Spherocytosis-Related L1340P Mutation in Ankyrin Affects Its Interactions with Spectrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids and Bacterial Strains
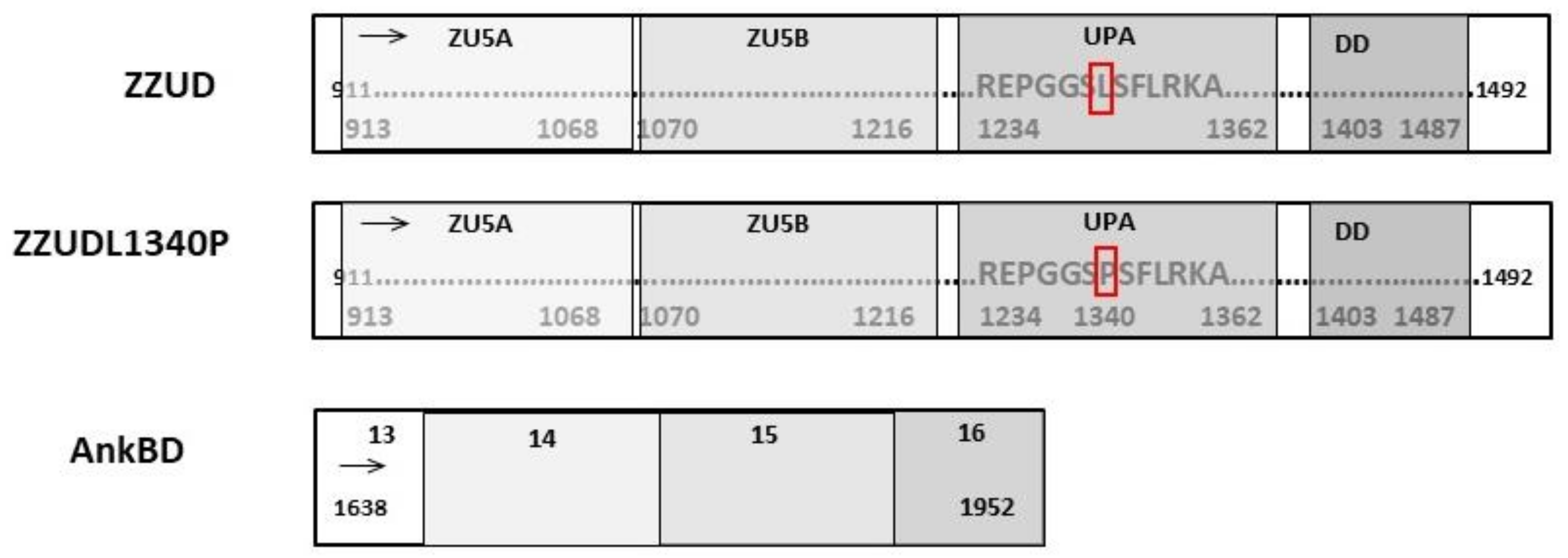
2.2. Cloning, Expression, and Purification of His(6)GFP–AnkBD and GST-ZZUD Fragments and the L1340P Mutant
2.3. CD Analysis
2.4. BLI Method
2.5. Sedimentation Assay
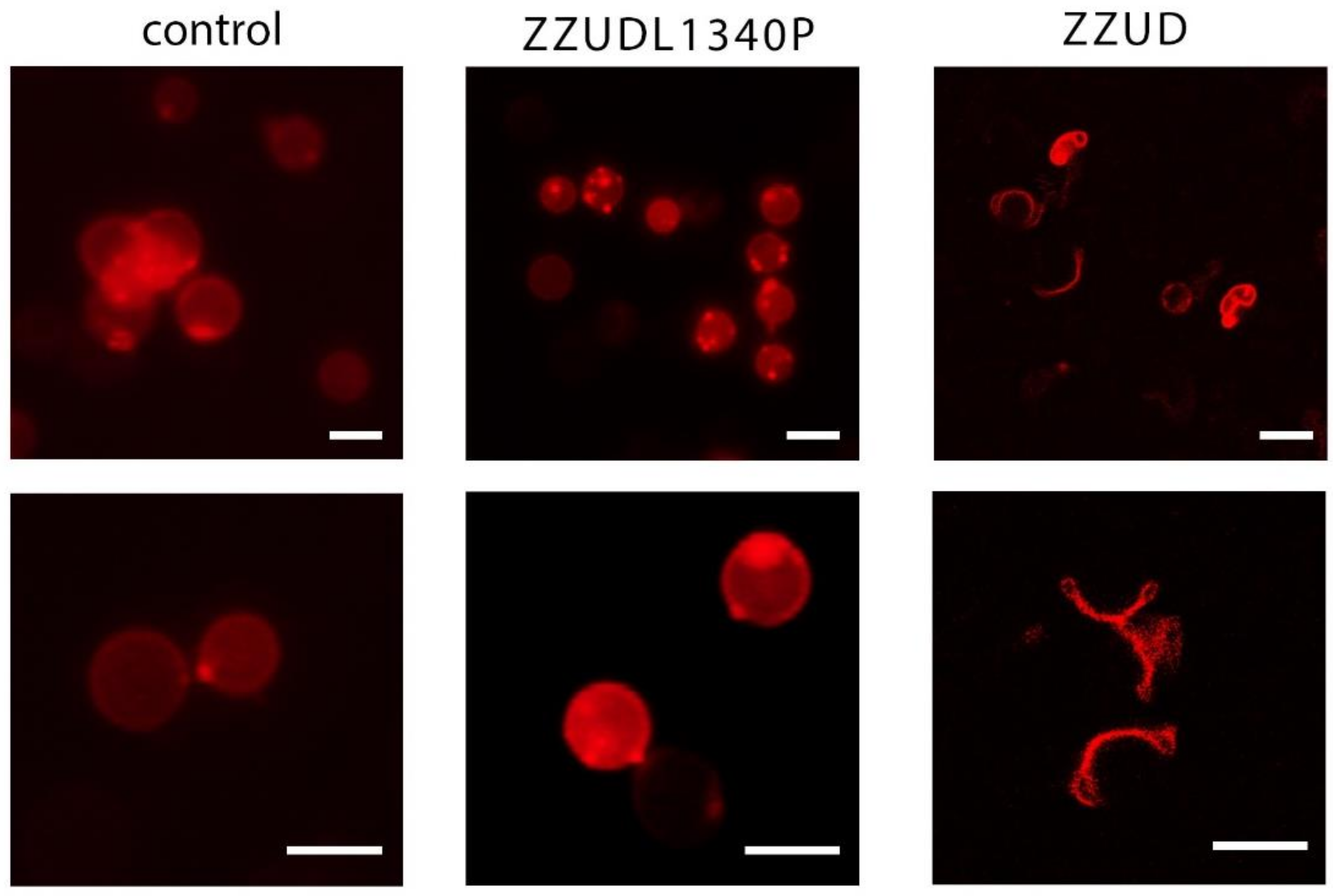
2.6. Resealed Erythrocyte Ghost Assay
2.7. Cloning of ZZUD and Its L1340P Mutant Domain into mEGPF–C1 and mRFP-C1 Plasmids
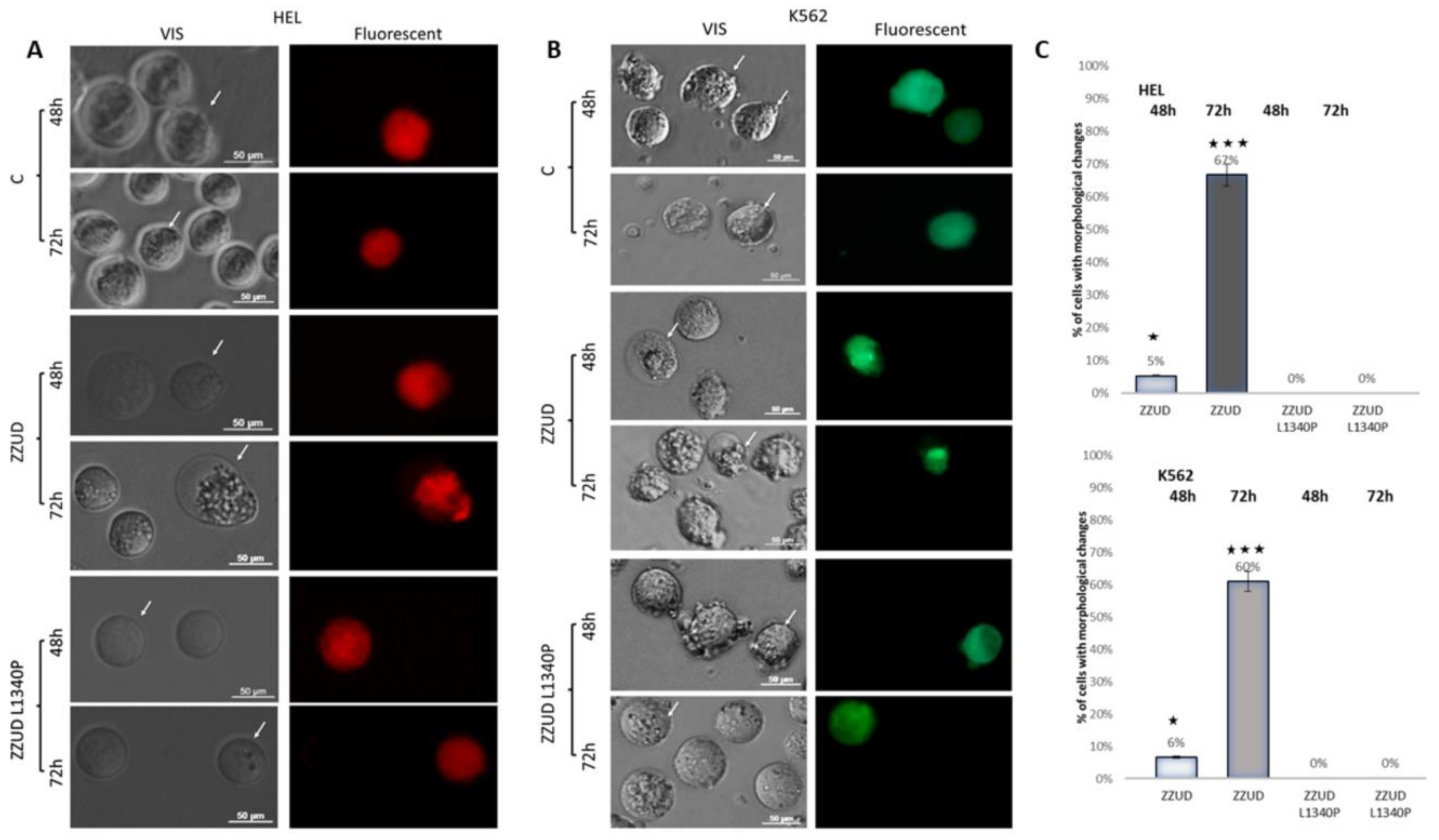
2.8. HEL and K562 Cell Transfection
3. Results
3.1. Binding Studies of Ankyrin and Spectrin
3.2. Effect of Mutations on the Morphology of Resealed Erythrocyte Ghosts
3.3. Effects of Mutations on the Shape of Transiently Transfected HEL and K562 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narla, J.; Mohandas, N. Red cell membrane disorders. Int. J. Lab. Hem. 2017, 39, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugi, J.; Drury, L.; Langer, J.; Butchart, S.; Fantauzzi, M.; Baker, J.; Blanchette, V.; Kirby-Allen, M.; Carcao, M. Genotype/Phenotype Correlations in 103 Children from 87 Families with Hereditary Spherocytosis. Blood 2016, 128, 2432. [Google Scholar] [CrossRef]
- Mohandas, N. Inherited hemolytic anemia: A possessive beginner’s guide. Hematology 2018, 2018, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ipsaro, J.; Huang, L.; Mondragón, A. Structures of the spectrin-ankyrin interaction binding domains. Blood 2009, 113, 5385–5393. [Google Scholar] [CrossRef] [Green Version]
- Ipsaro, J.; Mondragón, A. Structural basis for spectrin recognition by ankyrin. Blood 2010, 115, 4093–4101. [Google Scholar] [CrossRef] [Green Version]
