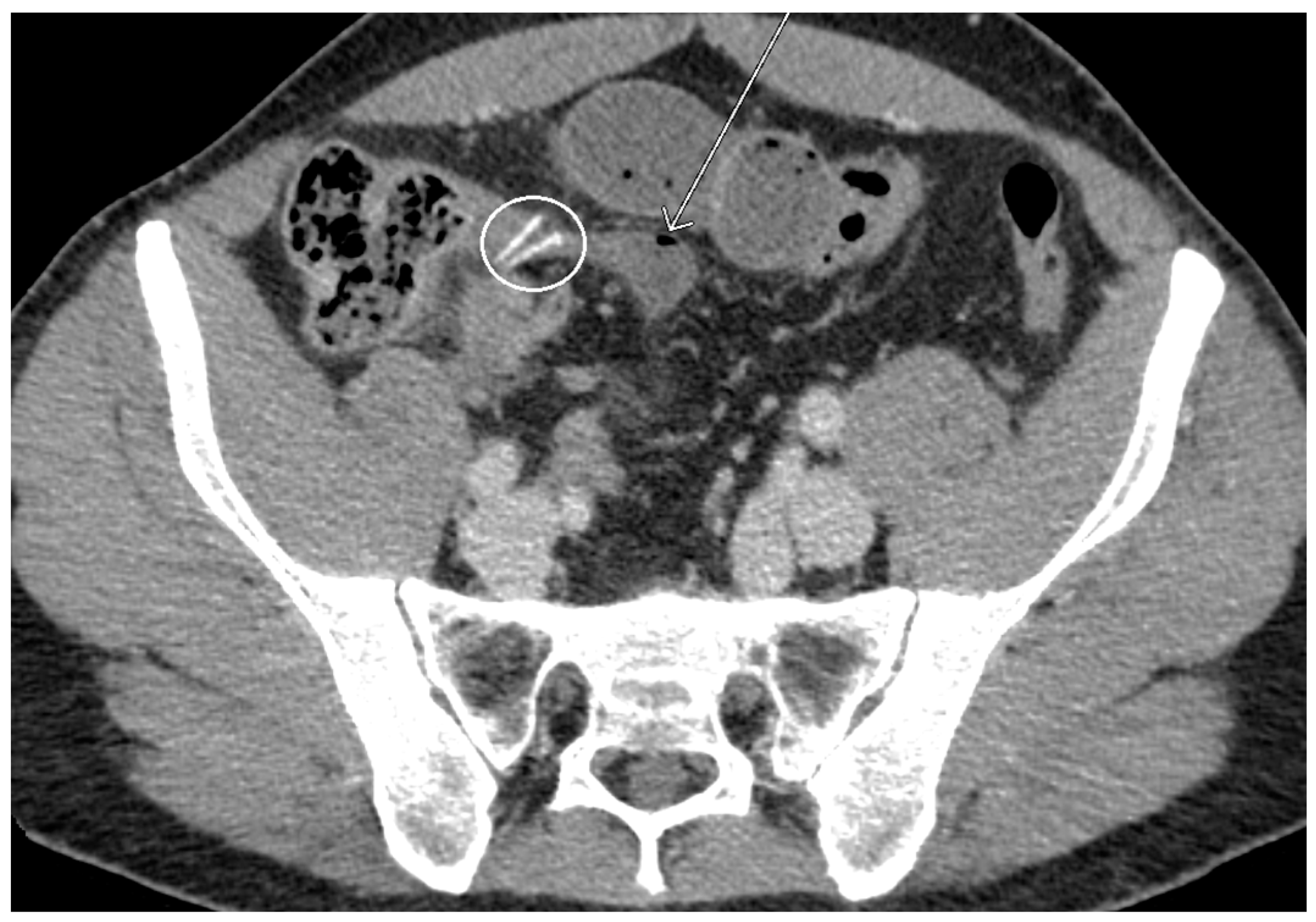
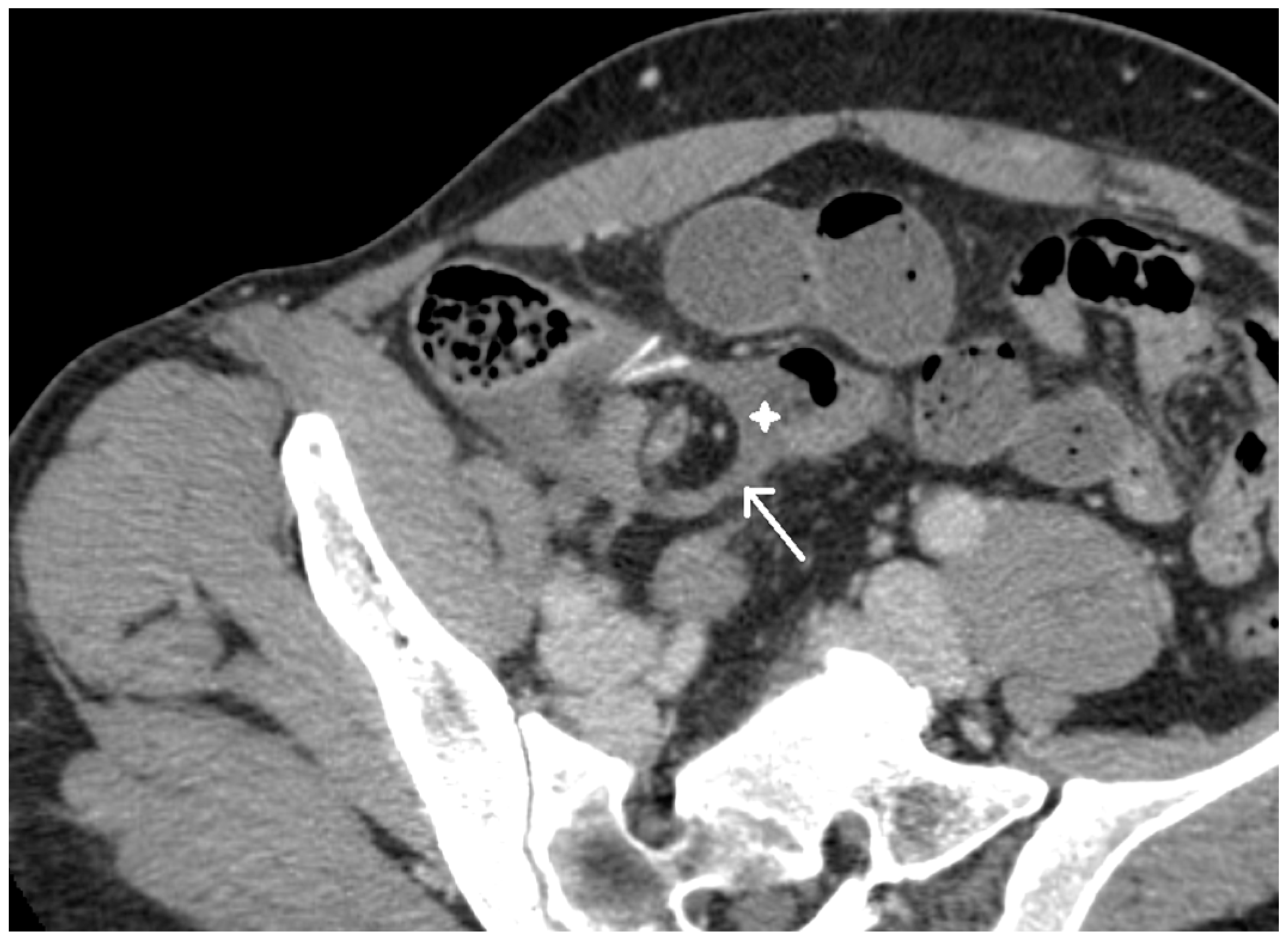
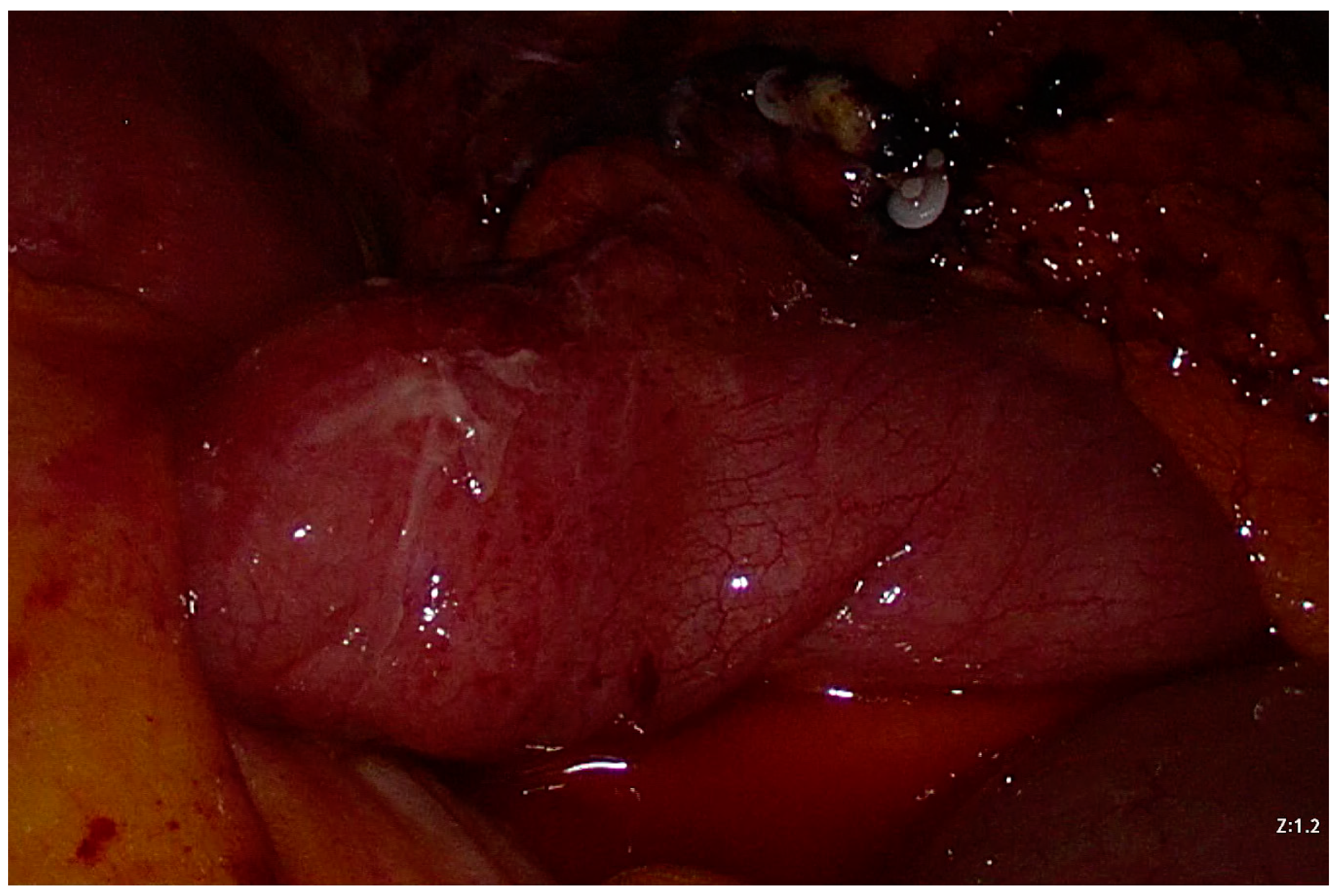
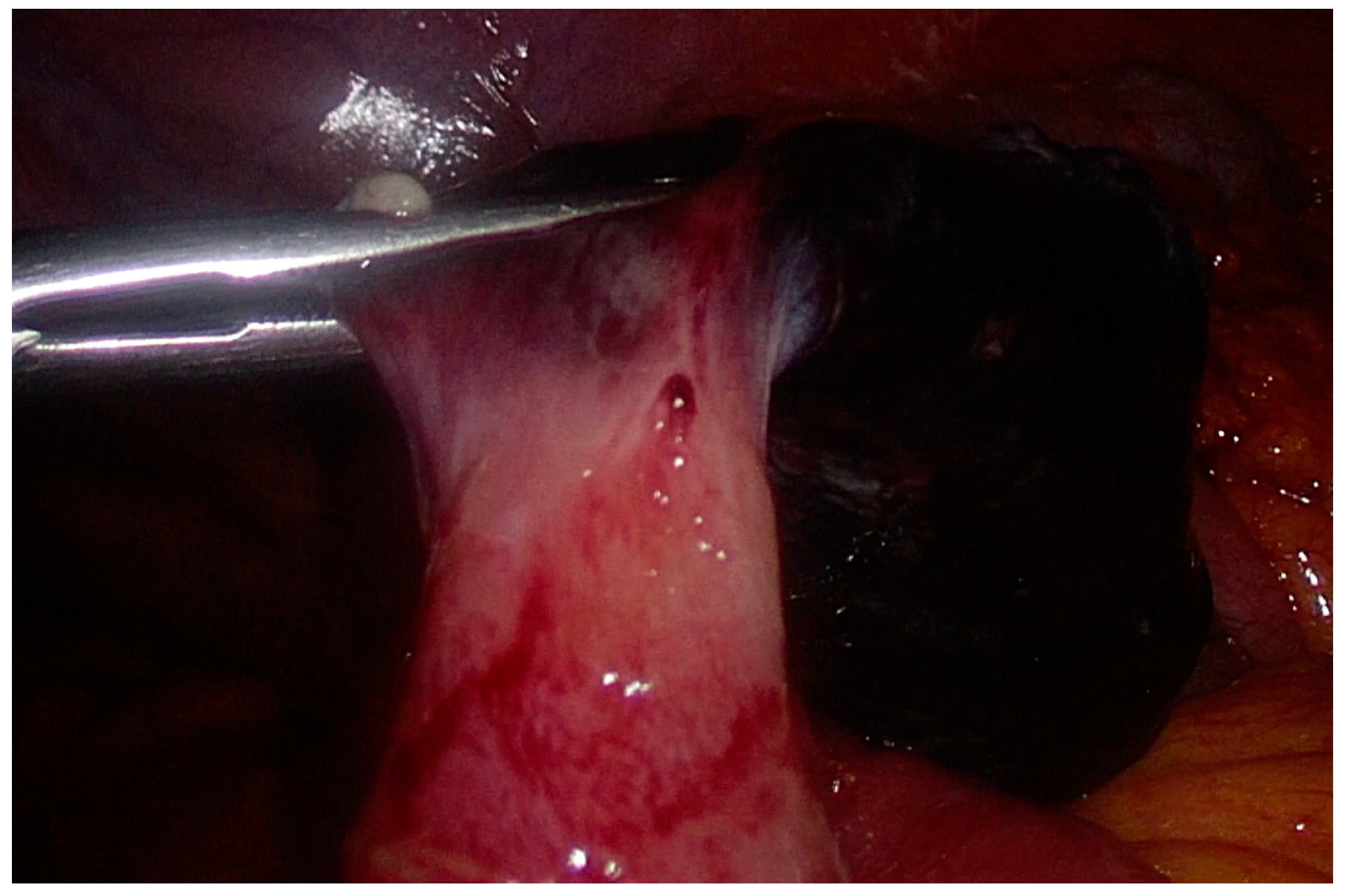
2. Case Presentation
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, C.C.; Søreide, K. Systematic review of epidemiology, presentation, and management of Meckel’s diverticulum in the 21st century. Medicine 2018, 97, e12154. [Google Scholar] [CrossRef]
- Sagar, J.; Kumar, V.; Shah, D.K. Meckel’s diverticulum: A systematic review. J. R. Soc. Med. 2006, 99, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Sancar, S.; Demirci, H.; Sayan, A.; Arıkan, A.; Candar, A. Meckel’s diverticulum: Ten years’ experience. Ulus. Cerrahi Derg. 2015, 31, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Srisajjakul, S.; Prapaisilp, P.; Bangchokdee, S. Many faces of Meckel’s diverticulum and its complications. Jpn. J. Radiol. 2016, 34, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kuru, S.; Kismet, K. Meckel’s diverticulum: Clinical features, diagnosis, and management. Rev. Esp. Enferm. Dig. 2018, 110, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, D.K.; Barnett, J.L. Meckel’s diverticulum. Am. J. Gastroenterol. 1990, 85, 777–781. [Google Scholar]
- Schaedlich, D.S.; Borges, P.C.; Lacombe, A.; Moron, R.A. Intestinal intussusception of Meckel’s diverticulum, a case report and literature review of the last five years. Einstein 2023, 21, eRC0173. [Google Scholar] [CrossRef]
- Kovacs, M.; Botstein, J.; Braverman, S. Angiographic diagnosis of Meckel’s diverticulum in an adult patient with negative scintigraphy. J. Radiol. Case Rep. 2017, 11, 22–29. [Google Scholar] [CrossRef]
- Uppal, K.; Tubbs, R.S.; Matusz, P.; Shaffer, K.; Loukas, M. Meckel’s diverticulum: A review. Clin. Anat. 2011, 24, 416–422. [Google Scholar] [CrossRef]
- Chen, J.J.; Lee, H.C.; Yeung, C.Y.; Chan, W.T.; Jiang, C.B.; Sheu, J.C.; Wang, N.L. Meckel’s Diverticulum: Factors Associated with Clinical Manifestations. ISRN Gastroenterol. 2014, 2014, 390869. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, Z.; Zhang, L.; Zhang, Y.; Pan, T.; Cai, D.; Xiong, Q.; Shu, Q.; Qian, Y. Multifaceted behavior of Meckel’s diverticulum in children. J. Pediatr. Surg. 2018, 53, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Leijonmarck, C.E.; Bonman-Sandelin, K.; Frisell, J.; Räf, L. Meckel’s diverticulum in the adult. Br. J. Surg. 1986, 73, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Fa-Si-Oen, P.R.; Roumen, R.M.; Croiset