A Rare Gastric Subepithelial Lesion Removed through Submucosal Tunneling Endoscopic Resection: Case Report and Literature Review
Abstract
1. Introduction
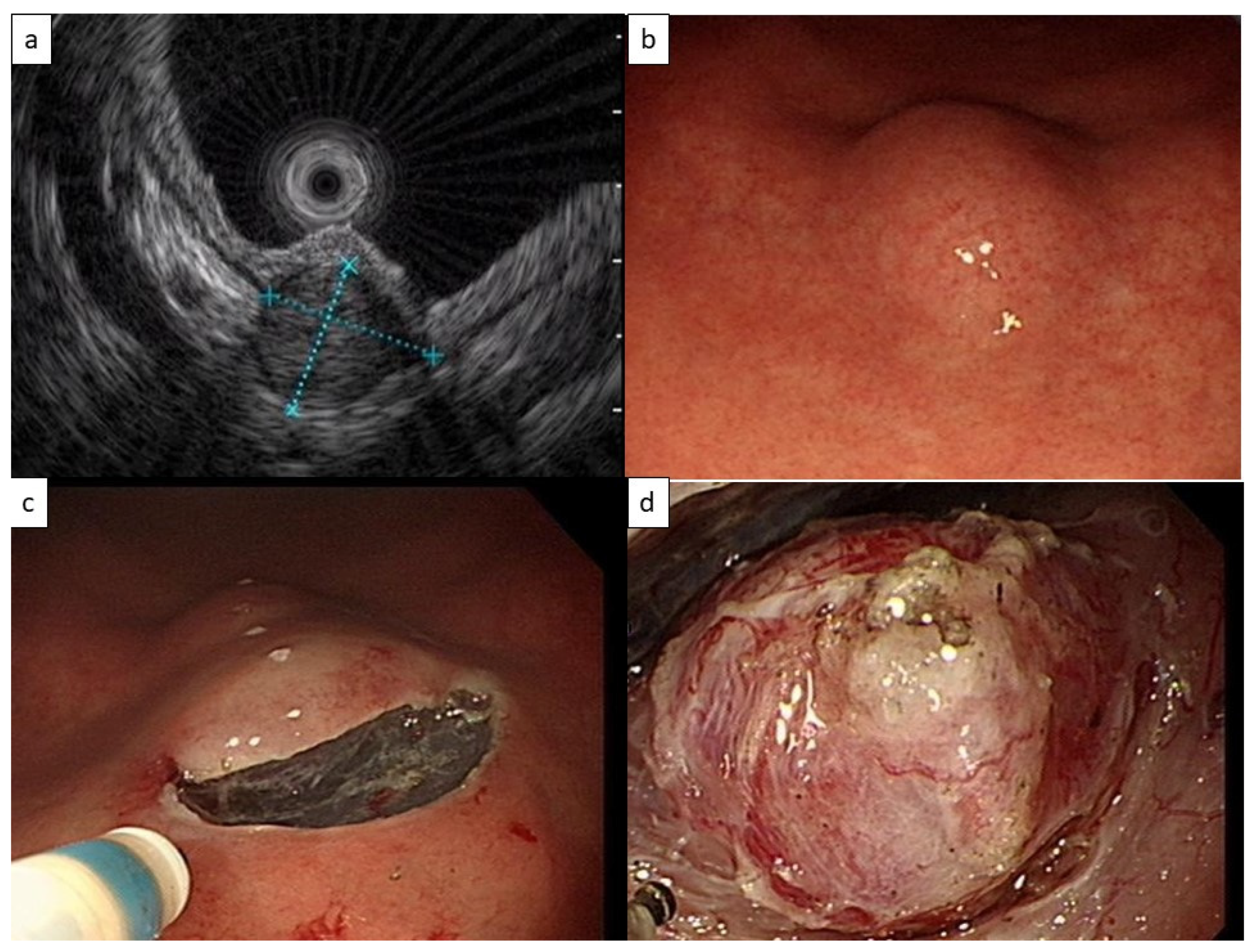
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H.; Lee, H.L.; Ahn, Y.W.; Lee, K.N.; Jun, D.W.; Lee, O.Y.; Han, D.S.; Yoon, B.C.; Choi, H.S. Prevalence of Gastric Subepithelial Tumors in Korea: A Single Center Experience. Korean J. Gastroenterol. 2015, 66, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Son, H.J.; Lee, J.S.; Byun, Y.H.; Suh, H.J.; Rhee, P.L.; Kim, J.J.; Rhee, J.C. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J. Gastroenterol. 2010, 16, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 2005, 37, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Deprez, P.H.; Moons, L.M.G.; O’Toole, D.; Gincul, R.; Seicean, A.; Pimentel-Nunes, P.; Fernandez-Esparrach, G.; Polkowski, M.; Vieth, M.; Borbath, I.; et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 412–429. [Google Scholar] [CrossRef] [PubMed]
- Standards of Practice Committee; Faulx, A.L.; Kothari, S.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Gurudu, S.R.; et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest. Endosc. 2017, 85, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Tsagkataki, E.S.; Flamourakis, M.E.; Gkionis, I.G.; Giakoumakis, M.I.; Delimpaltadakis, G.N.; Kazamias, G.M.; Giannikaki, E.S.; Christodoulakis, M.S. Gastric glomus tumor: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- ASGE Technology Committee; Aslanian, H.R.; Sethi, A.; Bhutani, M.S.; Goodman, A.J.; Krishnan, K.; Lichtenstein, D.R.; Melson, J.; Navaneethan, U.; Pannala, R.; et al. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE 2019, 4, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sharzehi, K.; Sethi, A.; Savides, T. AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Gastrointestinal Stromal Tumors (GISTs) (Version 2.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf (accessed on 2 December 2022).
- Koo, D.H.; Ryu, M.H.; Kim, K.M.; Yang, H.K.; Sawaki, A.; Hirota, S.; Zheng, J.; Zhang, B.; Tzen, C.Y.; Yeh, C.N.; et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res. Treat. 2016, 48, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Hirota, S.; Yanagisawa, A.; Sugino, Y.; Minami, M.; Yamamura, Y.; Otani, Y.; Shimada, Y.; Takahashi, F.; Kubota, T.; et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int. J. Clin. Oncol. 