- Kolondra, A.; Grzybek, M.; Chorzalska, A.; Sikorski, A. The 22.5kDa spectrin-binding domain of ankyrinR binds spectrin with high affinity and changes the spectrin distribution in cells in vivo. Protein Expr. Purif. 2008, 60, 157–164. [Google Scholar] [CrossRef]
- Kolondra, A.; Lenoir, M.; Wolny, M.; Czogalla, A.; Overduin, M.; Sikorski, A.; Grzybek, M. The role of hydrophobic interactions in ankyrin–spectrin complex formation. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 2084–2089. [Google Scholar] [CrossRef] [Green Version]
- La-Borde, P.; Stabach, P.; Simonović, I.; Morrow, J.; Simonović, M. Ankyrin recognizes both surface character and shape of the 14–15 di-repeat of β-spectrin. Biochem. Biophys. Res. Commun. 2010, 392, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Czogalla, A.; Sikorski, A. Do we already know how spectrin attracts ankyrin? Cell. Mol. Life Sci. 2010, 67, 2679–2683. [Google Scholar] [CrossRef]
- Bogusławska, D.; Skulski, M.; Machnicka, B.; Potoczek, S.; Kraszewski, S.; Kuliczkowski, K.; Sikorski, A. Identification of a Novel Mutation of β-Spectrin in Hereditary Spherocytosis Using Whole Exome Sequencing. Int. J. Mol. Sci. 2021, 22, 11007. [Google Scholar] [CrossRef]
- Delaunay, J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.; Galimand, J.; Fenneteau, O.; Mohandas, N. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev. 2013, 27, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Asimos, A.; Casella, J.; McMillan, C. Inheritance Pattern and Clinical Response to Splenectomy as a Reflection of Erythrocyte Spectrin Deficiency in Hereditary Spherocytosis. N. Engl. J. Med. 1986, 315, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Bogusławska, D.; Heger, E.; Baldy-Chudzik, K.; Zagulski, M.; Maciejewska, M.; Likwiarz, A.; Sikorski, A. (AC)n microsatellite polymorphism and 14-nucleotide deletion in exon 42 ankyrin-1 gene in several families with hereditary spherocytosis in a population of South-Western Poland. Ann. Hematol. 