van Uchelen, F.A. Complications and management of Meckel’s diverticulum—A review. Eur. J. Surg. 1999, 165, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Wolff, B.G.; Tollefson, M.K.; Walsh, E.E.; Larson, D.R. Meckel diverticulum: The Mayo Clinic experience with 1476 patients (1950–2002). Ann. Surg. 2005, 241, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, H.; Hall, M.; Desai, A.A.; St Peter, S.D.; Snyder, C.L. Demographic disparities of children presenting with symptomatic Meckel’s diverticulum in children’s hospitals. Pediatr. Surg. Int. 2014, 30, 649–653. [Google Scholar] [CrossRef]
- Kabir, S.A.; Raza, S.A.; Kabir, S.I. Malignant neoplasms of Meckel’s diverticulum; an evidence based review. Ann. Med. Surg. 2019, 43, 75–81. [Google Scholar] [CrossRef]
- Kloss, B.T.; Broton, C.E.; Sullivan, A.M. Perforated Meckel diverticulum. Int. J. Emerg. Med. 2010, 3, 455–457. [Google Scholar] [CrossRef][Green Version]
- Zani, A.; Eaton, S.; Rees, C.M.; Pierro, A. Incidentally detected Meckel diverticulum: To resect or not to resect? Ann. Surg. 2008, 247, 276–281. [Google Scholar] [CrossRef]
- Weinstein, E.C.; Cain, J.C.; Remine, W.H. Meckel’s diverticulum: 55 years of clinical and surgical experience. JAMA 1962, 182, 251–253. [Google Scholar] [CrossRef]
- Soltero, M.J.; Bill, A.H. The natural history of Meckel’s Diverticulum and its relation to incidental removal. A study of 202 cases of diseased Meckel’s Diverticulum found in King County, Washington, over a fifteen year period. Am. J. Surg. 1976, 132, 168–173. [Google Scholar] [CrossRef]
- Peoples, J.B.; Lichtenberger, E.J.; Dunn, M.M. Incidental Meckel’s diverticulectomy in adults. Surgery 1995, 118, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.A.; Hofeldt, M.J.; Campbell, J.E.; Vedula, G.; DeLuca, J.A.; Flaherty, S.K. Meckel diverticulum: Ten-year experience in adults. South. Med. J. 2004, 97, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Mackey, W.C.; Dineen, P. A fifty year experience with Meckel’s diverticulum. Surg. Gynecol. Obstet. 1983, 156, 56–64. [Google Scholar] [PubMed]
- Vane, D.W.; West, K.W.; Grosfeld, J.L. Vitelline duct anomalies. Experience with 217 childhood cases. Arch. Surg. 1987, 122, 542–547. [Google Scholar] [CrossRef] [PubMed]
- St-Vil, D.; Brandt, M.L.; Panic, S.; Bensoussan, A.L.; Blanchard, H. Meckel’s diverticulum in children: A 20-year review. J. Pediatr. Surg. 1991, 26, 1289–1292. [Google Scholar] [CrossRef]
