H2O2/DEM-Promoted Maft Promoter Demethylation Drives Nrf2/ARE Activation in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
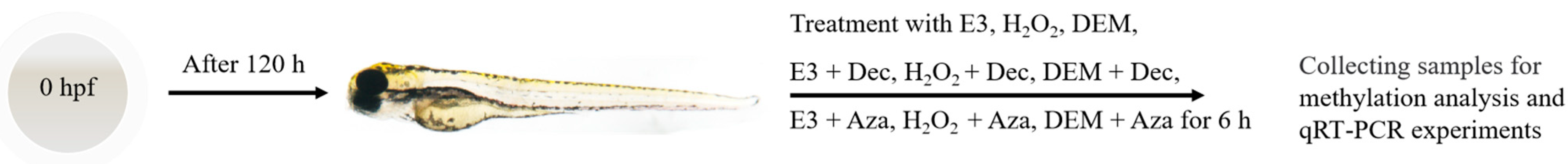
2.2. H2O2 and DEM Exposure of Zebrafish
2.3. Total RNA Extraction
2.4. Real-Time Quantitative PCR Assay
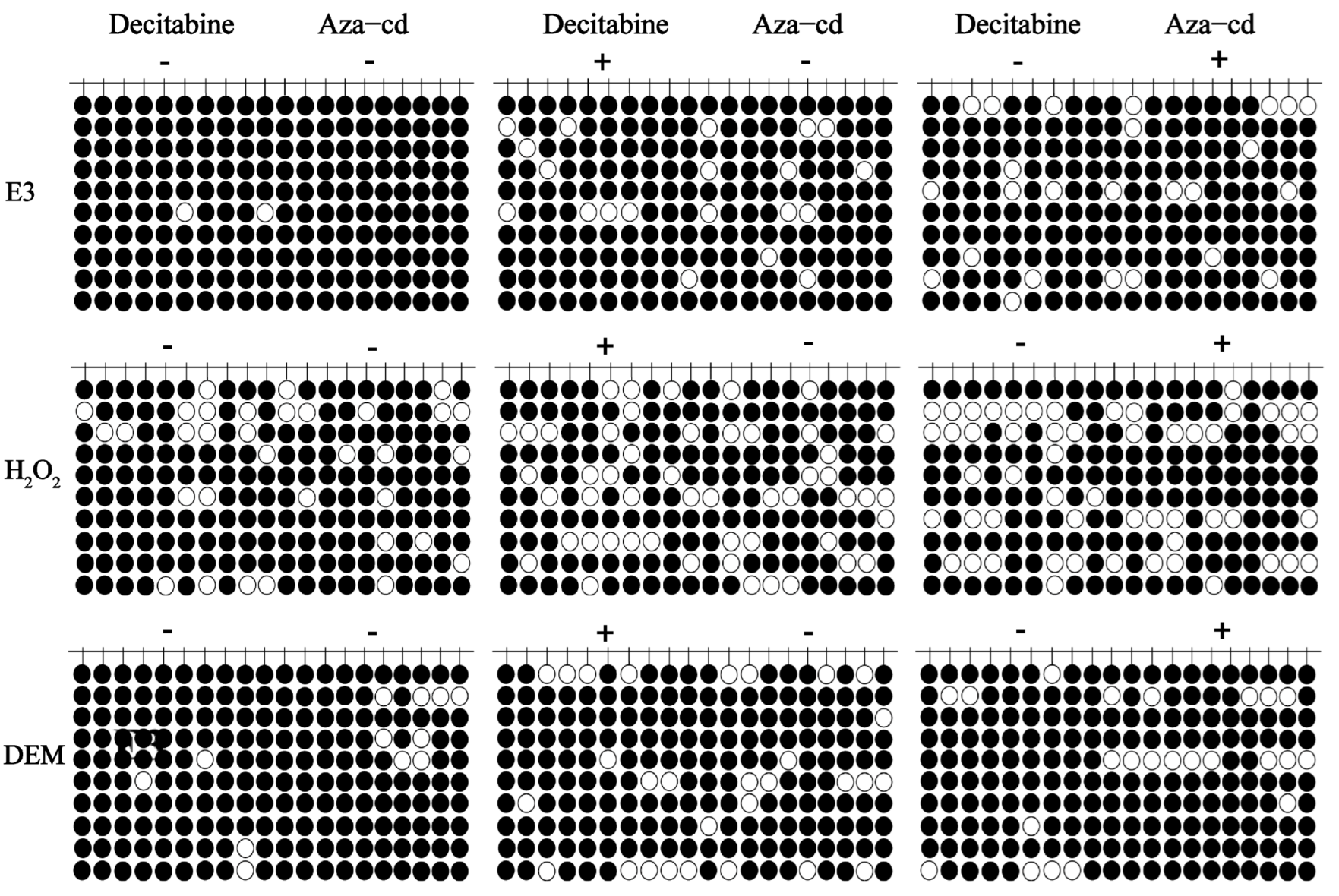
2.5. Genomic DNA Methylation Analysis
2.6. Statistical Analyze Software and Websites
3. Results
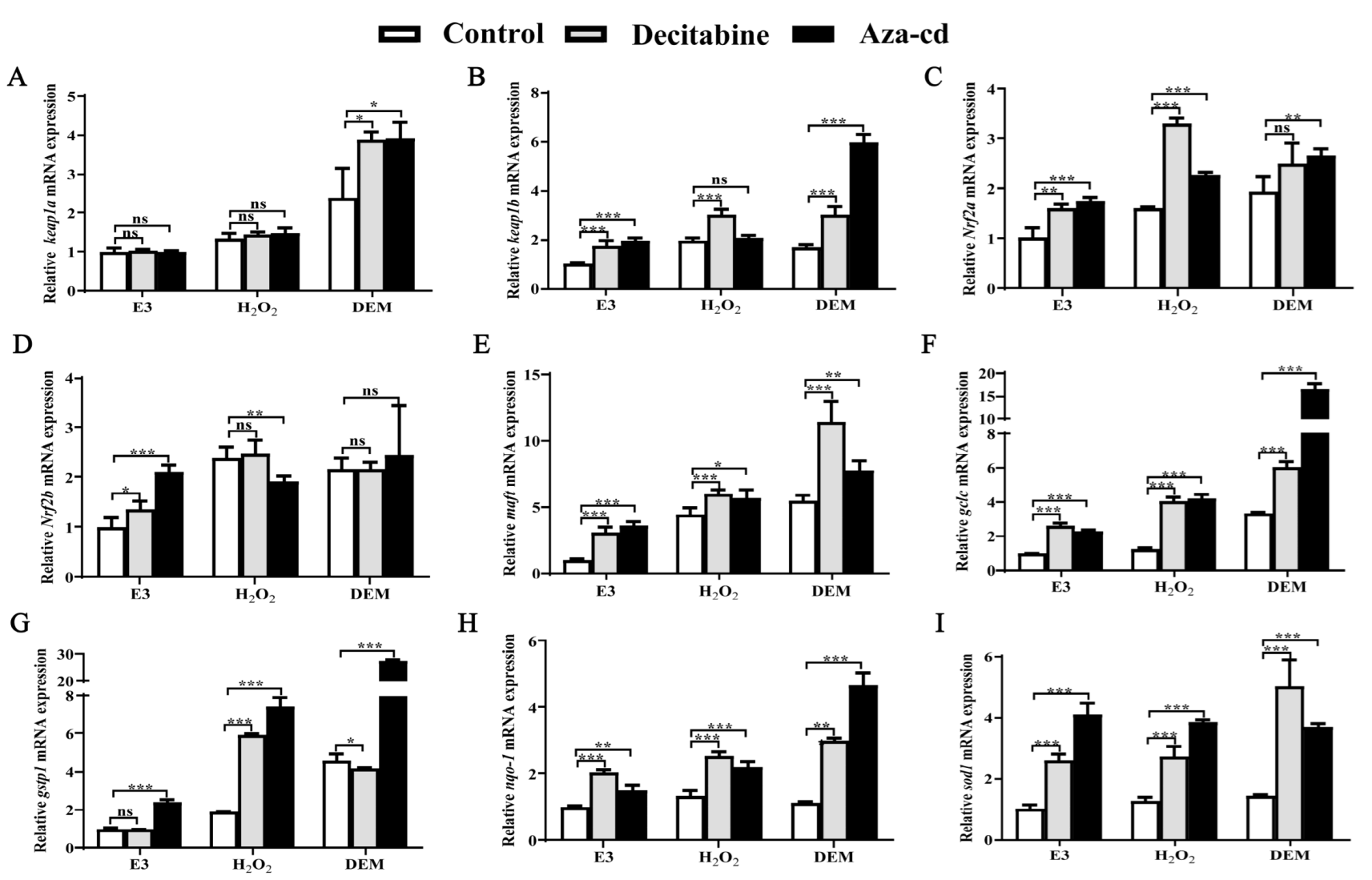
3.1. H2O2 and DEM Activate the Zebrafish Nrf2/ARE Signaling Pathway
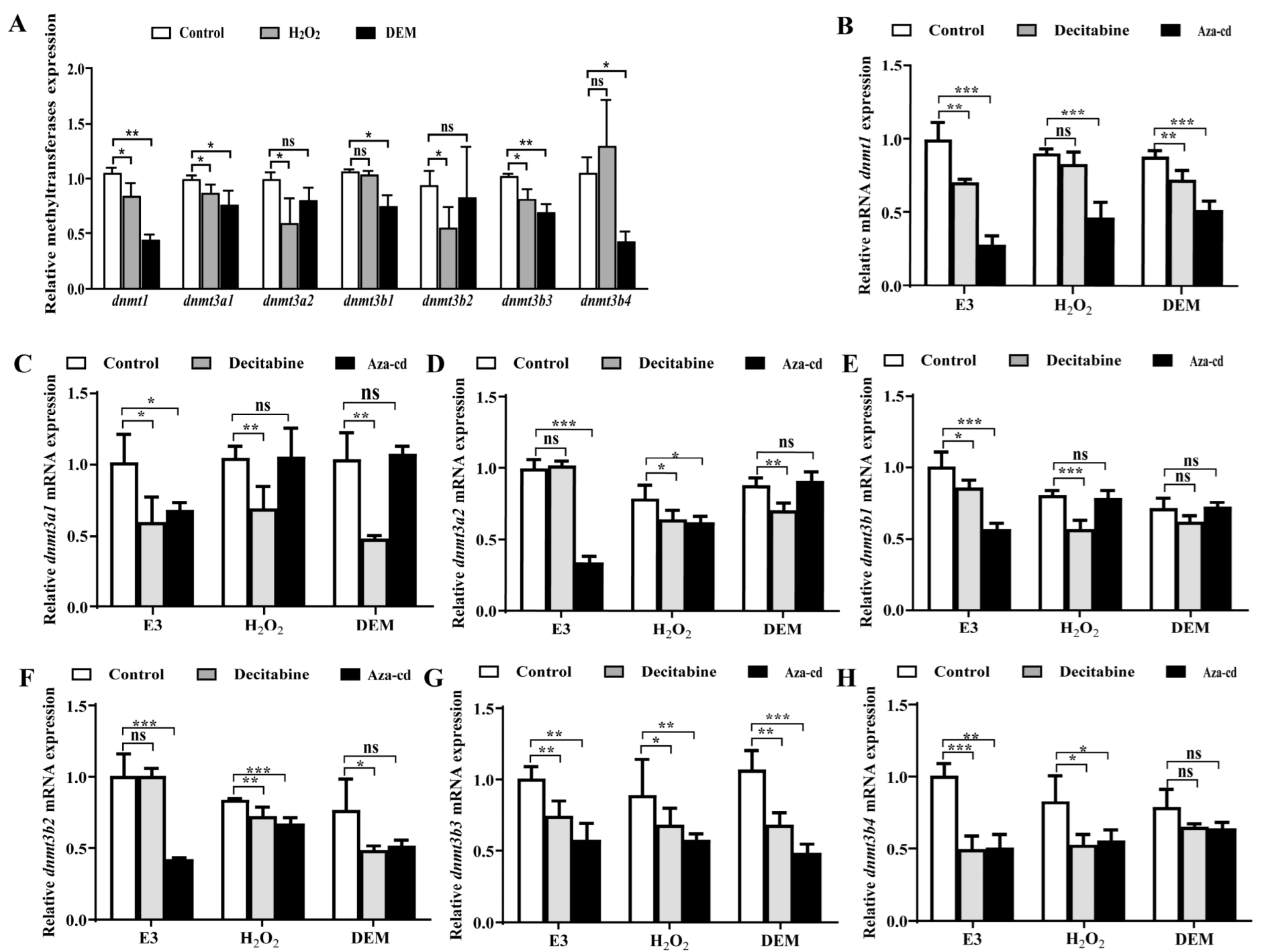
3.2. H2O2 and DEM Activate Nrf2/ARE Signaling Pathway Mechanism in Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, S.M.; Xin, J.C.; Liu, D.Y. Influence of reactive oxygen species on secretory component in the intestinal epithelium during hyperoxia. Exp. Ther. Med. 2017, 14, 4033–4040. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 23. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Scarpato, R.; Testi, S.; Colosimo, V.; Crespo, C.G.; Micheli, C.; Azzara, A.; Tozzi, M.G.; Ghirri, P. Role of oxidative stress, genome damage and DNA methylation as determinants of pathological conditions in the newborn: An overview from conception to early neonatal stage. Mutat. Res. Mutat. Res. 2020, 783, 11. [Google Scholar] [CrossRef]