2006, 85, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Eber, S.; Lux, S. Hereditary spherocytosis—Defects in proteins that connect the membrane skeleton to the lipid bilayer. Semin. Hematol. 2004, 41, 118–141. [Google Scholar] [CrossRef]
- Paździor, G.; Langner, M.; Chmura, A.; Bogusławska, D.; Heger, E.; Chorzalska, A.; Sikorski, A. The kinetics of haemolysis of spherocytic erythrocytes. Cell Mol. Biol. Lett. 2003, 8, 639–648. [Google Scholar]
- Demiralp, D.; Peker, S.; Turgut, B.; Akar, N. Comprehensive identification of erythrocyte membrane protein deficiency by 2D gel electrophoresis based proteomic analysis in hereditary elliptocytosis and spherocytosis. Prot. Clin. Appl. 2012, 6, 403–411. [Google Scholar] [CrossRef]
- Reliene, R.; Mariani, M.; Zanella, A.; Reinhart, W.; Ribeiro, M.; del Giudice, E.; Perrotta, S.; Iolascon, A.; Eber, S.; Lutz, H. Splenectomy prolongs in vivo survival of erythrocytes differently in spectrin/ankyrin- and band 3-deficient hereditary spherocytosis. Blood 2002, 100, 2208–2215. [Google Scholar] [CrossRef]
- King, M.-J.; Zanella, A. Hereditary red cell membrane disorders and laboratory diagnostic testing. Int. J. Lab. Hematol. 2013, 35, 237–243. [Google Scholar] [CrossRef]
- Tse, W.; Lux, S. Red blood cell membrane disorders. Br. J. Haematol. 1999, 104, 2–13. [Google Scholar] [CrossRef]
- Li, H.; Papageorgiou, D.; Chang, H.-Y.; Lu, L.; Yang, J.; Deng, Y. Synergistic Integration of Laboratory and Numerical Approaches in Studies of the Biomechanics of Diseased Red Blood Cells. Biosensors 2018, 8, 76. [Google Scholar] [CrossRef]
- He, B.-J.; Liao, L.; Deng, Z.-F.; Tao, Y.-F.; Xu, Y.-C.; Lin, F.-Q. Molecular Genetic Mechanisms of Hereditary Spherocytosis: Current Perspectives. Acta Haematol. 2018, 139, 60–66. [Google Scholar] [CrossRef]