- DiGiacomo, J.C.; Cottone, F.J. Surgical treatment of Meckel’s diverticulum. South. Med. J. 1993, 86, 671–675. [Google Scholar] [CrossRef]
- Cullen, J.J.; Kelly, K.A.; Moir, C.R.; Hodge, D.O.; Zinsmeister, A.R.; Melton, L.J., 3rd. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann. Surg. 1994, 220, 564–568. [Google Scholar] [CrossRef]
- Matsagas, M.I.; Fatouros, M.; Koulouras, B.; Giannoukas, A.D. Incidence, complications, and management of Meckel’s diverticulum. Arch. Surg. 1995, 130, 143–146. [Google Scholar] [CrossRef]
- Chiu, E.J.; Shyr, Y.M.; Su, C.H.; Wu, C.W.; Lui, W.Y. Diverticular disease of the small bowel. Hepatogastroenterology 2000, 47, 181–184. [Google Scholar]
- Groebli, Y.; Bertin, D.; Morel, P. Meckel’s diverticulum in adults: Retrospective analysis of 119 cases and historical review. Eur. J. Surg. 2001, 167, 518–524. [Google Scholar] [CrossRef]
- Onen, A.; Ciğdem, M.K.; Oztürk, H.; Otçu, S.; Dokucu, A.I. When to resect and when not to resect an asymptomatic Meckel’s diverticulum: An ongoing challenge. Pediatr. Surg. Int. 2003, 19, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bani-Hani, K.E.; Shatnawi, N.J. Meckel’s diverticulum: Comparison of incidental and symptomatic cases. World J. Surg. 2004, 28, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Ueberrueck, T.; Meyer, L.; Koch, A.; Hinkel, M.; Kube, R.; Gastinger, I. The significance of Meckel’s diverticulum in appendicitis--a retrospective analysis of 233 cases. World J Surg. 2005, 29, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Dumper, J.; Mackenzie, S.; Mitchell, P.; Sutherland, F.; Quan, M.L.; Mew, D. Complications of Meckel’s diverticula in adults. Can. J. Surg. 2006, 49, 353–357. [Google Scholar]
- Robijn, J.; Sebrechts, E.; Miserez, M. Management of incidentally found Meckel’s diverticulum a new approach: Resection based on a Risk Score. Acta Chir. Belg. 2006, 106, 467–470. [Google Scholar] [CrossRef]
- McKay, R. High incidence of symptomatic Meckel’s diverticulum in patients less than fifty years of age: An indication for resection. Am. Surg. 2007, 73, 271–275, Erratum in Am. Surg. 2007, 73, 1293. [Google Scholar]
- Zulfikaroglu, B.; Ozalp, N.; Zulfikaroglu, E.; Ozmen, M.M.; Tez, M.; Koc, M. Is incidental Meckel’s diverticulum resected safely? N. Z. Med. J. 2008, 22, 39–44. [Google Scholar]
- Thirunavukarasu, P.; Sathaiah, M.; Sukumar, S.; Bartels, C.J.; Zeh, H., 3rd; Lee, K.K.; Bartlett, D.L. Meckel’s diverticulum—A high-risk region for malignancy in the ileum. Insights from a population-based epidemiological study and implications in surgical management. Ann. Surg. 2011, 253, 223–230. [Google Scholar] [CrossRef]
