Extracellular Vesicles of Porphyromonas gingivalis Disrupt Trophoblast Cell Interaction with Vascular and Immune Cells in an In Vitro Model of Early Placentation
Abstract
:1. Introduction
2. Materials and Methods
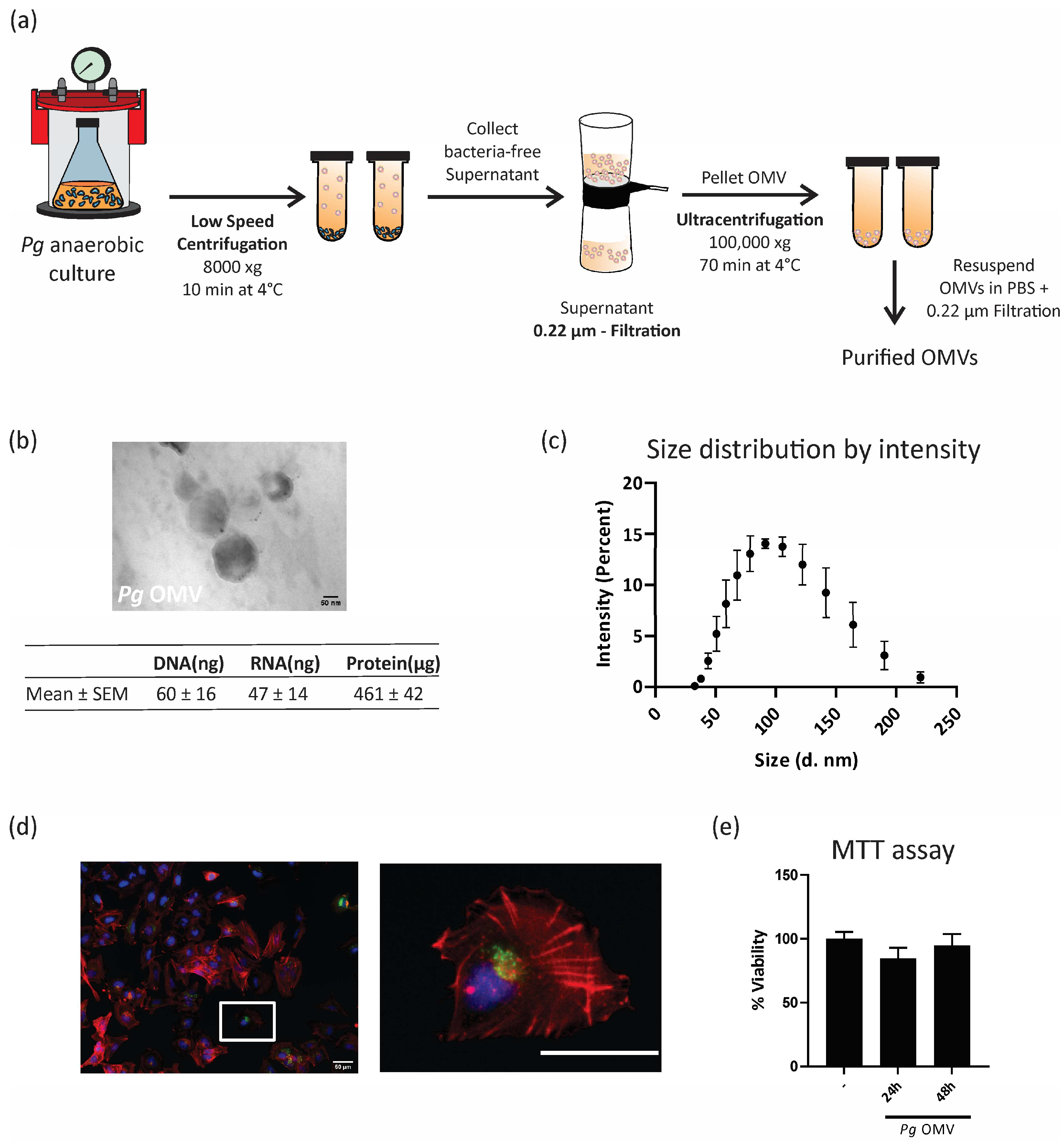
2.1. Porphyromonas gingivalis Outer Membrane Vesicle Isolation
2.2. Trophoblast, THP-1 Monocyte, and Endothelial Cell Cultures
2.3. Confocal Microscopy
2.4. Trophoblast-Conditioned Media Preparation
2.5. Neutrophil Isolation
2.6. Neutrophil Migration Assay
2.7. Reactive Oxygen Species (ROS) Production
2.8. qPCR
2.9. Antioxidant Enzyme Activity
2.10. MTT Assay
2.11. Endothelial Cell Migration
2.12. Monocyte Adhesion Assay
2.13. Statistical Analysis
3. Results
3.1. Porphyromonas gingivalis Outer Membrane Vesicle Isolation and Internalization by Trophoblast Cells
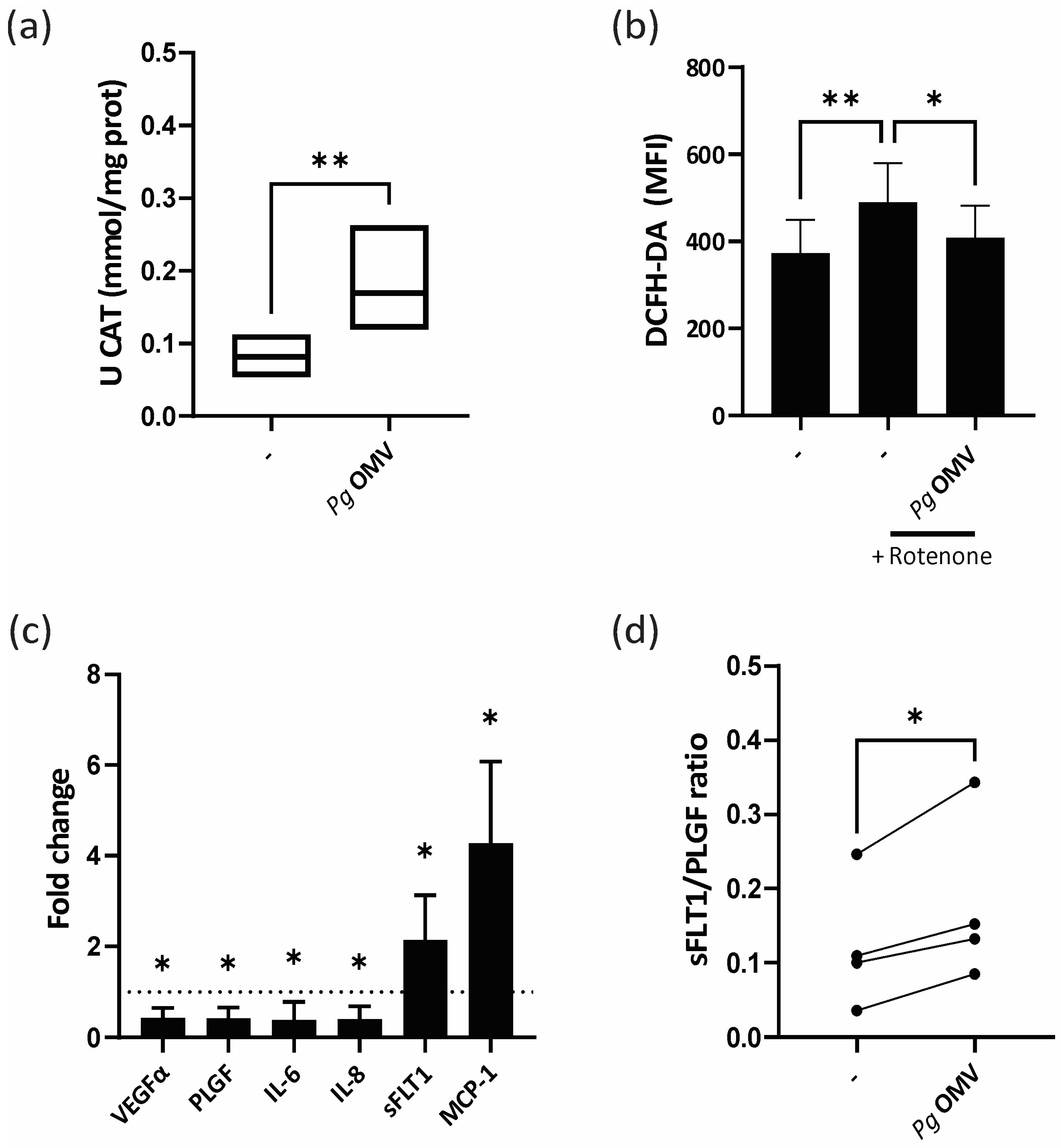
3.2. Pg OMVs Modulate Trophoblast Cell Oxidative State and Unbalance Trophoblast Marker Expression
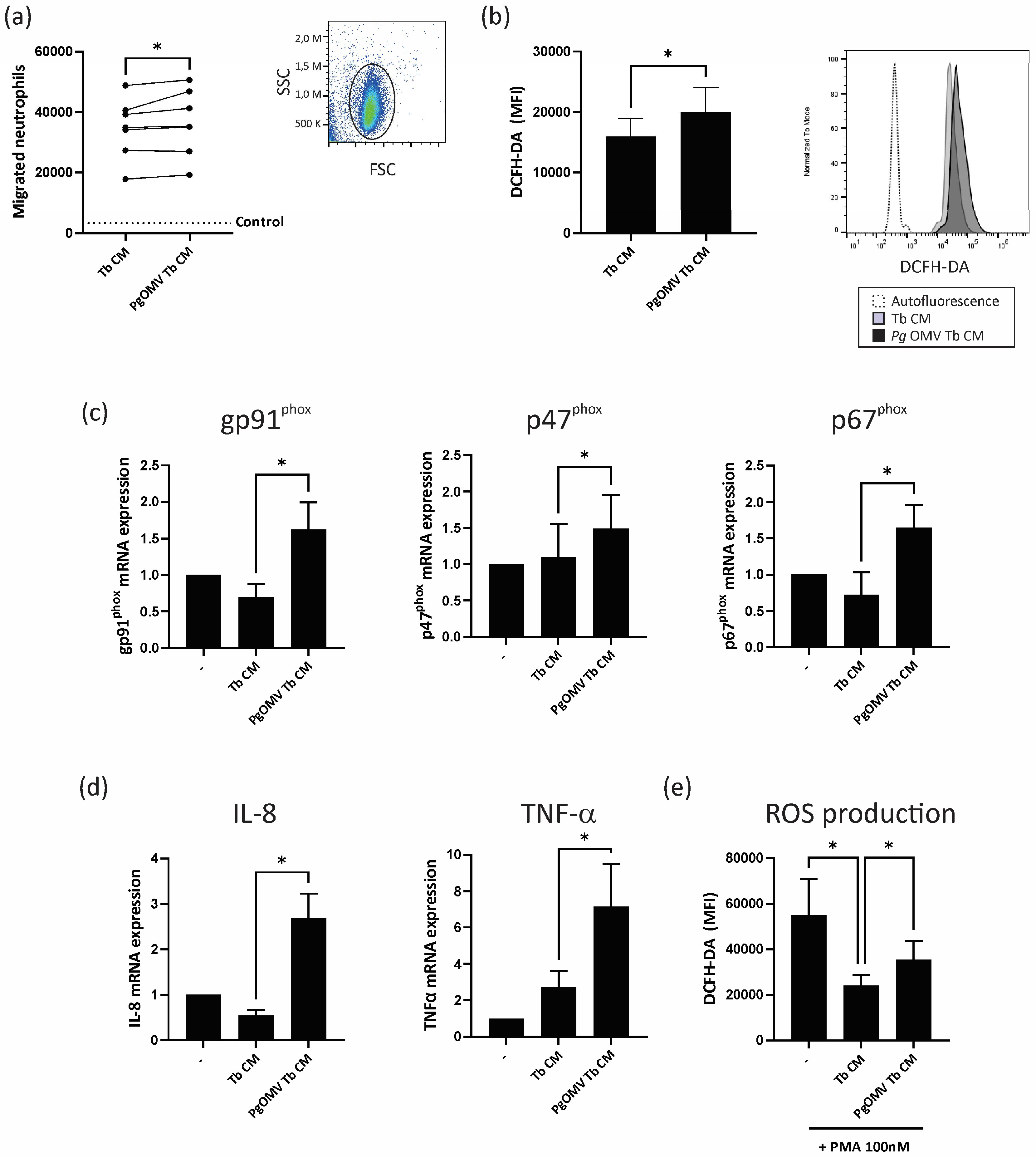
3.3. Trophoblast Cells Primed with Pg OMVs Enhance Neutrophil Chemoattraction and Activation
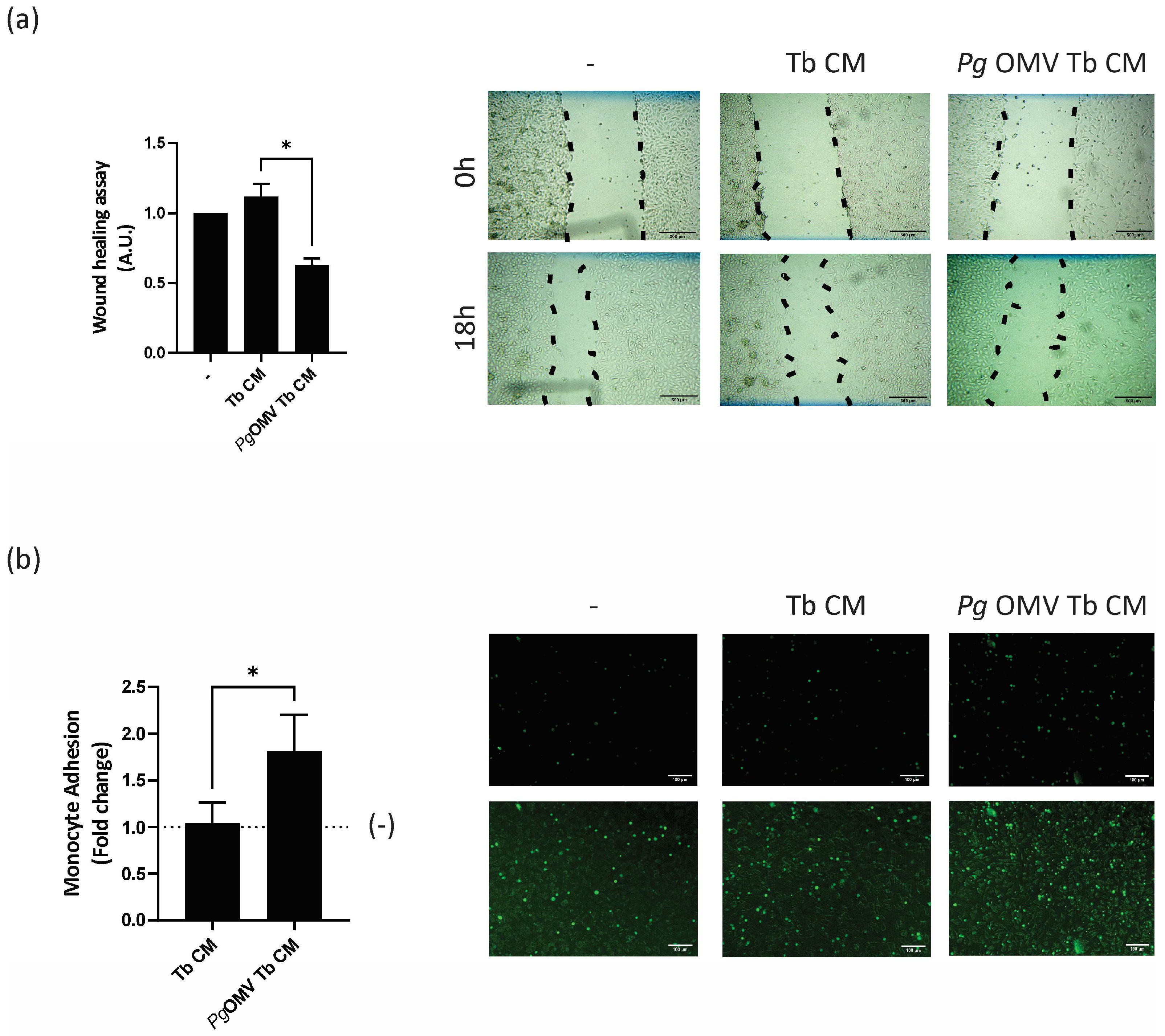
3.4. Trophoblast Cells Primed with Pg OMVs Impair Endothelial Cell Migration and Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuehn, M.J.; Kesty, N.C. Bacterial Outer Membrane Vesicles and the Host–Pathogen Interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.J.; Dashper, S.G.; Slakeski, N.; Chen, Y.-Y.; Reynolds, E.C. Spheres of Influence: Porphyromonas Gingivalis Outer Membrane Vesicles. Mol. Oral Microbiol. 