- Mann, G.E. Nrf2-mediated redox signalling in vascular health and disease. Free Radic. Biol. Med. 2014, 75, S1. [Google Scholar] [CrossRef]
- Hast, B.E.; Goldfarb, D.; Mulvaney, K.M.; Hast, M.A.; Siesser, P.F.; Yan, F.; Hayes, D.N.; Major, M.B. Proteomic Analysis of Ubiquitin Ligase KEAP1 Reveals Associated Proteins That Inhibit NRF2 Ubiquitination. Cancer Res. 2013, 73, 2199–2210. [Google Scholar] [CrossRef]
- Puglia, M.H.; Lillard, T.S.; Morris, J.P.; Connelly, J.J. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc. Natl. Acad. Sci. USA 2015, 112, 3308–3313. [Google Scholar] [CrossRef]
- Wolffe, A.P.; Matzke, M.A. Epigenetics: Regulation through repression. Science 1999, 286, 481–486. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y. DNA Methylation in Mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, 21. [Google Scholar] [CrossRef]
- Cocozza, S.; Scala, G.; Miele, G.; Castaldo, I.; Monticelli, A. A distinct group of CpG islands shows differential DNA methylation between replicas of the same cell line in vitro. BMC Genom. 2013, 14, 9. [Google Scholar] [CrossRef]
- Skinner, M.K.; Savenkova, M.I.; Zhang, B.; Gore, A.C.; Crews, D. Gene bionetworks involved in the epigenetic transgenerational inheritance of altered mate preference: Environmental epigenetics and evolutionary biology. BMC Genom. 2014, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Greally, J.M.; Jacobs, M.N. In Vitro and In Vivo Testing Methods of Epigenomic Endpoints for Evaluating Endocrine Disruptors. ALTEX-Altern. Anim. Exp. 2013, 30, 445–471. [Google Scholar] [CrossRef]