- Maciag, M.; Płochocka, D.; Adamowicz-Salach, A.; Burzyńska, B. Novel beta-spectrin mutations in hereditary spherocytosis associated with decreased levels of mRNA. Br. J. Haematol. 2009, 146, 326–332. [Google Scholar] [CrossRef]
- Gallagher, P.; Forget, B. Hematologically Important Mutations: Spectrin and Ankyrin Variants in Hereditary Spherocytosis. Blood Cells Mol. Dis. 1998, 24, 539–543. [Google Scholar] [CrossRef]
- Wang, C.; Yu, C.; Ye, F.; Wei, Z.; Zhang, M. Structure of the ZU5-ZU5-UPA-DD tandem of ankyrin-B reveals interaction surfaces necessary for ankyrin function. Proc. Natl. Acad. Sci. USA 2012, 109, 4822–4827. [Google Scholar] [CrossRef] [Green Version]
- Yasunaga, M.; Ipsaro, J.; Mondragón, A. Structurally Similar but Functionally Diverse ZU5 Domains in Human Erythrocyte Ankyrin. J. Mol. Biol. 2012, 417, 336–350. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Liu, S.; Zhou, Z. Structure, dynamics and assembly of the ankyrin complex on human red blood cell membrane. Nat. Struct. Mol. Biol. 2022, 29, 698–705. [Google Scholar] [CrossRef]
- Vallese, F.; Kim, K.; Yen, L.; Johnston, J.; Noble, A.; Calì, T.; Clarke, O. Architecture of the human erythrocyte ankyrin-1 complex. Nat. Struct. Mol. Biol. 2022, 29, 706–718. [Google Scholar] [CrossRef]
- Gallagher, P. Hematologically important mutations: Ankyrin variants in hereditary spherocytosis. Blood Cells Mol. Dis. 2005, 35, 345–347. [Google Scholar] [CrossRef]
- Agarwal, A. Ankyrin Mutations in Hereditary Spherocytosis. Acta Haematol. 2019, 141, 63–64. [Google Scholar] [CrossRef]