2016, 31, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Alpdundar Bulut, E.; Bayyurt Kocabas, B.; Yazar, V.; Aykut, G.; Guler, U.; Salih, B.; Surucu Yilmaz, N.; Ayanoglu, I.C.; Polat, M.M.; Akcali, K.C.; et al. Human Gut Commensal Membrane Vesicles Modulate Inflammation by Generating M2-like Macrophages and Myeloid-Derived Suppressor Cells. J. Immunol. 2020, 205, 2707–2718. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. The Inflammophilic Character of the Periodontitis-Associated Microbiota. Mol. Oral Microbiol. 2014, 29, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, N.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Pathogenesis of Important Virulence Factors of Porphyromonas Gingivalis via Toll-Like Receptors. Front. Cell. Infect. Microbiol. 2019, 9, 262. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The Role of Porphyromonas Gingivalis Outer Membrane Vesicles in Periodontal Disease and Related Systemic Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 585917. [Google Scholar] [CrossRef]
- Seyama, M.; Yoshida, K.; Yoshida, K.; Fujiwara, N.; Ono, K.; Eguchi, T.; Kawai, H.; Guo, J.; Weng, Y.; Haoze, Y.; et al. Outer Membrane Vesicles of Porphyromonas Gingivalis Attenuate Insulin Sensitivity by Delivering Gingipains to the Liver. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165731. [Google Scholar] [CrossRef]
- Farrugia, C.; Stafford, G.P.; Murdoch, C. Porphyromonas Gingivalis Outer Membrane Vesicles Increase Vascular Permeability. J. Dent. Res. 2020, 99, 1494–1501. [Google Scholar] [CrossRef]
