Induction of Triticale (×Triticosecale Wittmack) In Vitro Androgenesis in Anther Cultures of F1 Hybrid Combinations, Varieties and Homogeneity Testing of Offspring Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions of Donor Plants
2.2. Pre-Treatment of Donor Tillers
2.3. Isolation of Anthers and In Vitro AC
2.4. In Vitro Plant Regeneration and Acclimatization of Plantlets
2.5. Primers
2.6. DNA Isolation, PCR, Microsatellite Analysis, Allele Size Determination
2.7. Statistical Analysis
3. Results
3.1. Androgenesis Induction in Triticale (×Triticosecale Wittmack)
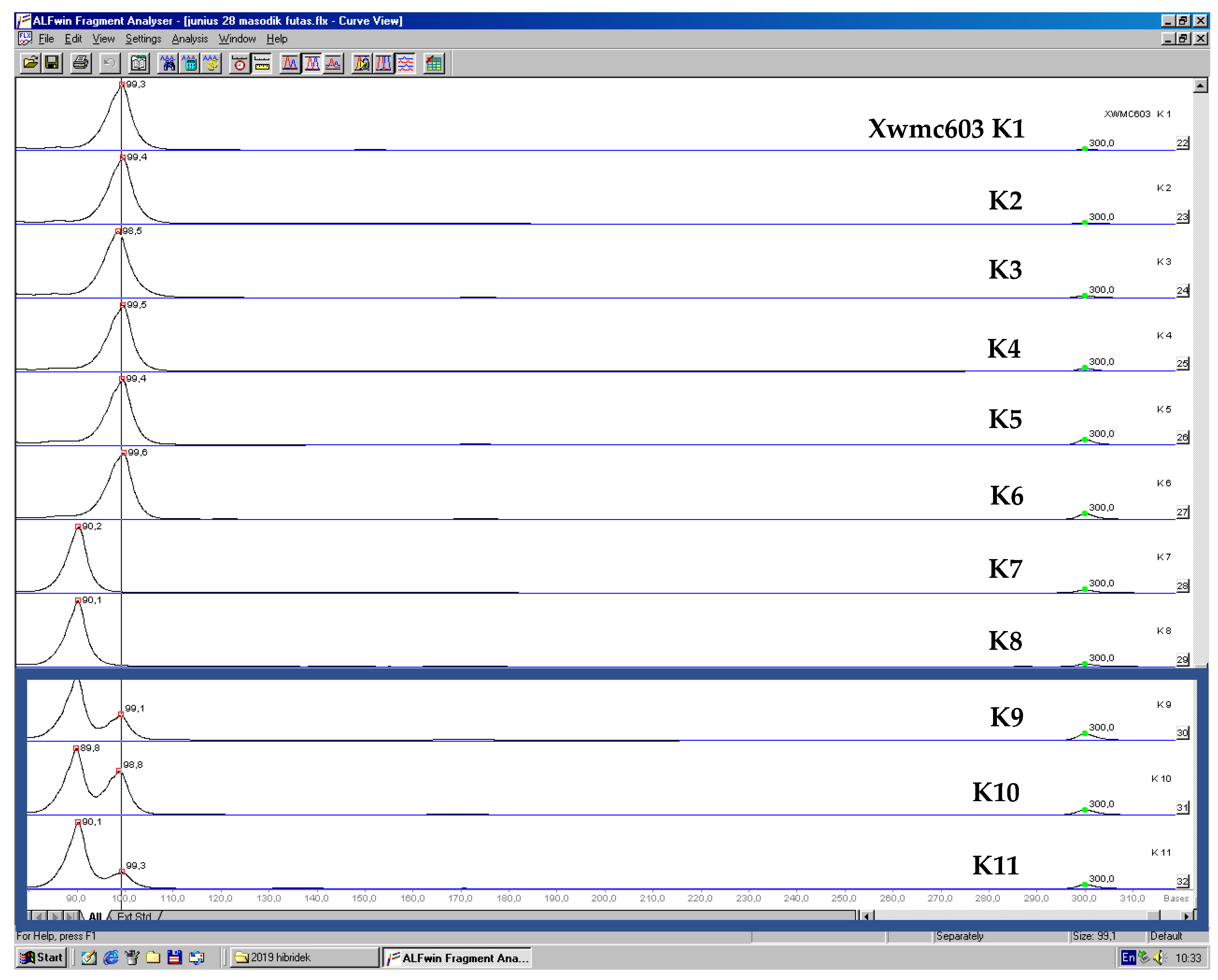
3.2. Testing of Homogeneity with Molecular Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunwell, J.M. Haploids in flowering plants: Origins and exploitation. Plant Biotechnol. J. 2010, 8, 377–424. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.S.; Wang, C.C.; Chu, C.C. Cytological studies on the androgenesis of Triticale. Acta Bot. Sin. 1973, 15, 145–154. [Google Scholar]
- Wang, Y.Y.; Sun, C.S.; Wang, C.C.; Chen, N.F. The induction of pollen plantlets of Triticale and Capsicum annum from anther culture. Scientia Sin. 1973, 16, 147–151. [Google Scholar]
- Pauk, J.; Poulimatka, M.; Lökös Tóth, K.; Monostori, T. In vitro androgenesis of triticale in isolated microspore culture. Plant Cell Tissue Organ. Cult. 2000, 61, 221–229. [Google Scholar] [CrossRef]
- Chien, Y.C.; Kao, K.N. Effects of osmolality, cytokinin and organic acid on pollen callus formation in triticale anthers. Can. J. Bot. 1983, 61, 639–641. [Google Scholar] [CrossRef]
- Hassawi, D.S.; Qi, J.; Liang, G.H. Effects of growth regulator and genotype on production of wheat and triticale polyhaploids from anther culture. Plant Breed. 1990, 104, 40–45. [Google Scholar] [CrossRef]
- Karsai, I.; Bedő, Z.; Hayes, P.M. Effect of induction medium, pH and maltose concentration on in vitro androgenesis of hexaploid winter triticale and wheat. Plant Cell Tissue Organ. Cult. 1994, 39, 49–53. [Google Scholar] [CrossRef]
- Ponitka, A.; Slusarkiewicz-Jarzina, A.; Wedzony, M.; Marcinska, I.; Wozny, J.; Wozna, J. The influence of various in vitro culture conditions on androgenetic embryo induction and plant regeneration from hexaploid triticale (×Triticosecale Wittm.). J. Appl. Genet. 1999, 40, 165–174. [Google Scholar]