- Caracappa, D.; Gullà, N.; Lombardo, F.; Burini, G.; Castellani, E.; Boselli, C.; Gemini, A.; Burattini, M.F.; Covarelli, P.; Noya, G. Incidental finding of carcinoid tumor on Meckel’s diverticulum: Case report and literature review, should prophylactic resection be recommended? World J. Surg. Oncol. 2014, 12, 144. [Google Scholar] [CrossRef][Green Version]
- Kilius, A.; Samalavicius, N.E.; Danys, D.; Zaldokas, G.; Seinin, D. Asymptomatic heterotopic pancreas in Meckel’s diverticulum: A case report and review of the literature. J. Med. Case Rep. 2015, 9, 108. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Bayron, J.; Marshall, W.T., 3rd. Is an Incidental Meckel’s Diverticulum Truly Benign? Case Rep. Surg. 2015, 2015, 679097. [Google Scholar] [CrossRef] [PubMed]
- Gezer, H.Ö.; Temiz, A.; İnce, E.; Ezer, S.S.; Hasbay, B.; Hiçsönmez, A. Meckel diverticulum in children: Evaluation of macroscopic appearance for guidance in subsequent surgery. J. Pediatr. Surg. 2016, 51, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Lequet, J.; Menahem, B.; Alves, A.; Fohlen, A.; Mulliri, A. Meckel’s diverticulum in the adult. J. Visc. Surg. 2017, 154, 253–259. [Google Scholar] [CrossRef]
- Blouhos, K.; Boulas, K.A.; Tsalis, K.; Barettas, N.; Paraskeva, A.; Kariotis, I.; Keskinis, C.; Hatzigeorgiadis, A. Meckel’s diverticulum in adults: Surgical concerns. Front. Surg. 2018, 5, 55. [Google Scholar] [CrossRef]
- Mora-Guzmán, I.; Muñoz de Nova, J.L.; Martín-Pérez, E. Neuroendocrine tumours within a Meckel’s diverticulum. Ann. R. Coll. Surg. Engl. Home 2018, 100, e10–e11. [Google Scholar] [CrossRef]
- Mora-Guzmán, I.; Muñoz de Nova, J.L.; Martín-Pérez, E. Meckel’s diverticulum in the adult: Surgical treatment. Acta Chir. Belg. 2019, 119, 277–281. [Google Scholar] [CrossRef]
- Demirel, B.D.; Hancıoglu, S.; Bicakci, U.; Bernay, F.; Ariturk, E. Complications of Meckel’s diverticulum in children: A 10-years’ experience. J. Exp. Clin. Med. 2020, 36, 67–71. [Google Scholar]
- Chen, Y.; Liu, Y.; Jiang, L.; Jiang, F.; Zhu, T. Axially torsional Meckel’s diverticulum accompanied by small bowel volvulus: A case report. J. Int. Med. Res. 2021, 49, 3000605211053554. [Google Scholar] [CrossRef]