- Yoshida, S.; Hatasa, M.; Ohsugi, Y.; Tsuchiya, Y.; Liu, A.; Niimi, H.; Morita, K.; Shimohira, T.; Sasaki, N.; Maekawa, S.; et al. Porphyromonas Gingivalis Administration Induces Gestational Obesity, Alters Gene Expression in the Liver and Brown Adipose Tissue in Pregnant Mice, and Causes Underweight in Fetuses. Front. Cell. Infect. Microbiol. 2022, 11, 745117. [Google Scholar] [CrossRef]
- Yang, I.; Claussen, H.; Arthur, R.A.; Hertzberg, V.S.; Geurs, N.; Corwin, E.J.; Dunlop, A.L. Subgingival Microbiome in Pregnancy and a Potential Relationship to Early Term Birth. Front. Cell. Infect. Microbiol. 2022, 12, 873683. [Google Scholar] [CrossRef]
- Daalderop, L.A.; Wieland, B.V.; Tomsin, K.; Reyes, L.; Kramer, B.W.; Vanterpool, S.F.; Been, J.V. Periodontal Disease and Pregnancy Outcomes: Overview of Systematic Reviews. JDR Clin. Transl. Res. 2018, 3, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.-A.; Akhter, R.; Coulton, K.M.; Vo, N.T.N.; Duong, L.T.Y.; Nong, H.V.; Yaacoub, A.; Condous, G.; Eberhard, J.; Nanan, R. Periodontitis and Preeclampsia in Pregnancy: A Systematic Review and Meta-Analysis. Matern. Child Health J. 2022, 26, 2419–2443. [Google Scholar] [CrossRef] [PubMed]
- Madianos, P.N.; Bobetsis, Y.A.; Offenbacher, S. Adverse Pregnancy Outcomes (APOs) and Periodontal Disease: Pathogenic Mechanisms. J. Clin. Periodontol. 2013, 40, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Phillips, P.; Wolfe, B.; Golos, T.G.; Walkenhorst, M.; Progulske-Fox, A.; Brown, M. Porphyromonas Gingivalis and Adverse Pregnancy Outcome. J. Oral Microbiol. 2017, 9, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Radhakrishnan, R.; Sharma, M. Porphyromonas Gingivalis and Adverse Pregnancy Outcomes: A Review on Its Intricate Pathogenic Mechanisms. Crit. Rev. Microbiol. 2020, 46, 213–236. [Google Scholar] [CrossRef]