2008, 13, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Hasuda, H.; Hu, Q.; Miyashita, Y.; Zaitsu, Y.; Tsuda, Y.; Hisamatsu, Y.; Nakashima, Y.; Ando, K.; Kimura, Y.; Yamada, Y.; et al. Gastric glomus tumor with a preoperative diagnosis by endoscopic ultrasonography-guided fine needle aspiration: A case report. Int. Cancer Conf. J. 2021, 10, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Pansa, A.; Sama, L.; Ruspi, L.; Sicoli, F.; Cananzi, F.C.M.; Quagliuolo, V. Glomus tumor of the stomach: A systematic review and illustrative case report. Dig. Dis. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L.; Fanburg-Smith, J.C.; Miettinen, M.; Weiss, S.W. Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am. J. Surg. Pathol. 2001, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Papke, D.J., Jr.; Sholl, L.M.; Doyle, L.A.; Fletcher, C.D.M.; Hornick, J.L. Gastroesophageal Glomus Tumors: Clinicopathologic and Molecular Genetic Analysis of 26 Cases With a Proposal for Malignancy Criteria. Am. J. Surg. Pathol. 2022, 46, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ge, N.; Wang, S.; Liu, X.; Guo, J.; Wang, G.; Sun, S. The Role of Endoscopic Ultrasound and Endoscopic Resection for Gastric Glomus: A Case Series and Literature Review. J. Transl. Int. Med. 2019, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, P.; Xu, M.; Chen, W.; Li, Q.; Ji, Y.; Yao, L. Endoscopic diagnosis and treatment of gastric glomus tumors. Gastrointest. Endosc. 2011, 73, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jiang, X.M.; He, Y.L.; Zhang, Y.L.; Xu, M.D.; Yao, L.Q. Glomus tumor of the stomach: A case treated by endoscopic submucosal dissection. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Ojima, T.; Takifuji, K.; Nakamura, M.; Nakamori, M.; Hayata, K.; Kitadani, J.; Yamaue, H. Endoscopic submucosal tunnel dissection versus conventional endoscopic submucosal dissection for early gastric cancers: Outcomes of 799 consecutive cases in a single institution. Surg. Endosc. 2020, 34, 5625–5631. [Google Scholar] [CrossRef] [PubMed]

| Society, Year | Timing for Tissue Acquisition | Gastric GIST, <20 mm, without High-Risk Features 1 | Gastric SELs with Unclear Diagnosis |
|---|---|---|---|
| ESGE, 2022 [4] |
| Surveillance or resection | <10 mm EGD at 3–6 months, then at 2–3 years interval 10–20 mm EGD at 3–6 months, then at 1–2 years interval. Diagnostic resection is an alternative for SELs <20 mm after failure of attempts to obtain diagnosis >20 mm EGD + EUS at 6 months, then at 6–12 months interval |