- Bogusławska, D.; Heger, E.; Listowski, M.; Wasiński, D.; Kuliczkowski, K.; Machnicka, B.; Sikorski, A. A novel L1340P mutation in the ANK1 gene is associated with hereditary spherocytosis? Br. J. Haematol. 2014, 167, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.; Appel, R.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Bilkova, E.; Pleskot, R.; Rissanen, S.; Sun, S.; Czogalla, A.; Cwiklik, L.; Róg, T.; Vattulainen, I.; Cremer, P.; Jungwirth, P.; et al. Calcium Directly Regulates Phosphatidylinositol 4,5-Bisphosphate Headgroup Conformation and Recognition. J. Am. Chem. Soc. 2017, 139, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Chorzalska, A.; Łach, A.; Borowik, T.; Wolny, M.; Hryniewicz-Jankowska, A.; Kolondra, A.; Langner, M.; Sikorski, A. The effect of the lipid-binding site of the ankyrin-binding domain of erythroid β-spectrin on the properties of natural membranes and skeletal structures. Cell. Mol. Biol. Lett. 2010, 15, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Ipsaro, J.; Huang, L.; Gutierrez, L.; MacDonald, R. Molecular Epitopes of the Ankyrin−Spectrin Interaction. Biochemistry 2008, 47, 7452–7464. [Google Scholar] [CrossRef] [Green Version]
- Chai, S.; Jiao, R.; Sun, X.; Fu, P.; Zhao, Q.; Sang, M. Novel nonsense mutation p. Gln264Ter in the ANK1 confirms causative role for hereditary spherocytosis: A case report. BMC Med. Genet. 2020, 21, 223. [Google Scholar] [CrossRef]
- Hao, L.; Li, S.; Ma, D.; Chen, S.; Zhang, B.; Xiao, D.; Zhang, J.; Jiang, N.; Jiang, S.; Ma, J. Two novel ANK1 loss-of-function mutations in Chinese families with hereditary spherocytosis. J. Cell Mol. Med. 2019, 23, 4454–4463. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Andolfo, I.; Manna, F.; Gambale, A.; Marra, R.; Rosato, B.; Caforio, P.; Pinto, V.; Pignataro, P.; Radhakrishnan, K.; et al. Multi-gene panel testing improves diagnosis and management of patients with hereditary anemias. Am. J. Hematol. 2018, 93, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Bogusławska, D.; Heger, E.; Machnicka, B.; Skulski, M.; Kuliczkowski, K.; Sikorski, A. A new frameshift mutation of the β-spectrin gene associated with hereditary spherocytosis. Ann. Hematol. 2017, 96, 163–165. [Google Scholar] [CrossRef]