- Aplin, J.D.; Myers, J.E.; Timms, K.; Westwood, M. Tracking Placental Development in Health and Disease. Nat. Rev. Endocrinol. 2020, 16, 479–494. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The Unique Immunological and Microbial Aspects of Pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Huppertz, B. Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. Int. J. Mol. Sci. 2020, 21, 289. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human Placenta and Trophoblast Development: Key Molecular Mechanisms and Model Systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Ramhorst, R.; Calo, G.; Paparini, D.; Vota, D.; Hauk, V.; Gallino, L.; Merech, F.; Grasso, E.; Leirós, C.P. Control of the Inflammatory Response during Pregnancy: Potential Role of VIP as a Regulatory Peptide. Ann. N. Y. Acad. Sci. 2019, 1437, 15–21. [Google Scholar] [CrossRef]
- Calo, G.; Sabbione, F.; Vota, D.; Paparini, D.; Ramhorst, R.; Trevani, A.; Leirós, C.P. Trophoblast Cells Inhibit Neutrophil Extracellular Trap Formation and Enhance Apoptosis through Vasoactive Intestinal Peptide-Mediated Pathways. Hum. Reprod. 2017, 32, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Calo, G.; Sabbione, F.; Pascuali, N.; Keitelman, I.; Vota, D.; Paparini, D.; Ramhorst, R.; Parborell, F.; Trevani, A.; Leirós, C.P. Interplay between Neutrophils and Trophoblast Cells Conditions Trophoblast Function and Triggers Vascular Transformation Signals. J. Cell. Physiol. 2020, 235, 3592–3603. [Google Scholar] [CrossRef] [PubMed]