- Immonen, S.; Robinson, J. Stress treatments and ficoll for improving green plant regeneration in triticale anther culture. Plant Sci. 2000, 150, 77–84. [Google Scholar] [CrossRef]
- Arzani, A.; Darvey, N.L. The effect of colchicine on triticale anther-derived plants: Microspore pre-treatment and haploid-plant treatment using a hydroponic system. Euphytica 2001, 122, 235–241. [Google Scholar] [CrossRef]
- Marciniak, K.; Kaczmarek, Z.; Adamski, T.; Surma, M. The anther-culture response of triticale line 9 tester progenies. Cell. Mol. Biol. Lett. 2003, 8, 343–351. [Google Scholar] [PubMed]
- Pauk, J.; Mihály, R.; Monostori, T.; Puolimatka, M. Protocol of triticale (×Triticosecale Wittmack) microspore culture. In Doubled Haploid Production in Crop Plants: A Manual Book; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 129–134. [Google Scholar]
- Oleszczuk, S.; Sowa, S.; Zimny, J. Direct embryogenesis and green plant regeneration from isolated microspores of hexaploid triticale (×Triticosecale Wittmack) cv. Bogo. Plant Cell Rep. 2004, 22, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Lantos, C.; Jancsó, M.; Pauk, J. Microspore culture of small grain cereals. Acta Physiol. Plant. 2005, 27, 523–531. [Google Scholar] [CrossRef]
- Eudes, F.; Amundsen, E. Isolated microspore culture of Canadian 6X triticale cultivars. Plant Cell Tissue Organ. Cult. 2005, 82, 233–241. [Google Scholar] [CrossRef]
- Zur, I.; Dubas, E.; Golemic, E.; Szechynska-Hebda, M.; Janowiak, F.; Wedzony, M. Stress-induced changes important for effective androgenic induction in isolated microspore culture of triticale (×Triticosecale Wittmack). Plant Cell Tissue Organ. Cult. 2008, 94, 319–328. [Google Scholar] [CrossRef]
- Zur, I.; Dubas, E.; Golemiec, E.; Szechynska-Hebda, M.; Golebiowska, G.; Wedzony, M. Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (× Triticosecale Wittm.). Plant Cell Rep. 2009, 28, 1279–1287. [Google Scholar] [CrossRef]
- Würschum, T.; Tucker, M.R.; Reif, J.C.; Maurer, H.P. Improved efficiency of doubled haploid generation in hexaploid triticale by in vitro chromosome doubling. BMC Plant Biol. 2012, 12, 109. [Google Scholar] [CrossRef]