- Williams, T.D.; Mirbahai, L.; Chipman, J.K. The toxicological application of transcriptomics and epigenomics in zebrafish and other teleosts. Brief. Funct. Genom. 2014, 13, 157–171. [Google Scholar] [CrossRef]
- Cheng, X.D.; Blumenthal, R.M. Mammalian DNA methyltransferases: A structural perspective. Structure 2008, 16, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kobayashi, M.; Kaneko, H.; Nakajima-Takagi, Y.; Nakayama, Y.; Yamamoto, M. Molecular evolution of Keap1-Two Keap1 molecules with distinctive intervening region structures are conserved among fish. J. Biol. Chem. 2008, 283, 3248–3255. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Karchner, S.I.; Franks, D.G.; Jenny, M.J.; Harbeitner, R.C.; Goldstone, J.V.; McArthur, A.G.; Hahn, M.E. Nrf2b, Novel Zebrafish Paralog of Oxidant-responsive Transcription Factor NF-E2-related Factor 2 (NRF2). J. Biol. Chem. 2012, 287, 4609–4627. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.K.; Cook, J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Kobayashi, M.; Li, L.; Suzuki, T.; Nishikawa, K.; Yamamoto, M. MafT, a new member of the small Maf protein family in zebrafish. Biochem. Biophys. Res. Commun. 2004, 320, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Christman, J.K. 5-Azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef]