- Weckman, A.M.; Ngai, M.; Wright, J.; McDonald, C.R.; Kain, K.C. The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes. Front. Microbiol. 2019, 10, 1924. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Khanipov, K.; Radnaa, E.; Ganguly, E.; Bento, G.F.C.; Urrabaz-Garza, R.; Kammala, A.K.; Yaklic, J.; Pyles, R.; Golovko, G.; et al. Amplification of Microbial DNA from Bacterial Extracellular Vesicles from Human Placenta. Front. Microbiol. 2023, 14, 1213234. [Google Scholar] [CrossRef]
- Hauk, V.; D’Eramo, L.; Calo, G.; Merech, F.; Doga, L.; Lara, B.; Gliosca, L.; Massone, C.; Molgatini, S.; Ramhorst, R.; et al. Gingival Crevicular Fluid from Pregnant Women Impairs Trophoblast Cell Function and Trophoblast-neutrophil Interaction. Am. J. Reprod. Immunol. 2022, 88, e13558. [Google Scholar] [CrossRef] [PubMed]
- Paparini, D.E.; Choudhury, R.H.; Vota, D.M.; Karolczak-Bayatti, M.; Finn-Sell, S.; Grasso, E.N.; Hauk, V.C.; Ramhorst, R.; Pérez Leirós, C.; Aplin, J.D. Vasoactive Intestinal Peptide Shapes First-Trimester Placenta Trophoblast, Vascular, and Immune Cell Cooperation. Br. J. Pharmacol. 2019, 176, 964–980. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Andrioli, N.B.; Nieves, M.; Poltronieri, M.; Bonzon, C.; Chaufan, G. Genotoxic Effects Induced for Sub-Cytotoxic Concentrations of Tebuconazole Fungicide in HEp-2 Cell Line. Chem. Biol. Interact. 2023, 373, 110385. [Google Scholar] [CrossRef]
- Wu, J.; Lin, X.; Xie, H. OxyR Is Involved in Coordinate Regulation of Expression of FimA and Sod Genes in Porphyromonas Gingivalis. FEMS Microbiol. Lett. 2008, 282, 188–195. [Google Scholar] [CrossRef]