- Lantos, C.; Bona, L.; Boda, K.; Pauk, J. Comparative analyses of in vitro anther- and isolated microspore culture in hexaploid triticale (×Triticosecale Wittmack) for androgenic parameters. Euphytica 2014, 197, 27–37. [Google Scholar] [CrossRef]
- Zur, I.; Dubas, E.; Krzewska, M.; Zielinski, K.; Fodor, J.; Janowiak, F. Glutathione provides antioxidaitve defence and promotes microspore-derived development in isolated microspore cultures of triticale (× Triticosecale Wittm.). Plant Cell Rep. 2019, 38, 195–209. [Google Scholar] [CrossRef]
- Maheshwari, P.; Laurie, J.D. Triticale isolated microspore culture for doubled haploid production. In Doubled Haploid Technology, Methods in Molecular Biology; Seguí-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 1, pp. 295–312. [Google Scholar]
- Pachota 2022 Pachota, K.A.; Orłowska, R.; Bednarek, P.T. Medium composition affects the tissue culture-induced variation in triticale regenerants. Plant Cell Tissue Organ Cult. 2022, 151, 35–46. [Google Scholar] [CrossRef]
- Orłowska, R. Triticale doubled haploid plant regeneration factors linked by structural equation modelling. J. Appl. Genet. 2022, 63, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Orlowska, R.; Zebrowski, J.; Dynkowska, W.M.; Androsiuk, P.; Bednarek, P.T. Metabolomic changes as key factors of green plant regeneration efficiency of triticale in vitro anther culture. Cells 2023, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Tuvesson, S.; Ljungberg, A.; Johansson, N.; Karlsson, K.E.; Suijs, L.W.; Josset, J.P. Large-scale production of wheat and triticale double haploids through the use of a single-anther culture method. Plant Breed. 2000, 119, 455–459. [Google Scholar] [CrossRef]
- Tuvesson, S.; von Post, R.; Ljungberg, A. Triticale anther culture. In Doubled Haploid Production in Crop Plants: A Manual Book; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 117–122. [Google Scholar]
- Wedzony, M. Protocol for anther culture in hexaploid triticale. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 123–128. [Google Scholar]
- Mozgova, G.V.; Zaitseva, O.I.; Lemesh, V.A. Structural changes in chloroplast genome accompanying albinism in anther culture of wheat and triticale. Cer. Res. Commun. 2012, 40, 467–475. [Google Scholar] [CrossRef]