- Tree, K.; Kotecha, K.; Reeves, J.; Aitchison, L.; Noeline Chui, J.; Gill, A.J.; Mittal, A.; Samra, J.S. Meckel’s diverticulectomy: A multi-centre 19-year retrospective study. ANZ J. Surg. 2023. ahead of print. [Google Scholar] [CrossRef]
- Ruscher, K.A.; Fisher, J.N.; Hughes, C.D.; Neff, S.; Lerer, T.J.; Hight, D.W.; Bourque, M.D.; Campbell, B.T. National trends in the surgical management of Meckel’s diverticulum. J. Pediatr. Surg. 2011, 46, 893–896. [Google Scholar] [CrossRef]
- Sharma, R.K.; Jain, V.K. Emergency surgery for Meckel’s diverticulum. World J. Emerg. Surg. 2008, 3, 27. [Google Scholar] [CrossRef]
- Mukai, M.; Takamatsu, H.; Noguchi, H.; Fukushige, T.; Tahara, H.; Kaji, T. Does the external appearance of a Meckel’s diverticulum assist in choice of the laparoscopic procedure? Pediatr. Surg. Int. 2002, 18, 231–233. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Ji, Z.; Zhang, J.; Wang, Q. Laparoscopic Management of Perforated Meckel’s diverticulum in adults. Int. J. Med. Sci. 2012, 9, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Piñero, A.; Martínez-Barba, E.; Canteras, M.; Rodriguez, J.M.; Castellanos, G.; Parrilla, P. Surgical management and complications of Meckel’s diverticulum in 90 patients. Eur. J. Surg. 2002, 168, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Brungardt, J.G.; Cummiskey, B.R.; Schropp, K.P. Meckel’s: A National Surgical Quality Improvement Program Survey in Adults Comparing Diverticulectomy and Small Bowel Resection. Am. Surg. 2021, 87, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, C.; Rangarajan, M.; Senthilkumar, R.; Madankumar, M.V.; Kavalakat, A.J. Laparoscopic management of symptomatic Meckel’s diverticula: A simple tangential stapler excision. JSLS J. Soc. Laparoendosc. Surg. 2008, 12, 66–70. [Google Scholar]
- Ezekian, B.; Leraas, H.J.; Englum, B.R.; Gilmore, B.F.; Reed, C.; Fitzgerald, T.N.; Rice, H.E.; Tracy, E.T. Outcomes of laparoscopic resection of Meckel’s diverticulum are equivalent to open laparotomy. J. Pediatr. Surg. 2018, 54, 507–510. [Google Scholar] [CrossRef]
- Rahmat, S.; Sangle, P.; Sandhu, O.; Aftab, Z.; Khan, S. Does an Incidental Meckel’s Diverticulum Warrant Resection? Cureus 2020, 12, e10307. [Google Scholar] [CrossRef]
| Author | Year | Conclusion |
|---|---|---|
| Weinstein et al. [19] | 1962 | Wide-mouthed MD were not considered dangerous, and therefore their resection would only add to the risk of surgical complications and do not provide any benefit. |