- Rocha, E.R.; Owens, G.; Smith, C.J. The Redox-Sensitive Transcriptional Activator OxyR Regulates the Peroxide Response Regulon in the Obligate Anaerobe Bacteroides Fragilis. J. Bacteriol. 2000, 182, 5059–5069. [Google Scholar] [CrossRef]
- Crapo, J.D. Oxidative Stress as an Initiator of Cytokine Release and Cell Damage. Eur. Respir. J. 2003, 22, 4s–6s. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the SFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Giaglis, S.; Hoesli, I.; Hasler, P. Neutrophil NETs in Reproduction: From Infertility to Preeclampsia and the Possibility of Fetal Loss. Front. Immunol. 2012, 3, 362. [Google Scholar] [CrossRef] [PubMed]
- Petty, H.R.; Kindzelskii, A.L.; Espinoza, J.; Romero, R. Trophoblast Contact Deactivates Human Neutrophils. J. Immunol. 2014, 176, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Paclet, M.-H.; Laurans, S.; Dupré-Crochet, S. Regulation of Neutrophil NADPH Oxidase, NOX2: A Crucial Effector in Neutrophil Phenotype and Function. Front. Cell Dev. Biol. 2022, 10, 945749. [Google Scholar] [CrossRef] [PubMed]
- Jenney, F.E.; Verhagen, M.F.J.M.; Cui, X.; Adams, M.W.W. Anaerobic Microbes: Oxygen Detoxification Without Superoxide Dismutase. Science 1999, 286, 306–309. [Google Scholar] [CrossRef]
- Lu, Z.; Imlay, J.A. When Anaerobes Encounter Oxygen: Mechanisms of Oxygen Toxicity, Tolerance and Defence. Nat. Rev. Microbiol. 2021, 19, 774–785. [Google Scholar] [CrossRef]