| Primer | Sequence |
|---|---|
| Sense Strand | ZZUD Xhol 5′-GACCTCGAGCGTTCCTGGTTTCTTTTATGG-3′ |
| Antisense strand | ZZUD HindIII 5′-CGTCAAGCTTCAACCACTACCTTCCAGC-3′ |
| EGFP-ZZUD/ZZUDL1340P | mRFP1-ZZUD/ZZUDL1340P | |
|---|---|---|
| Sense strand primers | EGFP-C 5′-CATGGTCCTGCTGGAGTTCGTG-3′ | DsRed1-C 5′-AGCTGGACATCACCTCCCACAACG-3′ |
| SV40p-A-Rs 5′-CCACGAAACTGGTGTATGC-3′ | RFP-2s 5′-GTCTGCTGTGCTCTGTGATTGG-3′ | |
| Antisense strand primers | EGFP-Ca 5′-CTGCCGTCAGTTCTTTATACAGC-3′ | RFP-1a 5′-GACAATCGGACAGCCAGAAACG-3′ |
| SV40p-A-R 5′-GAAATTTGTGATGCTATTGC-3′ | RFP-2a 5′-AACCTCTACAAATGTGGTATGGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machnicka, B.; Czogalla, A.; Bogusławska, D.M.; Stasiak, P.; Sikorski, A.F. Spherocytosis-Related L1340P Mutation in Ankyrin Affects Its Interactions with Spectrin. Life 2023, 13, 151. https://doi.org/10.3390/life13010151
Machnicka B, Czogalla A, Bogusławska DM, Stasiak P, Sikorski AF. Spherocytosis-Related L1340P Mutation in Ankyrin Affects Its Interactions with Spectrin. Life. 2023; 13(1):151. https://doi.org/10.3390/life13010151
Chicago/Turabian StyleMachnicka, Beata, Aleksander Czogalla, Dżamila M. Bogusławska, Piotr Stasiak, and Aleksander F. Sikorski. 2023. "Spherocytosis-Related L1340P Mutation in Ankyrin Affects Its Interactions with Spectrin" Life 13, no. 1: 151. https://doi.org/10.3390/life13010151
APA StyleMachnicka, B., Czogalla, A., Bogusławska, D. M., Stasiak, P., & Sikorski, A. F. (2023). Spherocytosis-Related L1340P Mutation in Ankyrin Affects Its Interactions with Spectrin. Life, 13(1), 151. https://doi.org/10.3390/life13010151