| Soltero and Bill [20] | 1976 | To save one patient’s life from the complications of MD, it would be necessary to remove approximately 800 asymptomatic MDs. This would be likely to incur a significant increase in postoperative morbidity. They suggest that the prophylactic removal of MD is rarely, if ever, justified. |
| Leijonmarck et al. [12] | 1986 | In adults, an incidentally discovered, symptomless MD should be left in place. |
| Peoples et al. [21] | 1995 | Incidental diverticulectomy in adults should be abandoned. |
| Stone et al. [22] | 2004 | Removal of asymptomatic MD, particularly in women, is not recommended. |
| Zani et al. [18] | 2008 | Leaving an incidentally detected MD in situ reduces the risk of postoperative complications without increasing late complications. A large number of MD resections would need to be performed to prevent 1 death from MD. The above evidence does not support the resection of incidentally detected MD. |
| Author | Year | Conclusion |
|---|---|---|
| Mackey et al. [23] | 1983 | Recommend resection when risk factors are present: age ≤ 40 years, length of the diverticulum ≥ 2 cm, presence of heterotopic tissue, and male sex. |
| Vane et al. [24] | 1987 | Resection of asymptomatic vitelline remnants in early childhood is reasonable at the time of laparotomy for other conditions. |
| St-Vil et al. [25] | 1991 | MD discovered incidentally should be resected if ectopic mucosa present or if attached to the umbilicus or to the mesentery by fibrous bands. |
| DiGiacomo et al. [26] | 1993 | Stapler diverticulectomy is appropriate and safe and should be done for diverticula that easily fit the device. Diverticula that are so broad-based or short that stapled excision cannot be easily accomplished should be left in situ, since they are at low risk for complications. |
| Cullen et al. [27] | 1994 | Recommend prophylactic resection regardless of age (providing no additional condition, such as generalized peritonitis, would make removal hazardous). |
| Matsagas et al. [28] | 1995 | Resection of the unexpected MD can be performed safely with a low complication rate, regardless of the patient’s age. |
| Chiu et at. [29] | 2000 | The small bowel diverticula, except for MD, do not need to be treated if there are no significant symptoms. |
| Groebli et al. [30] | 2001 | The criteria to resect incidental MD: male sex, age < 40, ASA score, the operation being performed, size and position of the MD, palpable mass, exploration for acute right lower quadrant pain showing no other abnormality. |