| AGA, 2022 [8] | Lesion arising from muscularis propria layer | Surveillance with EUS, 1 year interval | Not specifically mentioned |
| ESMO–EURACAN–GENTURIS, 2022 [9] | Size > 20 mm | Resection. Surveillance is an alternative | <20 mm Active surveillance. short interval (e.g., 3 months) then increased interval. Resection as an alternative ≥ 20 mm Biopsy/excision |
| NCCN, 2022 [10] | When surgical resection or oncological treatment is required | Periodic endoscopic or radiographic surveillance. Risk and benefit should be discussed with the patient | Not specifically mentioned |
| ASGE, 2017 [5] | Lesions arising from submucosal or muscularis propria layer | Surveillance with EUS, 6–12 months interval | Removal as an alternative to tissue acquisition |
| Asian consensus guidelines for GIST, 2016 [11] | When surgical resection or oncological treatment is required | Resection. Surveillance is an alternative after informing the risk of malignancy | Not specifically mentioned |
| Japan GIST guideline subcommittee, 2008 [12] | Not specifically mentioned | Resection | <20 mm EGD at 6–12 months interval. When tumor growth or high-risk feature is noted, further examination is suggested, while resection is an alternative 20–50 mm Meticulous examinations with CT, EUS, and EUS-FNAB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, M.-M.; Lin, Y.-H.; Chang, C.-C.; Chien, H.-Y. A Rare Gastric Subepithelial Lesion Removed through Submucosal Tunneling Endoscopic Resection: Case Report and Literature Review. Life 2023, 13, 179. https://doi.org/10.3390/life13010179
Chien M-M, Lin Y-H, Chang C-C, Chien H-Y. A Rare Gastric Subepithelial Lesion Removed through Submucosal Tunneling Endoscopic Resection: Case Report and Literature Review. Life. 2023; 13(1):179. https://doi.org/10.3390/life13010179
Chicago/Turabian StyleChien, Mu-Ming, Yun-Ho Lin, Chun-Chao Chang, and Hsi-Yuan Chien. 2023. "A Rare Gastric Subepithelial Lesion Removed through Submucosal Tunneling Endoscopic Resection: Case Report and Literature Review" Life 13, no. 1: 179. https://doi.org/10.3390/life13010179
APA StyleChien, M.-M., Lin, Y.-H., Chang, C.-C., & Chien, H.-Y. (2023). A Rare Gastric Subepithelial Lesion Removed through Submucosal Tunneling Endoscopic Resection: Case Report and Literature Review. Life, 13(1), 179. https://doi.org/10.3390/life13010179