- Zur, I.; Dubas, E.; Krzewska, M.; Kopec, P.; Nowicka, A.; Surowka, E.; Gawronska, K.; Golebiowska, G.; Juzon, K.; Malaga, S. Triticale and barley microspore embryogenesis induction requires both reactive oxygen species generation and efficient system of antioxidative defence. Plant Cell Tissue Organ. Cult. 2021, 145, 347–366. [Google Scholar] [CrossRef]
- Thomas, W.T.B.; Forster, B.P.; Gertsson, B. Doubled haploids in breeding. In Doubled Haploid Production in Crop Plants: A Manual Book; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 337–350. [Google Scholar]
- Oleszczuk, S.; Rabiza-Swider, J.; Zimny, J.; Lukaszewski, A.J. Aneuploidy among androgenic progeny of hexaploid triticale (×Triticosecale Wittmmack). Plant Cell Rep. 2011, 30, 575–586. [Google Scholar] [CrossRef][Green Version]
- Lagunovskaya, A.V.; Buracova, A.A.; Bushtevich, V.N.; Sakovich, V.I.; Lemesh, V.A.; Gryb, S.I. Analysis of the intralinear polymorphism and the homozygosity rate of triticale doubled haploid lines by microsatellite markers. Dokl. Natl. Acad. Sci. Belarus 2020, 64, 199–208. (In Russian) [Google Scholar] [CrossRef]
- Zimny, J.; Sowa, S.; Otreba, P.; Kozdoj, J.; Zimny, A.; Kaczmarek, J.; Oleszczuk, S.; Czapliczki, A.; Jedryczka, M. Pollen flow of winter triticale (× Triticosecale Wittm.) investigated with transgenic line expressing β-glucuronidase gene. Agronomy 2021, 11, 431. [Google Scholar] [CrossRef]
- Torjek, O.; Kiss, E.; Mázik-Tőkei, K.; Hutvagner, G.; Silhavy, D.; Banfalvi, Z.; Kertesz, Z.; Pauk, J.; Heszky, L. Comparative molecular analysis of winter wheat cultivars and their doubled haploid derivatives. Cer. Res. Commun. 2001, 29, 41–48. [Google Scholar] [CrossRef]
- Chege, P.; Kiss, E.; Lantos, C.; Palagyi, A.; Pauk, J. Doubled haploid production using an improved anther culture protocol for sorghum [Sorghum bicolor (L.) Moench]. Phyton–I. J. Exp. Bot. 2021, 90, 475–487. [Google Scholar] [CrossRef]
- Ouyang, J.W.; Jia, S.E.; Zhang, C.; Chen, X.; Fen, G. A new Synthetic Medium (W14) for Wheat Anther Culture; Annual Report; Institute of Genetics, Academia Sinica: Beijing, China, 1989; pp. 91–92. [Google Scholar]
- Lantos, C.; Weyen, J.; Orsini, J.M.; Gnad, H.; Schlieter, B.; Lein, V.; Kontowski, S.; Jacobi, A.; Mihaly, R.; Broughton, S.; et al. Efficient application of in vitro anther culture for different European winter wheat (Triticum aestivum L.) breeding programs. Plant Breed. 2013, 132, 149–154. [Google Scholar] [CrossRef]