| Onen et al. [31] | 2003 | Resection of the MD is recommended in all children younger than 8 years, including asymptomatic ones, in the absence of absolute contraindications. |
| Bani-Hani et al. [32] | 2004 | Resection of incidentally found MD is not associated with increased operative morbidity or mortality. |
| Park et al. [14] | 2005 | Recommend removal when risk factors are present: age < 50 years, male sex, diverticulum > 2 cm, and the presence of histologically abnormal tissue. |
| Ueberrueck et al. [33] | 2005 | In cases of gangrenous or perforated appendicitis, an incidentally discovered MD should be left in place, whereas in an only mildly inflamed appendix it should be removed. |
| Dumper et al. [34] | 2006 | Criteria to resect incidental MD: younger age at presentation, narrow diverticular neck, previous abdominal adhesions or obstructions, and any palpable or visual abnormality of the diverticulum. |
| Robijn et al. [35] | 2006 | Recommend resection with a Risk Score ≥ 6. Risk factors of the score: male sex, patients < 45 years, MD >2 cm, and the presence of a fibrous band. |
| McKay [36] | 2007 | Recommend prophylactic resection in patients under 50 years of age. |
| Zulfikaroglu et al. [37] | 2008 | Recommend prophylactic resection because it is not associated with increased operative morbidity or mortality. |
| Thirunavukarasu et al. [38] | 2011 | MD is a high-risk area for cancer in the ileum. With risk that increases with age and high possibility of curative resection with negligible operative mortality, incidental MD is best treated with resection. |
| Caracappa et al. [39] | 2014 | Recommend prophylactic resection. |
| Kilius et al. [40] | 2015 | Recommend prophylactic resection. |
| Jadlowiec et al. [41] | 2015 | Recommend prophylactic resection in patients of all ages. |
| Gezer et al. [42] | 2016 | Recommend prophylactic resection regardless of its macroscopic appearance. |
| Lequet et al. [43] | 2017 | Recommend resection when risk factors are present: male sex, age ≤ 40 years, diverticulum length > 2 cm, and presence of macroscopically mucosal alteration noted at surgery. |
| Blouhos et al. [44] | 2018 | Recommend resection when risk factors are present: age < 50 years, male sex, diverticulum length > 2 cm, and ectopic or abnormal features within a diverticulum. Diverticulectomy should be performed for long MD and wedge resection for short MD. |
| Chen et al. [11] | 2018 | Heterotopic tissue is the main cause of a complicated diverticulum, and it is safe and feasible to remove the incidentally found MD. |