- Knowles, A.A.; Campbell, S.G.; Cross, N.A.; Stafford, P. Dysregulation of Stress-Induced Translational Control by Porphyromonas Gingivalis in Host Cells. Microorganisms 2023, 11, 606. [Google Scholar] [CrossRef]
- Amsalem, H.; Kwan, M.; Hazan, A.; Zhang, J.; Jones, R.L.; Whittle, W.; Kingdom, J.C.P.; Croy, B.A.; Lye, S.J.; Dunk, C.E. Identification of a Novel Neutrophil Population: Proangiogenic Granulocytes in Second-Trimester Human Decidua. J. Immunol. 2014, 193, 3070–3079. [Google Scholar] [CrossRef]
- Giaglis, S.; Stoikou, M.; Grimolizzi, F.; Subramanian, B.Y.; van Breda, S.V.; Hoesli, I.; Lapaire, O.; Hasler, P.; Than, N.G.; Hahn, S. Neutrophil Migration into the Placenta: Good, Bad or Deadly? Cell Adhes. Migr. 2016, 10, 208–225. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence | |
|---|---|---|
| VEGF-a | Sense | CGCAGCTACTGCCATCCAAT |
| Antisense | GTGAGGTTTGATCCGCATAATCT | |
| PLGF | Sense | TGTTCAGCCCATCCTGTGTCTC |
| Antisense | CCCAGAACGAACGGATCTTTAGG | |
| IL-6 | Sense | TTCGGTACATCCTCGACGGC |
| Antisense | TCACCAGGCAAGTCTCCTCA | |
| IL-8 | Sense | CCAACACAGAAATTATTGTAAAGC |
| Antisense | CACTGGCATCTTCACTGATTC | |
| sFLT-1 | Sense | AGAGGTGAGCACTGCAACAA |
| Antisense | TCTCCTCCGAGCCTGAAAGT | |
| MCP-1 | Sense | CAGCAGCAAGTGTCCCAAAG |
| Antisense | GAGTGAGTGTTCAAGTCTTCGG | |
| TNF-α | Sense | GCCTCTTCTCCTTCCTGATCG |
| Antisense | CAGCTTGAGGGTTTGCTACA | |
| GP91phox | Sense | ACATTCAACCTCTGCCACCAT |
| Antisense | ACCCCAGCCAAACCAGAATG | |
| P47phox | Sense | CCCACGGACAACCAGACAAA |
| Antisense | TCTGACAGAACCACCAACCG | |
| P67phox | Sense | CTGTTTGCCTGTGAGGTGTT |
| Antisense | AGACACACTCCATCGCCTTG | |
| YHWAZ | Sense | CAGAGAGAAAATTGAGACGGAGC |