- Ouyang, J.W.; Hu, H.; Chuang, C.C.; Tseng, C.C. Induction of pollen plants from anthers of Triticum aestivum L. cultured in vitro. Sci. Sin. 1973, 16, 79–95. [Google Scholar]
- Pauk, J.; Mihály, R.; Puolimatka, M. Protocol of wheat (Triticum aestivum L.) anther culture. In Doubled Haploid Production in Crop Plants, a Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Kluwer: Dordrect, The Netherlands, 2003; pp. 59–64. [Google Scholar]
- Song, Q.J.; Fickus, E.W.; Cregan, P.W. Characterization of trinucleotide SSR motifs in wheat. Theor. Appl. Genet. 2002, 104, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, W.; Rhoads, R.E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989, 17, 8543–8551. [Google Scholar] [CrossRef] [PubMed]
- Van Deynze, A.E.; Sorrells, M.E.; Park, W.D.; Ayres, N.M.; Fu, H.; Cartinhour, S.W.; Paul, E.; McCouch, S.R. Anchor probes for comparative mapping of grass genera. Theor. Appl. Genet. 1998, 97, 356–369. [Google Scholar] [CrossRef]
- Kuleung, C.; Baenziger, P.S.; Dweikat, I. Transferability of SSR markers among wheat, rye, and triticale. Theor. Appl. Genet. 2004, 108, 1147–1150. [Google Scholar] [CrossRef]
- Somers, D.; Isaac, P.; Edwards, K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 1105–1114. [Google Scholar] [CrossRef]
- Hackauf, B.; Wehling, P. Identification of microsatellite polymorphisms in an expressed portion of the rye genome. Plant Breed. 2001, 121, 17–25. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Mi, X.F.; Melchinger, A.E.; Reif, J.C.; Wuerschum, T. Optimum allocation of test resources and comparison of breeding strategies for hybrid wheat. Theor. Appl. Genet. 2014, 127, 2117–2126. [Google Scholar] [CrossRef]
- Islam, S.M.S.; Tuteja, N. Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci. 2012, 182, 134–144. [Google Scholar] [CrossRef]
- Oleszczuk, S.; Lukaszewsky, A.J. The origin of unusual chromosome constitutions among newly formed allopolyploids. Am. J. Bot. 2014, 101, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lantos, C.; Pauk, J. Anther culture as an effective tool in winter wheat (Triticum aestivum L.) breeding. Rus. J. Genet. 2016, 52, 794–801. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Muniz, L.M.; Jouve, N. Mapping of QTLs for androgenetic response based on a molecular genetic map of ×Triticosecale Wittmack. Genome 2005, 48, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Krzewska, M.; Czyczylo-Mysza, I.; Dubas, E.; Golebiowska-Pikana, G.; Golemiec, E.; Stojalowski, S.; Chrupek, M.; Zur, I. Quantitative trait loci associated with androgenic responsiveness in triticale (× Triticosecale Wittm.) anther culture. Plant Cell Rep. 2012, 31, 2099–2108. [Google Scholar] [CrossRef]
- Gonzalez, M.; Hernandez, I.; Jouve, N. Analysis of anther culture response in hexaploid triticale. Plant Breed. 1997, 116, 302–304. [Google Scholar] [CrossRef]
- Warzecha, R.; Sowa, S.; Salak-Warzecha, K.; Oleszczuk, S.; Sliwinska, E.; Zimny, J. Doubled haploids in production of male sterility maintaining triticale (Triticosecale Wittmack) lines. Acta Physiol. Plant. 2005, 27, 245–250. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R.; Mankowski, D.R.; Zimny, J.; Kowalczyk, K.; Nowak, M.; Zebrowski, J. Glutathione and copper ions as critical factors of green plant regeneration efficiency of triticale in vitro anther culture. Front. Plant Sci. 2022, 13, 926305. [Google Scholar] [CrossRef]
- Niazian, M.; Shariatpanahi, M.E. In vitro—based doubled haploid production: Recent improvements. Euphytica 2020, 216, 69. [Google Scholar] [CrossRef]
- Asif, M.; Eudes, F.; Randhawa, H.; Amundsen, E.; Spaner, D. Phytosulfokine alpha enhances microspore embryogenesis in both triticale and wheat. Plant Cell Tissue Organ. Cult. 2014, 116, 125–130. [Google Scholar] [CrossRef]
- Slusarkiewicz-Jarzina, A.; Pudelska, H.; Wozna, J.; Pniewski, T. Improved production of doubled haploids of winter and spring triticale hybrids via combination of colchicine treatments on anthers and regenerated plants. J. Appl. Genet. 2017, 58, 287–295. [Google Scholar] [CrossRef][Green Version]


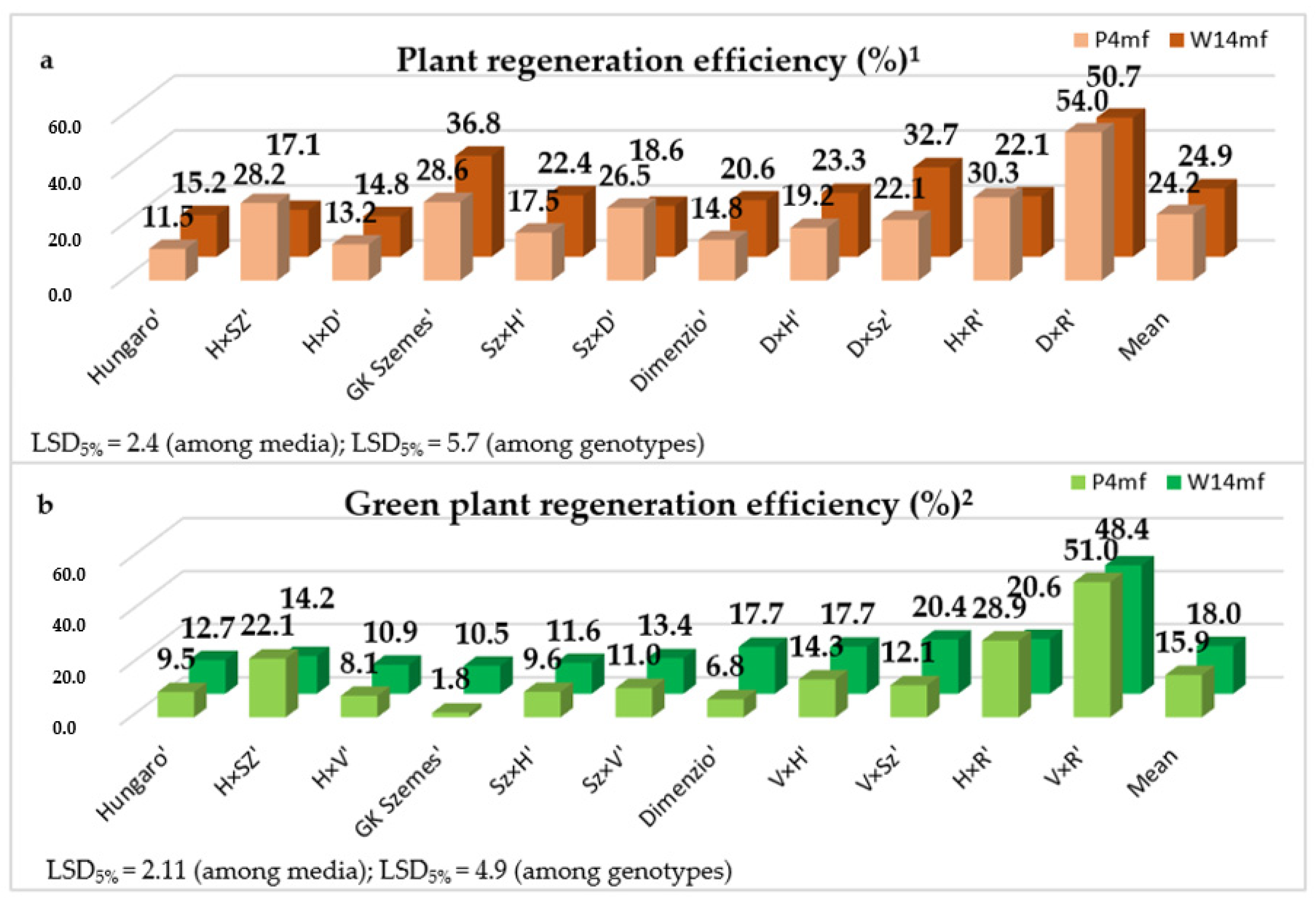

| Source of Variance | df | MS—ELS | MS—Green Plantlets | MS—Albinos | MS—Transplanted Plantlets |
|---|---|---|---|---|---|
| Induction medium | 1 | 4145.636 * | 159.1225 ns | 72.7273 * | 210.182 * |
| Genotype | 8 | 35,588.25 *** | 2926.648 *** | 42.2263 ** | 1019.25 *** |
| Interaction | 8 | 721.4058 ns | 94.3030 ns | 23.0884 ns | 103.5818 ** |