- Choi, S.H.; Byun, H.M.; Kwan, J.M.; Issa, J.P.J.; Yang, A.S. Hydroxycarbamide in combination with azacitidine or decitabine is antagonistic on DNA methylation inhibition. Br. J. Haematol. 2007, 138, 616–623. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; He, M.; Li, X.; Yu, L.; Liu, Y.; Yang, Y.; Li, L.; Jia, J.; Li, B. H2O2/DEM-Promoted Maft Promoter Demethylation Drives Nrf2/ARE Activation in Zebrafish. Life 2022, 12, 1436. https://doi.org/10.3390/life12091436
Chen C, He M, Li X, Yu L, Liu Y, Yang Y, Li L, Jia J, Li B. H2O2/DEM-Promoted Maft Promoter Demethylation Drives Nrf2/ARE Activation in Zebrafish. Life. 2022; 12(9):1436. https://doi.org/10.3390/life12091436
Chicago/Turabian StyleChen, Ce, Mingyue He, Xueting Li, Lidong Yu, Yi Liu, Yan Yang, Li Li, Jianbo Jia, and Bingsheng Li. 2022. "H2O2/DEM-Promoted Maft Promoter Demethylation Drives Nrf2/ARE Activation in Zebrafish" Life 12, no. 9: 1436. https://doi.org/10.3390/life12091436
APA StyleChen, C., He, M., Li, X., Yu, L., Liu, Y., Yang, Y., Li, L., Jia, J., & Li, B. (2022). H2O2/DEM-Promoted Maft Promoter Demethylation Drives Nrf2/ARE Activation in Zebrafish. Life, 12(9), 1436. https://doi.org/10.3390/life12091436