| Hansen et al. [1] | 2018 | Incidental MD should always be resected in the pediatric population and in the presence of risk factors in the adult population. |
| Mora-Guzmán et al. [45] | 2018 | Recommend prophylactic resection because the benefits of resection of this high-risk area for cancer outweigh the risks of surgery. |
| Mora-Guzmán et al. [46] | 2018 | Recommend prophylactic resection of incidentally found MD because of benefits outweighing the risks in this high-risk area for cancer. |
| Demirel et al. [47] | 2019 | Recommend prophylactic resection due to the risk of ectopic tissue that may cause a mass effect or a narrow neck that may predispose to obstruction and diverticulitis. |
| Chen et al. [48] | 2021 | Recommend prophylactic resection when MD is longer, with a fibrous band at the tip of the diverticulum and a narrow base. |
| Tree et al. [49] | 2023 | Recommend laparoscopic prophylactic resection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanchetta, M.; Inversini, D.; Pappalardo, V.; Grappolini, N.; Morabito, M.; Gianazza, S.; Carcano, G.; Ietto, G. Meckel’s Diverticulum Causing Ileal Volvulus and Peritonitis after a Recent Appendectomy: A Case Report and Literature Review—We Should Likely Resect an Incidental MD. Life 2023, 13, 1996. https://doi.org/10.3390/life13101996
Zanchetta M, Inversini D, Pappalardo V, Grappolini N, Morabito M, Gianazza S, Carcano G, Ietto G. Meckel’s Diverticulum Causing Ileal Volvulus and Peritonitis after a Recent Appendectomy: A Case Report and Literature Review—We Should Likely Resect an Incidental MD. Life. 2023; 13(10):1996. https://doi.org/10.3390/life13101996
Chicago/Turabian StyleZanchetta, Matteo, Davide Inversini, Vincenzo Pappalardo, Niccolo Grappolini, Marika Morabito, Simone Gianazza, Giulio Carcano, and Giuseppe Ietto. 2023. "Meckel’s Diverticulum Causing Ileal Volvulus and Peritonitis after a Recent Appendectomy: A Case Report and Literature Review—We Should Likely Resect an Incidental MD" Life 13, no. 10: 1996. https://doi.org/10.3390/life13101996
APA StyleZanchetta, M., Inversini, D., Pappalardo, V., Grappolini, N., Morabito, M., Gianazza, S., Carcano, G., & Ietto, G. (2023). Meckel’s Diverticulum Causing Ileal Volvulus and Peritonitis after a Recent Appendectomy: A Case Report and Literature Review—We Should Likely Resect an Incidental MD. Life, 13(10), 1996. https://doi.org/10.3390/life13101996