| Antisense | GTGACTGATCGACAATCCCTTTC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, B.; Sassot, M.; Calo, G.; Paparini, D.; Gliosca, L.; Chaufan, G.; Loureiro, I.; Vota, D.; Ramhorst, R.; Pérez Leirós, C.; et al. Extracellular Vesicles of Porphyromonas gingivalis Disrupt Trophoblast Cell Interaction with Vascular and Immune Cells in an In Vitro Model of Early Placentation. Life 2023, 13, 1971. https://doi.org/10.3390/life13101971
Lara B, Sassot M, Calo G, Paparini D, Gliosca L, Chaufan G, Loureiro I, Vota D, Ramhorst R, Pérez Leirós C, et al. Extracellular Vesicles of Porphyromonas gingivalis Disrupt Trophoblast Cell Interaction with Vascular and Immune Cells in an In Vitro Model of Early Placentation. Life. 2023; 13(10):1971. https://doi.org/10.3390/life13101971
Chicago/Turabian StyleLara, Brenda, Matías Sassot, Guillermina Calo, Daniel Paparini, Laura Gliosca, Gabriela Chaufan, Iñaki Loureiro, Daiana Vota, Rosanna Ramhorst, Claudia Pérez Leirós, and et al. 2023. "Extracellular Vesicles of Porphyromonas gingivalis Disrupt Trophoblast Cell Interaction with Vascular and Immune Cells in an In Vitro Model of Early Placentation" Life 13, no. 10: 1971. https://doi.org/10.3390/life13101971
APA StyleLara, B., Sassot, M., Calo, G., Paparini, D., Gliosca, L., Chaufan, G., Loureiro, I., Vota, D., Ramhorst, R., Pérez Leirós, C., & Hauk, V. (2023). Extracellular Vesicles of Porphyromonas gingivalis Disrupt Trophoblast Cell Interaction with Vascular and Immune Cells in an In Vitro Model of Early Placentation. Life, 13(10), 1971. https://doi.org/10.3390/life13101971