| Error | 54 | 910.6944 | 99.4634 | 12.5278 | 39.2424 |
| Source of Variance | df | MS—Plant Regeneration Efficiency | MS—Green Plant Regeneration Efficiency |
|---|---|---|---|
| Genotype | 8 | 234.7103 *** | 285.0326 *** |
| Induction medium | 1 | 3.2918 ns | 23.9410 ns |
| Error | 8 | 26.5776 | 19.6739 |
| Genotype | Number of Transplanted Plantlets | Haploids | Spontaneous DH | Percentage of Spontaneous Chromosome Doubling * (%) |
|---|---|---|---|---|
| ‘Hungaro’ | 175 | 131 | 44 | 25.1 |
| ‘H × Sz’ | 26 | 14 | 12 | 46.2 |
| ‘H × D’ | 43 | 14 | 29 | 67.4 |
| ‘GK Szemes’ | 3 | 2 | 1 | 33.3 |
| ‘Sz × H’ | 82 | 64 | 18 | 22.0 |
| ‘Sz × D’ | 36 | 29 | 7 | 19.4 |
| ‘Dimenzio’ | 7 | 5 | 2 | 28.6 |
| ‘D × H’ | 87 | 62 | 25 | 28.7 |
| ‘D × Sz’ | 55 | 42 | 13 | 23.6 |
| ‘H × R’ | 351 | 256 | 95 | 27.1 |
| ‘D × R’ | 187 | 125 | 62 | 33.2 |
| Total | 1052 | 744 | 308 | 29.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruppa, J.; Kanbar, O.Z.; Tóth-Lencsés, K.A.; Kiss, E.; Bóna, L.; Lantos, C.; Pauk, J. Induction of Triticale (×Triticosecale Wittmack) In Vitro Androgenesis in Anther Cultures of F1 Hybrid Combinations, Varieties and Homogeneity Testing of Offspring Generation. Life 2023, 13, 1970. https://doi.org/10.3390/life13101970
Kruppa J, Kanbar OZ, Tóth-Lencsés KA, Kiss E, Bóna L, Lantos C, Pauk J. Induction of Triticale (×Triticosecale Wittmack) In Vitro Androgenesis in Anther Cultures of F1 Hybrid Combinations, Varieties and Homogeneity Testing of Offspring Generation. Life. 2023; 13(10):1970. https://doi.org/10.3390/life13101970
Chicago/Turabian StyleKruppa, József, Osama Zuhair Kanbar, Kitti Andrea Tóth-Lencsés, Erzsébet Kiss, Lajos Bóna, Csaba Lantos, and János Pauk. 2023. "Induction of Triticale (×Triticosecale Wittmack) In Vitro Androgenesis in Anther Cultures of F1 Hybrid Combinations, Varieties and Homogeneity Testing of Offspring Generation" Life 13, no. 10: 1970. https://doi.org/10.3390/life13101970
APA StyleKruppa, J., Kanbar, O. Z., Tóth-Lencsés, K. A., Kiss, E., Bóna, L., Lantos, C., & Pauk, J. (2023). Induction of Triticale (×Triticosecale Wittmack) In Vitro Androgenesis in Anther Cultures of F1 Hybrid Combinations, Varieties and Homogeneity Testing of Offspring Generation. Life, 13(10), 1970. https://doi.org/10.3390/life13101970







